Back to Journals » Biologics: Targets and Therapy » Volume 12
A descriptive analysis of real-world treatment patterns of innovator (Remicade®) and biosimilar infliximab in an infliximab-naïve Turkish population
Authors Yazici Y, Xie L, Ogbomo A, Ellis LA , Goyal K, Teeple A, Mutlu EA, Simsek I
Received 25 April 2018
Accepted for publication 22 June 2018
Published 2 October 2018 Volume 2018:12 Pages 97—106
DOI https://doi.org/10.2147/BTT.S172241
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Doris Benbrook
Yusuf Yazici,1 Lin Xie,2 Adesuwa Ogbomo,2 Lorie A Ellis,3 Kavitha Goyal,4 Amanda Teeple,5 Ece A Mutlu,6 Ismail Simsek7
1Department of Internal Medicine, Division of Rheumatology, New York University Hospital for Joint Diseases, New York, NY, USA; 2STATinMED Research, Health Economics and Outcomes Research, Ann Arbor, MI, USA; 3Janssen Scientific Affairs, Real World Value and Evidence, Titusville, NJ, USA; 4Janssen Biotech Incorporated, Immunology Medical Affairs, Horsham, PA USA; 5Jassen Scientific Affairs, LLC, Health Economics and Outcomes Research, Horsham, PA, USA; 6Rush University, Medical College, Department of Medicine, Chicago, IL, USA; 7Guven Hospital, Department of Rheumatology, Ankara, Turkey
Purpose: Biosimilar IFX (CT-P13) received marketing approval in Turkey for treatment of rheumatologic diseases, including ankylosing spondylitis, psoriatic arthritis, rheumatoid arthritis (RA), inflammatory bowel disease (IBD), and psoriasis. Population data on real-world treatment patterns of CT-P13 following marketing approval in European countries are largely unreported. This study examined the prescribing and medication utilization patterns of innovator infliximab (IFX) and CT-P13 in Turkey for patients with RA or IBD naïve to either IFX.
Materials and methods: Adult patients with ≥1 diagnosis claim for RA or IBD and ≥1 claim for IFX or CT-P13 were identified in the Turkish Ministry of Health database during the following identification periods: 1 Oct 2014–30 May 2015 (RA) and 1 Oct 2014–31 Dec 2015 (IBD). Continuous medical and pharmacy coverage for ≥12 months before and after the date of the first dose (index) IFX or CT-P13 was also required. Separate analyses were done for each population.
Results: Seven hundred and seventy nine adult RA and 581 IBD patients met the selection criteria. The majority of RA (74%; n=575) and IBD patients (87%; n=504) were initiated on IFX. The average study observation period was 16 months for the RA and 12 months for the IBD population. Over the observation periods, discontinuation among RA patients occurred in 42% of IFX and 63% of CT-P13 while discontinuation in the IBD cohort occurred in 38% of IFX; and 62% of CT-P13.
Conclusion: In this population-based study, more IFX-naïve RA and IBD patients were initially treated with IFX than CT-P13. Discontinuation and switching were observed more often and earlier among patients treated with CT-P13 regardless of disease state. Further studies are needed to understand the reasons for these observed differences.
Keywords: discontinuation, switch, inflammatory bowel disease, rheumatoid arthritis
Plain language summary
Infliximab (IFX) is a biologic used in the management of autoimmune diseases, such as rheumatoid arthritis (RA), psoriasis, ankylosing spondylitis, and inflammatory bowel diseases (IBD) eg, Crohn’s disease and ulcerative colitis. CT-P13 is an IFX biosimilar, designed to have active properties similar to IFX. However, few real-world studies have been carried out on the prescribing and utilization patterns among patients using CT-P13. This descriptive study used the Turkish Ministry of Health database to provide insights into physician prescribing patterns and patient utilization of CT-P13 compared with IFX. The study included patients diagnosed with RA and IBD who not been previously treated with IFX or CT-P13. Patients were sorted into different groups: the IFX and the CT-P13 groups. Although CT-P13 is a biosimilar to IFX, the study results showed a higher proportion of patients in the CT-P13 group discontinued or switched from CT-P13 compared with patients in the IFX group regardless of the disease condition.
