Back to Archived Journals » Journal of Neurorestoratology » Volume 2
A long-term, complex, unitary appraisal regarding neurorestorative, including neurorehabilitative, outcomes in patients treated with Cerebrolysin®, following traumatic brain injury
Authors Daia C, Haras M, Spircu T, Anghelescu A, Onose L, Ciurea A, Mihăescu AS, Onose G
Received 30 December 2013
Accepted for publication 20 February 2014
Published 26 June 2014 Volume 2014:2 Pages 85—93
DOI https://doi.org/10.2147/JN.S49693
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Cristina O Daia,1,2 Monica Haras,1,2 Tiberiu Spircu,1 Aurelian Anghelescu,1,2 Liliana Onose,3 Alexandru Vlad Ciurea,1,2 Anca Sanda Mihaescu,2 Gelu Onose1,2
1Carol Davila University of Medicine and Pharmacy, Bucharest, Romania; 2Bagdasar-Arseni Teaching Emergency Hospital, Bucharest, Romania; 3Metrorex – The Medical Service, Bucharest, Romania
Background: Neuroprotection is a modern therapeutic concept that has some useful outcomes discussed in the literature, including for traumatic brain injury (TBI).
Scope and study design: This was a retrospective case-control study that was approved by the bioethics commission of the Bagdasar-Arseni Teaching Emergency Hospital, Bucharest, Romania. The aim of the study was to comparatively assess neurorestorative, including neurorehabilitative, outcomes obtained with or without Cerebrolysin®.
Materials and methods: Nineteen cases treated with Cerebrolysin versus 28 who did not receive this drug were included in this study. All cases had a subacute or post-acute status after TBI and were hospitalized (only at their first admission) between January 2005 and December 2010 in the hospital's NeuroRehabilitation Clinic Division. Epidemiological, clinical, paraclinical, and functional parameters were evaluated, using the: Functional Independence Measure (FIMTM), Glasgow Outcome Score (GOS), and Modified Rankin Scale.
Results: Patients in the Cerebrolysin group had, on average, higher (although not statistically significant) FIM evolution values (36.53) than the control group (29.64) (P=0.174, 95% confidence interval: -8.0 to 21.8). The effect size assessed on the GOS was 2.1%. Additionally, the mean FIM value at admission of the Cerebrolysin group (45.79) was lower than that of controls (61.50; P=0.076).
Discussion and conclusion: The clinical/functional evolution, comparatively evaluated in the studied inpatients, and taking into account the small sample and effect sizes – including for GOS – suggest that Cerebrolysin, correctly indicated and administered, may perhaps contribute to some improvement of post-TBI patients' overall neurorestorative/rehabilitative outcomes; this given the short period (approximately 1 month) over which the medicine's action was evaluated, the lower FIM mean value at admission in the Cerebrolysin group, and respectively that, for severe central nervous system lesions – including after TBI – and consequent conditions, it cannot yet be concluded that any therapeutic approaches, such as Cerebrolysin, can significantly improve post-injury outcomes.
Keywords: neuroprotection, Functional Independence Measure (FIM), brain trauma
Background
Brain and spinal cord injuries (traumatic or nontraumatic) generally entail lesions of the central nervous system (CNS), which, especially when severe, may result in impairments that can vary in severity and duration. These impairments may affect motor/neural muscles (tone decrease and/or trophicity); coordination; balance; sphincter control; and sensations. For traumatic brain injury (TBI) in particular, sensory, cognitive/consciousness, communication, and behavioral impairments can also occur, often with an altered general state.
