Back to Journals » International Medical Case Reports Journal » Volume 16
A Masquerade Case: Choroidal Hemangioma Misdiagnosed As Central Serous Retinopathy
Authors Lai L, Javier T, Lee S, Gallemore RP
Received 13 December 2022
Accepted for publication 28 February 2023
Published 4 April 2023 Volume 2023:16 Pages 239—244
DOI https://doi.org/10.2147/IMCRJ.S398844
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Leo Lai, Therese Javier, Sol Lee, Ron P Gallemore
Department of Clinical Research, Retina Macula Institute, Torrance, CA, USA
Correspondence: Ron P Gallemore, Department of Clinical Research, Retina Macula Institute, 4201 Torrance Blvd, Torrance, CA, 90503, USA, Tel +1 310 - 944 – 9393, Fax +1 310 - 944 – 3393, Email [email protected]
Purpose: To report a case of misdiagnosed choroidal hemangioma, initially treated as central serous retinopathy (CSR) complicated by choroidal neovascularization (CNV), and to improve the proper identification of this disorder.
Observations: Fundus images revealed a subtle, elevated choroidal lesion with an associated exudative detachment and choroidal vascular lesion on indocyanine green (ICG) angiography. Combined treatment with photodynamic therapy (PDT) and anti-VEGF therapy led to resolution of fluid and improvement in VA from 20/50 to 20/25.
Conclusion: It is critical to understand the clinical features of choroidal hemangiomas and their physical presentation on retinal testing to diagnose and treat them in a timely and appropriate manner.
Keywords: macular edema, subretinal fluid, CSR, central serous retinopathy, diagnostic testing
Introduction
A choroidal hemangioma is a benign congenital vascular tumor that is often sight threatening.1 It can be difficult to diagnose and may mimic other disorders due to the nonspecific findings it may be associated with, including macular edema, exudative retinal detachment and leakage on fluorescein angiography (FA).2 The lesion presents both a therapeutic and a diagnostic challenge. Here, we present a case which was initially misdiagnosed and mistreated as CSR complicated by CNV, and we hope to improve the diagnosis and appropriate treatment of this disorder.
Methods
Retrospective chart review of a patient being treated for choroidal hemangioma, documented with optical coherence tomography (OCT) on the Cirrus HD-OCT (Carl Zeiss Meditec AG, Jena, Germany), A-scan and B-scan ultrasonography (Ellex Eye-Cubed, Adelaide, Australia), and wide-field fluorescein and indocyanine green angiography (Optos, Dunfermline, United Kingdom).
Case Report
A 56-year-old Caucasian female presented for a second opinion regarding the diagnosis and treatment of vision loss in her left eye. She noted blurry vision in only the left eye which started about five years prior. She was initially seen elsewhere and diagnosed with central serous retinopathy complicated by choroidal neovascularization. She was treated with intravitreal injections of bevacizumab (n=1) and aflibercept (n=1) over a period of two months with no improvement.
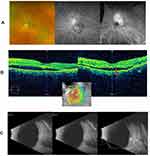
On presentation to our office, visual acuity (VA) was 20/20 in the right eye (OD) and 20/50 in the left eye (OS) with pinhole VA to 20/40 OS. Intraocular pressure (IOP) was 17 mmHg OD and 20 mmHg OS. On exam, there was an area of subtle elevation of the retina above and adjacent to the disc, measuring approximately six disc areas in size. FA studies revealed leakage from an ill-defined lesion superotemporal to the disc, while the indocyanine-green (ICG) studies showed diffuse and intense hyperfluorescence of choroidal vessels, consistent with a choroidal hemangioma (Figure 1A). Baseline OCT studies over the lesion revealed a smooth dome-like elevation of the retinal pigment epithelium (RPE), associated with a shallow exudative detachment (Figure 1B). B-scan ultrasonography demonstrated a mass lesion with a maximal apical height of 2.32 mm, maximal basal dimensions of 6.68 mm x 7.30 mm, and areas of medium to high internal reflectivity on the A-scan (Figure 1C).