Introduction
Infliximab (IFX), a chimeric, monoclonal antibody with high affinity for tumor necrosis factor-alpha (TNF-α), neutralizes the biological activity of soluble and transmembrane TNF-α and inhibits binding of TNF-α with its receptor.1,2 IFX was first approved in 1998 in the USA for the treatment of Crohn’s disease (CD) and received subsequent approvals for the treatment of rheumatoid arthritis (RA),3 ankylosing spondylitis (AS),4 psoriasis,5 psoriatic arthritis (PsA),6 and ulcerative colitis (UC).7 CT-P13, a biosimilar of IFX, was approved in 2013 by the European Medicines Agency, and most recently by the Food and Drug Administration (FDA) in the USA, for use in adult patients with the same conditions for which IFX is indicated.8–10
On a molecular basis, CT-P13 is considered biosimilar to IFX based on amino acid sequence and physicochemical properties. Pharmacokinetics, efficacy and safety of CT-P13 have been assessed in randomized clinical trials for AS (PLANETAS) and RA (PLANETRA).11–13 Post-marketing reports of small case series describing experiences with CT-P13 in several European countries, predominantly in RA and CD, have been presented. These are characterized by small sample size, lack of comparator groups, and inconsistent data collection, hence questions remain regarding performance of CT-P13 in a real-world setting.14–22 Real-world evidence can provide insights into physician prescribing patterns and patient utilization of CT-P13 compared with IFX, but rigorous studies require evidence from large numbers of patients in countries where both IFX and CT-P13 are approved, marketed, and prescribed without restriction of one product vs another, and where reliable utilization data can be obtained. This study employed the Turkish National Ministry of Health database, which covers about 80% of the population and has well-documented IFX and CT-P13 utilization. We conducted a population-level analysis to describe the extent to which physicians prescribe IFX or CT-P13 for IFX-naïve RA or IBD patients. The study aims were to determine whether the characteristics of patients prescribed CT-P13 or IFX were similar, and to understand medication utilization patterns, including persistency and switching.
Materials and methods
Experimental design and data source
A retrospective, descriptive study of treatment patterns for IFX and CT-P13, was conducted in Turkish RA and IBD patient populations. The Turkish Ministry of Health database, a nationwide medical information collection system, established under the 2007 Health Budget Law, was used for this study. The dataset is comprised of pharmacy, inpatient, outpatient, and laboratory claims from >17,800 pharmacies, 5,600 general practitioners, 4,500 medical centers, 1,200 government hospitals, and 338 private hospitals, covering about 80% of the population in Turkey. The data have been used in previous research studies.23,24
Patient identification
Adult patients (aged ≥18 years) with continuous medical and pharmacy eligibility and ≥1 claim for IFX or CT-P13 were identified. RA patients were identified using ICD, 10th revision, clinical modification (ICD-10-CM) diagnosis codes (M05.X or M06.X) during the RA patient identification period (1 Oct 2014–30 May 2015), and IBD patients were identified with ICD-10-CM diagnosis codes for CD: (K50.X) or UC: (K51.X) during the IBD patient identification period (1 Oct 2014–31 Dec 2015). The identification period began after 1 Oct 2014 to ensure both IFX and CT-P13 were available for prescribing. Identification period length was selected to maximize the number of patients observed in a given time period. For RA, all patients were studied for at least 12 months after the date of their first IFX or CT-P13 dose, and IBD patients were studied for at least 6 months. A shorter period of follow-up was required for IBD compared to RA patients because of the small IBD sample size. The study index date was designated as the first claim for IFX or CT-P13 observed in the identification period. Patients were excluded if they had a prescription claim for IFX or CT-P13 during the 12 months prior to the index date (baseline period), had a cancer diagnosis (ICD-10-CM: C00-D49) during the follow-up period, or were pregnant during the study period, as these latter 2 conditions were likely to confound treatment patterns. Patients in the IBD cohort also required evidence of at least 1 additional non-biologic IBD-related medication to support the diagnosis, such as aminosalicylates (mesalamine, sulfasalazine, balsalazide, and olsalazine), immunosuppressants (mercaptopurine, azathioprine, methotrexate, tacrolimus, and cyclophosphamide), antibiotics (metronidazole and ciprofloxacin), corticosteroids (prednisone, methylprednisolone, budesonide, and hydrocortisone), and anti-diarrheals (loperamide and diphenoxylate-atropine) during the baseline period.