The major lesions/pathophysiological conditions following primary injuries and involved in the development of secondary brain damage are currently considered to be the following:
- hemorrhage (extradural, subdural, subarachnoid, intracerebral, and intraventricular);
- brain tissue swelling;
- reduced local/regional blood flow leading to ischemia;
- possible infection.1
It should be noted that intracranial pressure (especially that over 40 mmHg, or persistent intracranial pressure values above 20 mmHg)2 aggravates TBI severity – leading to cerebral mass herniation – and, especially, brain tissue swelling and ischemia, with consequent hydrocephalus and hypoxia,3 respectively. The points listed above correspond, at cellular and subcellular levels, to what modern research has identified as a series of events that lead, cyclically, to secondary injuries. These events include the following: “impact depolarization” – effusion from the disrupted cells of the potassium ions and of the neurotransmitter glutamate – resulting in early and very dangerous excitotoxicity;4 failure of cellular energy metabolism, interconditioned with local intense generation of reactive oxygen species/oxidative stress5 (including with the detrimental role of bleeding/iron)6 followed by biological membranes’ lipid peroxidation, DNA and protein damage/misfolding, immune shifts/inflammatory processes/acidosis, global and/or focal multifactorial ischemia, ischemic penumbra and alteration of regional microcirculation, ionic disturbances (leading to cell swelling, including massive edema – due to sudden osmolysis, and cell-induced necrosis), with added excitotoxicity (excess influx of intracellular calcium ions), related activation of apoptotic genes, and, thus, of different pathways of delayed mechanisms of cell death: apoptosis and apoptosis-like processes.1,7–11
Thereby, the cascade of secondary events entails an extremely complex and extended reaction, from a gene level to a macroscopic/clinical level. Therefore, the concept of secondary CNS (including brain) injuries has become the basis for developing an array of neuroprotective modern therapies in traumatic, ischemic, and degenerative injuries of the CNS (including both the brain and the spinal cord).
Based on a deeper and more comprehensive knowledge of detailed neural functioning, it is considered that the approaches most likely to lead to positive results are those that would best merge with the continuous activity of the endogenous defense system; this system acts continuously in the nervous system, simultaneously performing and integrating neurobiological processes of neurotrophicity, neuroprotection, neuroplasticity, neurogenesis and synaptogenesis.12
Historical and general data
In 1986, Rita Levi-Montalcini and Stanley Cohen received the Nobel Prize for discovering nerve growth factor (NGF) and epidermal growth factor, respectively.13 Since that time, many other neurotrophic factors have been identified; they are naturally synthesized polypeptides. Their activity is crucial for nervous system development and neural cells’ natural survival, including resistance to noxious factors, retaining phenotypes during their lifetime and neurotransmitter production, especially for sympathetic neuron peripheral extensions.14
The neurotrophic factor concept appeared several decades ago, mainly generated by experimental observations regarding tight relationships between the amount of target tissues and the size of related neuronal populations: NGF, discovered in the 1950s, was the first known molecular proof. This concept basically refers to the feedback-type property of innervated tissues to generate signals for their innervating neurons in order to selectively limit neuronal death, which occurs in the course of development; in fact, it is an application in living organisms of a more general principle for any well-functioning state (including in society/economy – for instance, feasibility studies). Hence, this is a simple and clever way to match the size of neuronal and target cell populations, by conditioning the related neurons’ survival.14
Neurotrophic factors generally belong to one of three families: neurotrophins; glial cell-line derived neurotrophic factor family ligands; or neuropoietic cytokines – all generally with synergistic mechanisms/additive interactions – although not entirely common, and only partially overlapped.15
Neurotrophic factors also stimulate neural plasticity and synaptic activity, therefore are important for both learning processes and the nervous system’s ability to spontaneously reorganize and thus clinically adapt (mainly by taking over/substitution) after different injuries, a process that is known as (self-)neurorecovery.12
The identification of the neurotrophic factors and their importance has led to new perspectives for the therapy of TBI and neurodegenerative and cerebrovascular diseases. The major disadvantage of these polypeptides is their low rate of penetration through the hemato-neurraxial/blood–brain barrier, due to their relatively high molecular weight.
By its molecular structure, Cerebrolysin® (EVER Neuro Pharma GmbH, Unterach, Austria) has the potential to overcome this biochemical/pharmacodynamic hurdle. Different aspects of Cerebrolysin’s properties have been presented elsewhere.10,16–19
Herein we only reiterate the well-known mechanisms of action by which Cerebrolysin – through its NGF-like activity – protects against neuronal damage, including apoptosis/necrosis, and stimulates neuroplasticity and axonal/dendritic growth. In summary, Cerebrolysin:
- protects against excitotoxicity;
- inhibits the caspase protease pathway and protects against apoptosis;
- inhibits the calpain protease (Ca2+-dependent) pathway and protects against apoptosis;
- protects neuronal cytoskeleton elements against degradation;
- prevents free radical formation after cellular insults;
- decreases beta-amyloid production; and
- stimulates neuroplasticity, neurogenesis (axonal and dendritic growth) and synaptogenesis.