She was initially scheduled for treatment with PDT, in conjunction with intravitreal aflibercept to counteract any associated inflammation the treatment might cause.3,4 A half-fluence PDT protocol was utilized with 25 Joules, spot size of 5200, and 83s duration.5,6 Post-operative inflammation was managed with a course of topical anti-inflammatory eyedrops. An NSAID (bromfenac 0.075% ophthalmic solution) eyedrop and a steroid (difluprednate 0.05% ophthalmic emulsion) eyedrop were both initially used twice daily and tapered off several weeks thereafter.
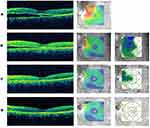
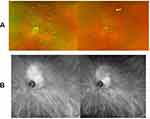
On the day of treatment, OCT studies documented a significant amount of subretinal fluid (SRF) within the macula associated with the choroidal hemangioma (Figure 2A). Three weeks after treatment with PDT, intravitreal aflibercept, and topical anti-inflammatory eyedrops, there was dramatic improvement in SRF on the OCT difference map (Figure 2B). Five weeks after treatment, OCT studies showed near complete resolution of SRF (Figure 2C) and VA remained stable at 20/50 OS with stable intraocular pressures of 14 mmHg OD and 18 mmHg OS. Seven and a half weeks after treatment, there was complete resolution of SRF (Figure 2D) and improvement in VA to 20/30 OS; intraocular pressures were 17 mmHg OD and 20 mmHg OS. Eleven and a half weeks after treatment, fundus photos showed a decrease in elevation of the lesion (Figure 3A), and ICG studies showed a decrease in choroidal perfusion associated with hemangioma (Figure 3B). VA ultimately improved to 20/25 OS.
Discussion
Choroidal hemangiomas can present in two different forms: diffuse or circumscribed. Diffuse hemangiomas are commonly found at birth and are usually linked to Sturge-Weber syndrome.1,7 Circumscribed hemangiomas, on the other hand, do not have systemic associations and are usually diagnosed in adults during routine eye exams or when patients experience visual symptoms.8 A combination of ancillary testing - such as ultrasonography, optical coherence tomography, and angiographic studies - and the examination of key clinical features are needed to correctly diagnose choroidal hemangiomas.7 Clinically, diffuse choroidal hemangiomas present with red-orange posterior choroidal thickening.1,9 Circumscribed hemangiomas are characterized as red-orange elevated masses with blended margins and are usually found at the posterior pole.1,8 In symptomatic cases of circumscribed hemangiomas, subretinal fluid is usually present and can lead to more serious complications, such as an exudative retinal detachment. A circumscribed hemangioma may be misdiagnosed as an amelanotic choroidal melanoma, central serous retinopathy, metastatic tumor, exudative macular degeneration, or other processes (infectious, infiltrative, inflammatory, or vascular) that may be associated with vascular leakage and an exudative detachment. Ancillary testing can lead to increased accuracy in diagnosis and selecting the appropriate therapeutic interventions.
Angiographic studies with fluorescein and indocyanine green dyes, ultrasonography, and optical coherence tomography, in particular, can be used for accurate diagnosis of choroidal hemangiomas. In fluorescein angiography, circumscribed hemangiomas appear as lacy hyperfluorescent masses in the (early) pre-arterial and arterial phase but increase in overall hyperfluorescence in later phases along with variable quantities of late leakage.1,7,8 Diffuse hyperfluorescence in the pre-arterial phase is seen in diffuse hemangiomas.8 In indocyanine green angiography, the intrinsic vascular pattern of the hemangioma is better visualized, and a distinct hyperfluorescence can be seen within 30 seconds of the dye injection.1,8 During the late phase, the subsequent departure of the dye from the hemangioma leads to a “washout” effect and a decrease in initial hyperfluorescence.1,7,8 This late stage “washout” is commonly seen in circumscribed hemangiomas but is clinically absent in diffuse hemangiomas.9 On B-scan ultrasonography, circumscribed hemangiomas typically appear as a dome-shaped mass with acoustic solidity, with high internal reflectivity between 50% and 100% on the A-scan ultrasonography.1,8 In contrast, diffuse hemangiomas are portrayed as diffuse, marked thickening of the choroid instead of a dome-shaped mass.9 Optical coherence tomography is utilized to study retinal changes secondary to the choroidal hemangiomas, such as cystoid macular edema and subretinal fluid.8 Combined data from clinical findings and ancillary testing are key in properly diagnosing choroidal hemangiomas and starting effective treatment.