Study variables
Patient demographic and clinical characteristics
Demographic and clinical characteristics were evaluated for the baseline period and included age, sex, geographic region (Aegean, Central Anatolia, Eastern Anatolia, Southeastern Anatolia, Black Sea, Marmara, and Mediterranean), Charlson comorbidity index (CCI) score, individual comorbidities based on ICD-10-CM codes, prior biologic use, and concomitant disease-modifying anti-rheumatic drug use.
Medication prescribing patterns (ie, the proportion of eligible patients who initiated IFX or CT-P13) and medication utilization patterns (the number of administrations, number of vials per administration, time between administrations, and measures of persistency, including mean treatment duration and the proportion of patients who discontinued, switched from IFX to CT-P13, switched from CT-P13 to IFX, or remained on IFX or CT-P13) were evaluated. The average time to discontinuation was assessed for patients who had a confirmed discontinuation. A confirmed discontinuation was defined as a direct switch to another biologic medication or absence of a claim for the index biologic for at least 120 days. In this study, the number of days of supply or clinical benefit period for 1 dose of IFX or CT-P13 was assumed to be 56 days (8 weeks).
Statistical analysis
Statistical analyses were conducted with Statistical Analysis System Version 9.3. All study variables were examined descriptively. Numbers and percentages were provided for dichotomous and polychotomous variables. Means and SDs were provided for continuous variables. For dichotomous variables, P-values were calculated using the chi-squared test, and for continuous variables, independent samples t-tests were used to calculate the P-values.
Ethics approval and informed consent
Since the data used for this study were de-identified and only aggregate results were reported, the study was exempt from Institutional Review Board review.
Results
RA cohort
Baseline descriptive and clinical characteristics
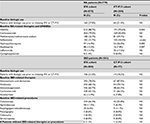
A total of 779 adult RA patients met the study selection criteria and were included for analysis. Physicians prescribed IFX to 73.8% (n=575) of the study population (IFX cohort) while 26.2% (n=204) were prescribed CT-P13 (CT-P13 cohort). The mean age was not significantly different between the cohorts IFX=42 years vs CT-P13=43 years; P=not significant [NS]). Over half of the patients were women (IFX=54.1% vs CT-P13=58.8%; P=NS). The Marmara region had the greatest proportion of RA patients and notably is the region with the greatest population density.25,26 Geographic distribution of the IFX and CT-P13 populations was also greatest in Marmara followed by the Central Anatolia and Mediterranean regions (Table 1).
In the 12 months prior to initiation of either IFX or CT-P13, both cohorts demonstrated a mean CCI score of 1.3. Common comorbidities included gastrointestinal ulcers (esophageal, gastric, duodenal, gastrojejunal, and peptic ulcer of unspecific site) and gastroesophageal reflux disease, hypertension, and depression. While the defined study population was based on a diagnosis code of RA, a sizeable proportion of patients also had a diagnosis of AS at baseline (IFX=42.4% vs CT-P13=55.9%; P<0.001; Table 1). Diagnoses of psoriasis, PsA, or IBD were less often observed.
Use of non-IFX biologics during the baseline period were similar in RA patients who initiated IFX or CT-P13 (IFX=27.8% vs CT-P13=31.4%; P=NS; Table 2). Most patients in both cohorts had received non-steroidal anti-inflammatory drugs (IFX=88.7% vs CT-P13=93.6%; P=0.044) and corticosteroids (IFX=79.0% vs CT-P13=82.8%; P=NS) during the baseline period. A higher proportion of patients in the IFX cohort had a prescription for azathioprine (IFX=15.3% vs CT-P13=7.8%; P=0.007) and a lower proportion had a prescription for hydroxychloroquine (IFX=16.9% vs CT-P13=26.0%; P=0.005) or sulfasalazine (IFX=31.7% vs CT-P13=50.0%; P<0.001) during the baseline period (Table 2).