Taking into account Cerebrolysin’s main reported effects and the pathways of the secondary injury cascades it targets/counteracts, it has been considered as a pleiotropic drug, but, as it seems also to stimulate neuroplasticity, neurogenesis, and synaptogenesis, it is reasonable to consider that it may have a multimodal mechanism of action.10,16–20
Materials and methods
Scope of the study
The present study aimed to assess neurorestorative, including neurorehabilitative, outcomes obtained in inpatients of the Physical (neuromuscular) and Rehabilitation Medicine/NeuroRehabilitation Clinic Division of the Bagdasar-Arseni Teaching Emergency Hospital, Bucharest, Romania with subacute/post-acute conditions following TBI and treated with Cerebrolysin, compared to inpatients who did not receive this multimodal drug.
Study design
This was a retrospective case-control study, approved by The Bio-ethics Commission of the Bagdasar-Arseni Teaching Emergency Hospital, comprising a comparative analysis: post-TBI subacute/post-acute inpatients who received Cerebrolysin (10 mL per day) for an average of almost 14 days versus controls (post-TBI subacute/post-acute inpatients not treated with Cerebrolysin), during only their first admission (with a global mean hospital-stay duration of 29.5 days, which was within the mean hospitalization duration agreed by Romania’s national social health insurance system). All inpatients also received complex pharmacological and physical therapy, as necessary.
Patients
Forty-seven inpatients, admitted to the Physical (neuromuscular) and Rehabilitation Medicine/NeuroRehabilitation Clinic Division of the Bagdasar-Arseni Teaching Emergency Hospital between January 2005 and December 2010, were included in the study and divided into two groups. Nineteen patients were treated with Cerebrolysin (five females, 14 males; mean age 36.58 years [median 31, standard deviation 19.37, minimum 18, maximum 80]), and there were 28 controls (six females, 22 males; mean age 38.46 years [median 33.5, standard deviation 15.69, minimum 19, maximum 74]).
Inclusion criteria for both groups were:
- inpatients aged over 18 years, diagnosed with varying degrees of TBI (initial stratification according to Glasgow Coma Scale [GCS]:21 severe, moderate, or mild);
- up to 2.5 months since the TBI; and
- first admission to the clinic.
- antithrombotic/antiaggregant prophylactic therapy;
- antibiotics for concurrent infections, if necessary;
- spasticity medication, if necessary;
- urinary antiseptics, to prevent urinary infections;
- pain medication, if necessary (nonsteroidal anti-inflammatory drugs, acetaminophen/paracetamol, GABAergic agonists);
- adequate hydroelectrolytic repletion;
- mucolytics, if necessary;
- psychotropic drugs (antidepressants, neuroleptics, sedatives, hypnotics, CNS stimulants), if necessary;
- anticonvulsant drugs (only as prophylactic, post-brain surgery medication);
- antioxidants;
- nootropics, such as pramiracetam; and
- pharmaceutical preparations containing B vitamins and α-lipoic acid.
The following concomitant medications were allowed:
Thereby, all patients received necessary and appropriate treatment for their conditions and comorbidities, in accordance with current good practice and their therapeutic needs.
Allowed medicines and/or physio-/kinesitherapy procedures (including administration initially – in supra-acute/acute stages – prior to the admission into our ward) could interfere to some extent with the studied patients’ functional level at baseline and/or with the related outcomes, but, considering that the aforementioned types of drugs and/or minimal physio-/kinesitherapy procedures were used similarly in both groups (as needed – most of them included in the common medical approach after TBI), we consider this to be an acceptable bias/limitation of the study, and one that was unlikely to prevent the observation of Cerebrolysin effectiveness.
All patients in this study followed a rehabilitation program adapted to their neurologic/functional deficits and specific needs. Programs included:
- kinesitherapy;
- use of adapted orthoses and/or other assistive devices;
- prophylaxis and/or care of skin and subcutaneous/hypodermic lesions (pressure sores, surgical wounds, skin/nail infections);
- bladder and bowel management and training, if necessary;
- non-kinesiological physiotherapy, if necessary; and
- psychotherapy, if needed.
- a TBI requiring multimodal therapy, with patients having poor neuro-motor-psycho-cognitive function.
- a TBI with no indication/not requiring multimodal therapy and/or had contraindication(s) for Cerebrolysin; and
- (temporarily) impossible for such a medicine to be purchased.
- severe, life-threatening comorbidities: heart failure, lung failure, renal failure, liver failure;
- stroke with TBI;
- epilepsy; and
- pregnancy or lactation.