In contrast, central serous retinopathy or central serous chorioretinopathy presents in a different fashion. Clinical features are divided into the acute and chronic stage. In the acute stage, which lasts and resolves within 3–4 months, CSR presents with a distinct serous macular detachment. Yellowish subretinal material from fibrin, RPE detachments, and RPE defects may also be identified. The chronic stage, which extends beyond the 3–4 month period, presents with RPE degeneration, schitic or cystoid retinal edema, and shallow SRF.10 Cases where the SRF initially resolves and then returns are known as “recurrent CSR.”
Optical coherence tomography and angiographic studies with fluorescein and indocyanine green dyes are also used to aid the diagnosis of CSR. Optical coherence tomography is the first line of defense in correctly diagnosing CSR and can provide information on the presence of SRF, pigment epithelial detachments (PED), and retinal thickening.11,12 SRF can present as a hyporeflective spot between the RPE and neurosensory retina (NSR) in acute CSR. The form of the NSR remains intact with acute SRF, but prolonged SRF can lead to the elongation of the photoreceptor outer segment. Intraretinal cysts and schitic spaces are seen in chronic CSR cases on the OCT.10 There is also increased choroidal thickness, with a mean of 505μm, suggesting increased hydrostatic pressure in the choroid of CSR patients.11,13 In fluorescein angiography, leaks in the RPE are shown and are commonly seen in the macula. An “ink-blot” or “smoke-stack” pattern around the mid and late phase are classic presentations of CSR.10,11 There is usually a single leakage point with acute CSR; however, though less common, multifocal point leaks are possible and are usually adjacent to the area of SRF, making it differentiable from a choroidal hemangioma. Chronic CSR commonly shows multifocal leakage sites, leading to mottled or granular hyperfluorescence in the mid and late phase of the fluorescein angiography.10 Indocyanine green angiography provides information on the abnormal choroidal circulation seen in patients with CSR.11 There is choroidal filling delay in the early phase while the mid-phase shows focal choroid hyperfluorescence due to choroidal vascular hyperpermeability. In the late phase, the hyperfluorescent areas usually fade, though they can also lead to persistent hyperfluorescence or centrifugal displacement of hyperfluorescence in some cases.10 Broad areas of atrophy and loss of choriocapillaris may occur with chronic CSR, creating window defects on FA and ICG studies. This can be similar to a choroidal hemangioma but a choroidal mass is not present in CSR, so broad-dome elevations of choroid and RPE on the OCT and B-scan, as seen in circumscribed choroidal hemangiomas, can be used to distinguish its diagnosis from multifocal chronic CSR.
Based on the clinical findings with the above tests, our patient clearly presented with a choroidal hemangioma rather than central serous retinopathy. Treatment options for choroidal hemangioma include observation in the cases for lesions not visually significant, PDT, steroidal and nonsteroidal anti-inflammatory treatment, anti-VEGF therapy, thermal laser, radioactive plaques, or a combination of the above.7,9 In this case, we used a combination of PDT and anti-VEGF therapy, followed by topical anti-inflammatory (NSAID and corticosteroid) eyedrops, leading to the resolution of the subretinal fluid over the course of seven and a half weeks.