RA follow-up period
The IFX cohort had 1 month longer average follow-up period compared with CT-P13 (IFX=16.5 months vs CT-P13=15.5 months; P<0.001). There was no significant difference in concomitant medication use between the cohorts during the follow-up period (Table 3).
Dosing patterns
Patients in the IFX cohort had a significantly higher average number of infusions (IFX=5.3 infusions vs CT-P13=4.1; P<0.001) and a significantly lower number of vials per infusion (VPI) (IFX=4.9 VPI vs CT-P13=5.8; P<0.001). The mean number of days between administrations was shorter for IFX compared with CT-P13 (IFX=60 days vs CT-P13=67; P=0.025). When examined in further detail, there were differences in the mean number of days between-the first and second infusions (IFX=40 days vs CT-P13=56; P<0.001); whereas there were no differences in the mean number of days between the second and third infusions (IFX=56 days vs CT-P13=61.6; P=NS); nor for the mean number of days between the third and fourth infusions (IFX=69.1 days vs CT-P13=72.4; P=NS).
Discontinuation patterns
Mean time to discontinuation for all patients was significantly longer in the IFX cohort compared with the CT-P13 cohort (IFX=263 days vs CT-P13=207; P<0.001). Additionally, the proportion of patients with a confirmed discontinuation was significantly lower in the IFX cohort compared with the CT-P13 (IFX=42.1% vs CT-P13=62.8%; P<0.001; Figure 1). In the IFX cohort, 27.3% (n=157) had discontinued their treatment after the third dose. In the CT-P13, 45.6% (n=93) had discontinued after the third dose (P<0.001).
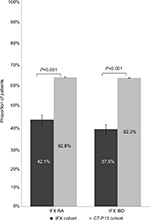  | Figure 1 Proportion of IFX or CT-P13 cohort with index therapy discontinuation. Abbreviations: IBD, inflammatory bowel disease; IFX, infliximab; RA, rheumatoid arthritis. |
Switching patterns
Fewer patients in the IFX cohort switched to another biologic therapy compared with the CT-P13 (IFX=23.5% vs CT-P13=35.8%; P<0.001). In the IFX cohort, patients who switched initially switched to CT-P13 (34.8% [n=47/135]), or to other biologics (65.2% [n=88/135]), primarily adalimumab (23.7% [32/135]). In the CT-P13 cohort, 56.2% [n=41/73] of patients initially switched to IFX, and the remainder switched to other biologics (43.8% [n=32/73]), primarily etanercept (13.7% [10/73]) (Figure 2). Switching patterns varied somewhat regionally; the highest proportion of switching occurred in Central Anatolia in both cohorts (IFX=42.6% vs CT-P13=27.9%; P=NS; Figure 3).
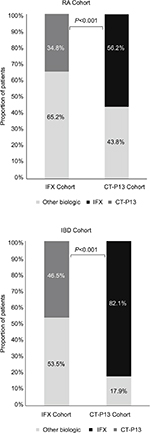  | Figure 2 Proportion of RA and IBD switchers selecting alternative therapies. Abbreviations: IBD, inflammatory bowel disease; IFX, infliximab; RA, rheumatoid arthritis. |
  | Figure 3 Geographical distribution of first switch by study cohort. Note: *P-value<0.05. Abbreviations: IBD, inflammatory bowel diseases; IFX, infliximab; RA, rheumatoid arthritis. |
To more specifically analyze treatment patterns in RA, a sensitivity analysis evaluating RA patients without a coincident AS diagnosis was conducted and showed findings consistent with those observed in the main analysis. In the sensitivity analysis, 79% of the patients were initiated on IFX, and 21% were initiated on CT-P13. Confirmed discontinuation was significantly lower in the IFX cohort compared with the CT-P13 (IFX=43.2% vs CT-P13=72.1%; P<0.001)
IBD cohort
Baseline descriptive and clinical characteristics
A total of 581 adult IBD patients met the study selection criteria. Physicians prescribed IFX to 87% (n=504) of the study population (IFX cohort), and CT-P13 (CT-P13 cohort) to 13% (n=77). The proportion of patients with UC did not differ between the cohorts (IFX=55.6% vs CT-P13=59.7%; P=NS); however, more patients in the IFX cohort were diagnosed with CD during the baseline period (IFX=66.5% vs CT-P13=53.3%; P=0.024). Diagnostic codes for both UC and CD for the same patient were observed in both cohorts to a similar extent (IFX=24.6% vs CT-P13=15.6%; P=NS). The mean age of the IFX cohort was slightly lower than the CT-P13 (IFX=38 years vs CT-P13=41 years; P=NS) and the proportion of male patients was slightly higher in the IFX cohort than in the CT-P13 (IFX=58% vs CT-P13=52%; P=NS). However, there were no statistically significant differences in demographic characteristics noted between groups. The largest proportion of IBD patients resided in the Marmara region followed by Central Anatolia for both cohorts (Table 1).