Inclusion for the Cerebrolysin-treated group in particular required:
Inclusion criteria specifically for the control group were:
Exclusion criteria for both groups were:
Note that previous cranial/brain surgical intervention(s) did not constitute an exclusion criterion.
All the patients in this study were evaluated individually with the same clinical/paraclinical and functional criteria and scales.
Evaluated parameters
Evaluated parameters were of the following types:
- epidemiological: age, sex distribution, etiology of TBI (traffic accidents, work-related accidents, falls, suicide attempts, diving events, assault, unknown causes), hospitalization duration;
- clinical/evolution signs/symptoms: motor deficit (hemiparesis, tetraparesis, diparesis, monoparesis), headache, memory disorders, confusion, aphasia, intracranial hypertension/dizziness, drowsiness, behavioral changes (agitation), vomiting, anisocoria, epistaxis;
- paraclinical: computed tomography (CT) scan pathological findings (contusion, laceration, sub-/epi-/extradural hematoma, subarachnoid/intraventricular hemorrhage, diffuse axonal injury, cerebral edema, hygroma, skull fractures, maxillary ethmoidal hemosinus);
- therapeutic (surgery post-TBI: yes/no; Cerebrolysin [only in the study group]);
- dysfunction/disability, measured by three standardized, well-known assessment tools:
- Functional Independence Measure ([FIM™] “the most widely accepted functional assessment measure in use in the rehabilitation community”),22 measured at admission (aFIM) and at discharge ([dFIM] usually after 30 days).
- Glasgow Outcome Score (GOS), with scoring as follows: 1= dead; 2= persistent vegetative state; 3= severe disability; 4= moderate disability; 5= “good recovery”.23 GOS was measured at admission (GOS_1), on day 10 (GOS_10), and on or near the 30th day (GOS_30) of hospitalization.
- Modified Rankin Scale/Rankin Disability Score (mRDS), with scoring as follows: 0= no symptoms; 1= no significant disability; 2= slight disability; 3= moderate disability; 4= moderately severe disability; 5= severe disability; 6= dead).22 mRDS was measured at admission, on day 10, and on or near the 30th day of hospitalization.
Having less quantification items, GOS and mRDS are easier and less time-consuming to administer than FIM; therefore, they were administered three times during hospitalization (at baseline, after 10 days, and at discharge), whereas FIM was administered only twice (at admission and at discharge).
Statistical analysis
In order to perform population distribution comparative analysis, and because of the small group sizes, we used χ2 goodness-of-fit test, median test, t-tests, and Pearson correlation coefficient.24 The software used was the SPSS (IBM Corporation, Armonk, NY, USA).25
Results
Age at admission
The age distribution of the patients in the two groups at admission is presented in Figure 1.
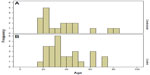  | Figure 1 Histograms of age at admission distribution. |
The mean age at admission for the entire sample was 37.6 years (standard deviation 16.9), with a median of 33. The two groups did not significantly differ by age (P=0.859 [median test]; P= 0.715 [t-test]).
Sex distribution
There were five females and 14 males in the Cerebrolysin-treated group and six females and 22 males in the control group.
TBI etiology
In both groups, the main cause of TBI was car crash (eleven cases in the Cerebrolysin group and 17 in the control group).
Hospitalization duration
Hospitalization duration was longer in the Cerebrolysin group (32.95 days) than in the control group (25.29 days), due to the overall greater severity of the cases in the former group, but this difference was not significant (P=0.154 [t-test]). Pearson coefficient between hospitalization duration and aFIM value in the Cerebrolysin group was −0.63, and, in the control group −0.42, showing relatively high correlation.
Signs and symptoms
The most frequent clinical signs and symptoms in both groups were: motor deficit (hemiparesis was prevalent in both groups, with eleven cases in the Cerebrolysin group and 19 in the control group); headache (six cases in Cerebrolysin, eight in controls); memory disorders (four cases in each group); and confusion (six cases in Cerebrolysin, three in controls).
CT scan pathological findings
The most frequent types of lesions found in CT examinations were contusion (nine cases in the Cerebrolysin group and 18 in the control group) and laceration (five cases in the Cerebrolysin group and nine in controls). The control group presented more hemorrhagic lesions, mainly subarachnoid hemorrhage (four cases in Cerebrolysin, eight in controls) and subdural hematoma (no cases in Cerebrolysin, nine in controls).
Nine patients in each of the groups had undergone head and/or brain surgery.