While the distinct characteristics of a choroidal hemangioma are well-documented in the literature, choroidal hemangiomas continue to be misdiagnosed.14,15 In a 2001 review, Shields et al found that of 200 consecutive patients with a choroidal hemangioma, 142 cases (or 71%) were misdiagnosed prior to referral – 7% of which were misdiagnosed as central serous retinopathy.13 The treatment options for a choroidal hemangioma compared to other ocular problems are vastly different, and accurate diagnosis is crucial. In this case, the patient underwent many unnecessary, ineffective sequential anti-VEGF injections without response to treatment, while the addition of PDT early on would likely have resolved the condition and restored vision. Some permanent vision loss may also have been prevented. Careful clinical observation and supporting diagnostic data are key components that can be used to properly diagnose a patient with choroidal hemangioma and to appropriately administer treatment in a timely manner.
Patient Consent
The authors confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies. Informed written consent has been obtained from all patients to have case details and any accompanying images published.
Acknowledgments
No institutional approval was required for the publishing of this case series.
Funding
No grant support or funding was given for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Singh A, Kaiser P, Sears J. Choroidal hemangioma. Ophthalmol Clin North Am. 2005;18,1:151–161. doi:10.1016/j.ohc.2004.07.004
2. Mashayekhi A, Shields CL. Circumscribed choroidal hemangioma. Curr Opin Ophthalmol. 2003;14,3:142–149. doi:10.1097/00055735-200306000-00006
3. Sagong M, Lee J, Chang W. Application of intravitreal bevacizumab for circumscribed choroidal hemangioma. Korean J Ophthalmol. 2009;23:127–131. doi:10.3341/kjo.2009.23.2.127
4. Kim H, Wang A, Mititelu M. Case series of anti-vascular endothelial growth factor and photodynamic therapy in the treatment of circumscribed choroidal hemangiomas. J Vitreoretin Dis. 2017;1:133–137. doi:10.1177/2474126416687424
5. Van Dijk E, van Rijssen T, Subhi Y, Boon C. Photodynamic therapy of chorioretinal diseases; a practical approach. Ophthalmol Ther. 2020;9:329–342. doi:10.1007/s40123-020-00250-0
6. Nagesha C, Walinjkar J, Khetan V. Choroidal neovascular membrane in a treated choroidal hemangioma. Indian J Ophthalmol. 2016;64:606–608.
7. Manayath GJ, Ranjan R, Shah VS, et al. Central serous chorioretinopathy: current update on pathophysiology and multimodal imaging. Oman J Ophthalmol. 2018;11(2):103–112. doi:10.4103/ojo.OJO_75_2017
8. Karimi S, Nourinia R, Mashayekhi A, et al. Circumscribed choroidal hemangioma. J Ophthalmic Vis Res. 2015;10,3:320–328.
9. Ramasubramanian A, Shields C. The current management of choroidal hemangioma. Retina Today. 2010;1:52–55.
10. Ross A, Ross AH, Mohamed Q. Review and update of central serous chorioretinopathy. Curr Opin Ophthalmol. 2011;22(3):166–173. doi:10.1097/ICU.0b013e3283459826
11. van Velthoven MEJ, Verbraak FD, Garcia PM, et al. Evaluation of central serous retinopathy with en face optical coherence tomography. Br J Ophthalmol. 2005;89,11:1483–1488. doi:10.1136/bjo.2005.073056
12. Imamura Y, Fujiwara T, Margolis RON, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29:1469–1473. doi:10.1097/IAE.0b013e3181be0a83
13. Shields CL, Honavar SG, Shields JA, Cater J, Demirci H. Circumscribed choroidal hemangioma: clinical manifestations and factors predictive of visual outcome in 200 consecutive cases. Ophthalmology. 2001;108:2237–2248. doi:10.1016/S0161-6420(01)00812-0
14. Pérez-González D, Goldstein M, Iglicki M, et al. Half-dose photodynamic therapy as a novel treatment protocol for circumscribed choroidal hemangioma. Life. 2022;12(11):1748. doi:10.3390/life12111748
15. Rahman W, Horgan N, Hungerford J. Circumscribed choroidal haemangioma mimicking chronic central serous chorioretinopathy. J Fr Ophtalmol. 2013;36(3):e37–e40. doi:10.1016/j.jfo.2012.05.006
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.