In the 12 months prior to initiating index medication, both IFX and CT-P13 cohorts demonstrated a mean CCI score of 0.7. Common comorbidities included gastrointestinal ulcers, hypertension, depression, and connective tissue disease. During the baseline period, a lower proportion of patients in the IFX cohort had a co-diagnosis of AS (IFX =17.3% vs CT-P13=40.3%; P<0.001), PsA or psoriasis (IFX=3.4% vs CT-P13=10.4%; P=0.005), or RA (IFX=11.3% vs CT-P13=19.5%; P=0.043). (Table 1).
Baseline period concomitant medication patterns and procedures were similar in IBD patients who initiated IFX and in patients who initiated CT-P13 (Table 1). Most patients in both cohorts had received 5-aminosalicylates, immunosuppressants, anti-diarrheals, and corticosteroids. Anti-diarrheal medications were typically observed in combination with other IBD therapies. Evidence of colonoscopy was observed in numerically fewer patients initiating CT-P13 (IFX=66.3% vs CT-P13=55.8%; P=NS). Use of other procedures was infrequent; sigmoidoscopy (IFX=11.5% vs CT-P13=6.5%; P=NS) and esophagogastroduodenoscopy (IFX=6.0% vs CT-P13=11.7%; P=NS) were the most commonly administered procedures other than colonoscopy. Baseline biologic use was similar in both cohorts (IFX=21.0% vs CT-P13=18.2%; P=NS), although numerically higher in the IFX cohort (Table 2).
IBD cohort follow-up results
The IFX cohort had a longer mean follow-up period (IFX=13.2 months vs CT-P13=11.6 months; P=0.001). Concomitant medication use in the follow-up period was similar to that observed during the baseline period (Table 3).
Dosing patterns
IFX cohort patients had a higher mean number of infusions (IFX=6.5 infusions vs CT-P13=3.8; P<0.001) with a significantly lower number of VPI (IFX=4.2 VPI vs CT-P13=6.1; P<0.001) compared with the CT-P13 cohort. The mean time between administrations was shorter for the IFX cohort overall (IFX=52 days vs CT-P13=63; P<0.001). Specifically, there were differences in the mean number of days between the first and second infusions (IFX=27 days vs CT-P13=50; P<0.001); and the second and third infusions (IFX=42 days vs CT-P13=60; P<0.001); and the third and fourth infusions (IFX=60 days vs CT-P13=72; P=0.001).
Discontinuation patterns
Mean time to discontinuation or end of the follow-up for all patients was longer in the IFX cohort compared with the CT-P13 as well (IFX=288 days vs CT-P13=177; P<0.001). The proportion of patients with a confirmed discontinuation was significantly lower in the IFX cohort. (IFX=37.5% vs CT-P13=62.3%; P<0.001; Figure 1). In the IFX cohort, 21.8% (n=110) had discontinued their treatment after the third dose, whereas in the CT-P13, 53.2% (n=41) had discontinued after the third dose (P<0.001).
Switching patterns
The proportion of patients with a switch to another biologic therapy was lower in the IFX cohort compared with the CT-P13 (IFX=14.1% vs 50.6%; P<0.001). In the IFX cohort, most first switches were to CT-P13 (46.5% [n=33/71]) and the remainder were to other biologics (53.5% [n=38/71]), primarily adalimumab (43.7% [n=31/71]). In the CT-P13 cohort, most first switches were to IFX (82.1% [n=32/39]) and the remainder were to other biologics (17.9% [n=7/39]), primarily adalimumab (10.3%; n=4/39; Figure 2). Of those who switched from IFX to CT-P13, 36.4% (n=12/33) returned to IFX, and of those who switched from CT-P13 to IFX, 40.6% (n=13/32) returned to CT-P13 (P=NS).