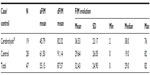
FIM scores
The descriptive statistics for aFIM values of patients in both groups are summarized in Table 1.
  | Table 1 Functional Independence Measure at admission distribution – descriptive statistics |
The distribution of patients in the two groups, according to aFIM values/scores, is presented in Figure 2.
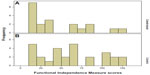  | Figure 2 Histograms of Functional Independence Measure at admission distribution. |
Patients in the control group had, on average, greater FIM values/scores at admission (61.50) than patients in the Cerebrolysin-treated group (45.79). This is supported by an independent samples t-test (P=0.076). This result, although not classically significant, was significant when testing at 0.1 level (ie, such a level accounts for a 10% likelihood of random results that could explain the outcomes; for the 0.05 level, this likelihood is half as likely, at one in 20).26
Because the design of our study was rigorous, including clinically (in terms of the aforementioned indications for the prescription of Cerebrolysin and inclusion criteria), the two groups differed at baseline in terms of the severity of clinical/functional impairment (objectified by the average aFIM values/scores).
Thus, it can be assumed that the effect sizes we determined might have been larger if, in our comparative appraisal, we had evaluated similar groups in terms of severity and related FIM values/scores at baseline.
The functional evolution of the inpatients was quantified by the following formula:
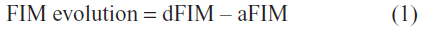
Larger FIM evolution values corresponded to better improvement in patient status.
The descriptive statistics for FIM evolution values/scores, according to the considered groups, together with the means of aFIM and dFIM values, are summarized in Table 2.
In the Cerebrolysin-treated group, a significant improvement, expressed in FIM mean value of 36.53 points, was registered (paired t-test P-value <0.001, 95% confidence interval [CI]: 25.4–47.7). Similarly, the control group experienced a significant increase of 29.64 FIM points on average (paired t-test P-value <0.001, 95% CI: 19.5–37.7).
It is apparent – though not statistically significant – that patients in the control group had, on average, smaller FIM evolution values (29.64) than patients in the Cerebrolysin-treated group (36.53) (P=0.174 [independent samples t-test]).
We consider a gain of 18 points on the FIM scale, in the approximately 1-month period over which we made our unitary evaluation, to be a clinical/functional “satisfactory improvement,” because, as is well known, the FIM scale consists of 18 items, with the possibility of ranking from 1 (“total assist” needed) to 7 (“complete independence – timely, safely”/no disability). More specifically, a completely independent person should score 18 × 7 = 126 points; conversely, a patient in a very severe clinical/functional state, needing “total assist” (the subject can achieve less than 25% of a tested task),27 could be quantified with 18 points. This suggests that every 18-point score increase (including for its means) on the FIM scale should equate to one degree of improvement (in independence, and decrease of dependence, respectively); for example, from level 2 (maximum assistance needed, whereby the patient is placed within the “complete dependence” level) to level 3 (the patient scores within the moderate assistance needed level).27
The effect size we determined was 6.88 FIM points (95% CI: −8.0 to 21.8); this encompasses the abovementioned clinical/functional “threshold” of 18 FIM points – in its positive benefit-indicating part – and is more than one-third of the abovementioned threshold of value/score gain.
GOS
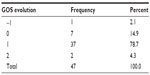
Based on the initial observed values (day 1) of the GOS, Table 3 shows that most patients scored 3 in GOS_1, which does not allow us to assert either similarity or dissimilarity (χ2 goodness-of-fit test asymptotic P-value =0.469) between the two groups.
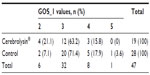  | Table 3 Glasgow Outcome Score at admission (GOS_1) distribution values |
GOS_10 values were compared to GOS_1. In order to do the comparison, we first evaluated the GOS difference according to the following formula:
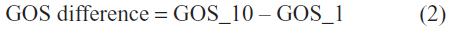
Only two possible GOS difference values were obtained: 0 (meaning stationary state of the patient) and 1 (meaning one degree of improvement).
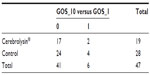
Two patients from the Cerebrolysin-treated group and four controls showed an increase in GOS degrees (Table 4).
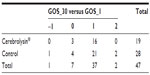
GOS evolution between admission and day 30 was evaluated through the difference between GOS_30 and GOS_1 (Table 5), according to the following formula:
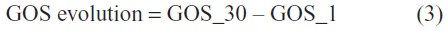
Only four possible GOS evolution values were obtained: −1 (meaning a one-degree decrease in GOS); 0 (no change); 1 (meaning a one-degree increase); and 2 (meaning a two-degree increase, ie, strong improvement).