Switching patterns varied regionally. The highest proportion of switching from IFX to CT-P13 was observed in the Marmara region (34.3 %), whereas the highest proportion of switching from CT-P13 to IFX was observed in the Black Sea region (28.1%) (Figure 3).
Similar trends in dosing, discontinuation, and switching patterns were observed in a sensitivity analysis conducted among IBD patients at 6 months of follow-up. Patients in the IFX cohort had higher mean number of infusions (IFX=4.1 infusions vs CT-P13=2.8; P<0.001) with significantly lower VPI (IFX=4.2 VPI vs CT-P13=6.1; P<0.001) compared with the CT-P13 cohort. Mean time to discontinuation was longer in the IFX compared with the CT-P13 cohort (IFX=157 days vs CT-P13=112; P<0.001). The proportion of patients with a confirmed discontinuation was significantly lower in the IFX cohort (IFX=13.9% vs CT-P13=48.1%; P<0.001), and the proportion of patients with a switch to another biologic therapy was also lower (IFX=8.7% vs CT-P13=40.3%; P<0.001).
Discussion
This study describes prescribing and medication utilization patterns in RA and IBD patients initiating IFX or CT-P13 in Turkey. In both RA and IBD groups, IFX was more commonly prescribed than CT-P13. Notable differences in medication utilization patterns were observed between the IFX and CT-P13 cohorts. In both RA and IBD groups, the CT-P13 cohort had higher discontinuation rates and discontinuation occurred earlier compared with the IFX cohort. Drug discontinuation was the most notable difference in medication utilization patterns observed between the cohorts. Switching patterns in this study were limited to the initial switch. This was necessary as very few patients were observed to have multiple switches during the follow-up period and due to the limited follow-up period in which to observe multiple switches.
Evaluation of innovator and biosimilar IFX in a large national cohort study of RA and IBD patients has not been reported previously. In Turkey, which is one of the largest countries of Europe, physicians are free to prescribe brand name drugs regardless of cost, although, in some cases, patients may be obligated to pay the difference between the higher and lower-priced medications.27 It is unlikely that patients in this study shouldered any medication cost since patients who had a chronic condition certified by their physician can have their medications fully reimbursed by the Turkish government.24 Furthermore, the list price for IFX per vial was ₮1,229.49 Turkish Lira and for CT-P13, was ₮1,205.87 Turkish Lira, which represents less than a 2% difference between the products. The lack of a substantial difference between the prices for IFX and CT-P13 in Turkey could be considered a strength of this study and provided an opportunity to gain insight into factors other than price that potentially contribute to observed differences in practice patterns.
Another potential strength of the study is careful selection of those patients not previously treated with IFX or CT-P13 in the prior year, allowing an opportunity to observe practice patterns in patients who are unlikely to have prior perception or clinical experience with IFX. Beyond the difference in group sizes, the IFX and CT-P13 groups were generally balanced: no differences in overall co-morbidity indices or prior biologic exposure were present at baseline. Thus, observed differences in utilization are potentially free from bias related to disease severity. Moreover, the sensitivity analysis for IBD patients, using data from the first 6 months of follow-up showed that dosing, discontinuation, and switching patterns were similar to those observed for patients during the entire follow-up period.
A key limitation of this study is that the Turkish Ministry of Health database does not capture reasons for discontinuation or switching. Therefore, we could not ascertain if the high rate of discontinuation observed in the CT-P13 cohort was because of immunogenicity, adverse events, drug inefficacy, or patient/physician choice. However, a study of non-medical switches from originator IFX to biosimilar CT-P13 in patients with inflammatory arthritis observed a higher discontinuation rate for patients who switched to CT-P13. The study showed that 50% of patients reported lack of effect, and 28% reported adverse events as the reason for discontinuation.28 Similarly, it is not possible to determine whether clinical or non-clinical factors, including market dynamics, such as regional purchasing contracts or availability of drug supply could influence prescribing practices or medication utilization. Discrepancies in coding and the lack of validated identification algorithms for this dataset somewhat limit the ability to accurately confirm patient diagnosis. In this study, nearly two-thirds of the RA population also had a diagnosis code for AS during the study period limiting our ability to confirm whether patients were prescribed their biologic for RA or AS.