When stratification frequencies are considered, it appears that only one patient, from the control group, experienced a worsening evolution. Sixteen patients from the Cerebrolysin group improved (84.2%), compared to 23 controls (82.1%) (see Table 6). However, this slight difference in improvement is not significant (χ2 test P-value =0.853); on the contrary, it emphasizes similar behavior.
The effect size, determined by the GOS evolution values (as absolute risk reduction; note that this scale has a smaller number of score levels), through comparative analysis based on the GOS, was weaker: only 2.1%.
For the analysis based on mRDS values/scores, the same quantification principle and methods were applied, and the results obtained were similar to those for GOS – so, they will not be detailed further – except for the effect size (evaluated as absolute risk increase) of 12.2%.
Discussion and conclusion
One limitation of the study concerns the low number of patients in both groups. This affects the power of the statistical testing, because, as discussed in the literature, sample size would preclude achieving significance.26 More specifically, such reduced values (for example N<20) would not be sufficient quantitatively to test various statistical assumptions, and would only detect very large effects as statistically significant.26
Comparing the Cerebrolysin group with the controls, in terms of the FIM evolution mean values/scores (36.53 points versus 29.64 points), the statistical t-test gave the P-value 0.174. On the other hand, the effect size was 6.88 FIM points, with a 95% CI −8.0 to 21.8; this encompasses the clinical/functional “threshold” of 18 FIM points – in the positive/benefit-indicating part – and this value is more than one third of the abovementioned threshold of score gain, which we have considered a “satisfactory improvement”. Although weaker, a still positive evaluation regarding the effect size – determine as absolute risk reduction –, ie, one favoring the Cerebrolysin-treated group, was also obtained for the related GOS data; in this respect, beneficial outcomes were observed on two of the three scales by which we made our assessments. Therefore, the results of our study cannot be excluded as insignificant.
Thereby, the clinical/functional evolution, as determined by the comparative analyses in the inpatients we studied, according to the “exploratory (suggestive) findings” presented, allows for the assumption that Cerebrolysin, correctly indicated and administered, might perhaps be useful, according to each specialist’s clinical expertise, in improving the overall neurorestorative/rehabilitative outcome in subacute/post-acute stages after TBI.
We consider this, also because the rather short duration (only of the first admission: of approximately 1 month) on which the respective outcomes were evaluated – given that, in cases like those of post-TBI, the neurorestorative and neurorehabilitative processes usually extend to months or, more likely, years – has to be taken into account, the relatively lower aFIM mean value in the Cerebrolysin group than in the controls. Due to the large complexity of issues associated with severe CNS lesions, including those following TBIs, at present there is no therapeutic approach that can provide a total cure or even lead to major improvement in such cases.
For further considerations and more extended conclusions, larger groups would be necessary in future studies.
Disclosure
The distributor of Cerebrolysin (EVER Neuro Pharma GmbH) administered in this study is an old and constant partner of ours, and contributed to the support of scientific meetings, training courses, summer schools, had some participation with accepted papers to congress/conferences/symposia, but they did not contribute in any way, to this study (neither in data collection or processing, nor in the conclusions or editing process). The authors report no other conflicts of interest in this work.
References
Teasdale GM, Bannan PE. Neuroprotection in head injury. In: Reilly P, Bullock R, editors. Head Injury. London: Chapman and Hall; 1997:423–438. | |
Boake C, Francisco GE, Ivanhoe CB, Kothari S. Brain injury rehabilitation. In: Braddom RL, editor. Physical Medicine and Rehabilitation. Toronto: Saunders Company; 2000:1073–1116. | |
Dawodu ST. Traumatic brain injury (TBI) – definition, epidemiology, pathophysiology [webpage on the Internet]. Medscape [updated March 6, 2013]. Available from: http://emedicine.medscape.com/article/326510. Accessed February 7, 2014. | |
Kochanek PM, Clark RSB, Jenkins LW. TBI: pathobiology. In: Zasler ND, Katz DI, Zafonte RD, editors. Brain Injury Medicine: Principles and Practice. New York, NY: Demos Medical Publishing, LLC; 2007:81–96. | |
Jia Z, Zhu H, Li J, Wang X, Misra H, Li Y. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord. 2012;50(4):264–274. | |
Youdim MB, Buccafusco JJ. Multi-functional drugs for various CNS targets in the treatment of neurodegenerative disorders. Trends Pharmacol Sci. 2005;26(1):27–35. | |
Muresanu D, Buzoianu A, Florian SI, von Wild T. Towards a roadmap in brain protection and recovery. J Cell Mol Med. 2012;16(12):2861–2871. | |
Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004;1:80–100. | |
Onose G, Daia-Chendreanu C, Haras M, Ciurea AV, Anghelescu A. Traumatic brain injury: current endeavors and trends for neuroprotection and related recovery. Romanian Neurosurgery. 2011;18(1):11–30. | |
Onose G, Mureşanu DF, Ciurea AV, et al. Neuroprotective and consequent neurorehabilitative clinical outcomes, in patients treated with the pleiotropic drug Cerebrolysin. J Med Life. 2009;2(4):350–361. | |
Onose G, Haras M, Anghelescu A, et al. Integrative emphases on intimate, intrinsic propensity/pathological processes – causes of self recovery limits and also, subtle related targets for neuroprotectionl pleiotropicity/multimodal actions, by accessible therapeutic approaches – in spinal cord injuries. J Med Life. 2010;3(3):262–274. | |
Muresanu D. Neuroplasticity and neurorecovery. In: Bornstein NM, editor. Stroke: Practical Guide for Clinicians. Basel: Karger Medical and Scientific Publishers; 2009:37–49. | |
The Nobel Prize in Physiology or Medicine 1986 [webpage on the Internet]. Nobel Media; 2014. Available from: http://www.nobelprize.org/nobel_prizes/medicine/laureates/1986. Accessed February 7, 2014. | |
Korsching S. The neurotrophic factor concept: a reexamination. J Neurosci. 1993;13(7):2739–2748. | |
Deister C, Schmidt CE. Optimizing neurotrophic factor combinations for neurite outgrowth. J Neural Eng. 2006;3(2):172–179. | |
Tatebayashi Y, Lee MH, Li L, Iqbal K, Grundke-Iqbal I. The dentate gyrus neurogenesis: a therapeutic target for Alzheimer’s disease. Acta Neuropathol. 2003;105:225–232. | |
Schauer E, Wronski R, Patockova J, et al. Neuroprotection of cerebrolysin in tissue cultures models of brain ischemia: post lesion application indicates a wide therapeutic window. J Neural Transm. 2006;113:855–868. | |
Damulin IV. [Neuroplasticity: main mechanisms and their clinical significance]. Zh Nevrol Psikhiatr Im S S Korsakova. 2009;109(4):4–8. Russian. | |
Jianu DC, Muresanu DF, Bajenaru O, et al. Cerebrolysin adjuvant treatment in Broca’s aphasics following first acute ischemic stroke of the left middle cerebral artery. J Med Life. 2010;3(3):297–307. | |
Muresanu D. Neuromodulation with pleiotropic and multimodal drugs – future approaches to treatment of neurological disorders. Acta Neurochir Suppl. 2010;106:291–294. | |
Teasdale G, Jennette B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1972;304(7872):81–84. Available from: http://www.bt.cdc.gov/masscasualties/pdf/glasgow-coma-scale.pdf. | |
Modified Rankin Scale (MRS). Internet Stroke Center. Available from: http://www.strokecenter.org/wp-content/uploads/2011/08/modified_rankin.pdf. Accessed February 7, 2014. | |
Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1(7905):480–484. | |
Armitage P, Berry G, Matthews JNS. Statistical Methods in Medical Research. 4th ed. Oxford: Blackwell Science; 2002;134–137. | |
IBM SPSS Statistics [webpage on the Internet]. Bucharest: IBM Romania. Available from: http://www-01.ibm.com/software/ro/analytics/spss/products/statistics/. Accessed February 7, 2014. Romanian. | |
Fleishman A. Significant P-values in small samples [webpage on the Internet]. Marlborough, MA: Allen Fleishman Biostatistics Inc.; 2012. Available from: http://allenfleishmanbiostatistics.com/Articles/2012/01/13-p-values-in-small-samples/. Accessed February 7, 2014. | |
Granger C, Black T, Braun S. Quality and outcome measures for medical rehabilitation. In: Braddom RL. editor. Physical Medicine and Rehabilitation. 3rd ed. Philadelphia, PA: Saunders; 2007:159. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.