In Turkey, tendering for pharmaceuticals occurs in all public hospitals,24 and this may, in part, explain the regional variation observed in prescribing and switching practices. Factors, such as disease severity or patient preference for one product over another, unmeasured in this study, may also have contributed to the results observed here. The non-standard dosing intervals observed in both RA and IBD cohorts could have contributed to the discontinuation or switching rates. Finally, clinical factors, such as effectiveness and adverse events, could have contributed to these findings; however, these data were not recorded in the database. Future studies are needed to investigate each of these possibilities.
To our knowledge, this study is the largest sample in which real-world treatment patterns and medication persistency for CT-P13 has been evaluated. While the lack of information on reasons for discontinuations hampers interpretation, the results of this study demonstrate that medication utilization patterns for CT-P13 are not similar to those for IFX. Additional large population-based studies are needed to explore potential differences in the real-world setting between IFX and its biosimilar CT-P13.
Acknowledgments
The abstract for this study was previously presented at the 2016 American College of Rheumatology/Association of Rheumatology Professionals Annual Meeting, November 11–16, 2016 in Washington, DC and at the Academy of Managed Care Pharmacy (AMCP) Nexus 2017, October 16–19, 2017, Dallas, TX and was published online.
Disclosure
AO and LX are paid employees of STATinMED Research, which is a paid consultant to Janssen Scientific Affairs, LLC. EAM is an employee of Rush University and was a paid consultant to Janssen in connection with this study and the development of this manuscript. YY is an employee of New York University and was a paid consultant to Janssen in connection with this study and development of this manuscript. During the conduct of this study and drafting of this manuscript, YY was an employee of Guven Hospital and was a paid consultant to Janssen Scientific Affairs, LLC. LAE, KG, and AT are employees of Janssen Scientific Affairs, LLC, and are stockholders in Johnson and Johnson. The authors report no other conflicts of interest in this work.
References
Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541–1549. | ||
Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354(9194):1932–1939. | ||
Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345(15):1098–1104. | ||
de Keyser F. Infliximab in Ankylosing Spondylitis. Drugs. 2005;65(9):1292–1294. | ||
Gottlieb AB. Infliximab for psoriasis. J Am Acad Dermatol. 2003;49(2 Suppl):112–117. | ||
Antoni C, Manger B. Infliximab for psoriasis and psoriatic arthritis. Clin Exp Rheumatol. 2002;20(6 Suppl 28):S122–125. | ||
Mckeage K. A review of CT-P13: an infliximab biosimilar. BioDrugs. 2014;28(3):313–321. | ||
Blair HA, Deeks ED. Infliximab Biosimilar (CT-P13; Infliximab-dyyb): A Review in Autoimmune Inflammatory Diseases. BioDrugs. 2016;30(5):469–480. | ||
Jha A, Upton A, Dunlop WC, Akehurst R. The Budget Impact of Biosimilar Infliximab (Remsima®) for the Treatment of Autoimmune Diseases in Five European Countries. Adv Ther. 2015;32(8):742–756. | ||
Biosimilar infliximab receives approval in Japan and Turkey. GaBI Online; 2014. Available from: http://www.gabionline.net/Biosimilars/News/Biosimilar-infliximab-receives-approval-in-Japan-and-Turkey. Accessed Accessed January 15, 2018. | ||
Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72(10):1605–1612. | ||
Glintborg B, Kringelbach T, Høgdall E, et al. Non-Medical Switch from Originator To Biosimilar Infliximab among Patients with Inflammatory Rheumatic Disease – Impact on S-Infliximab and Antidrug-Antibodies. Results from The National Danish Rheumatologic Biobank and The Danbio Registry. Ann Rheum Dis. 2016;75:224. | ||
Yoo DH, Prodanovic N, Jaworski J, et al. Efficacy and safety of CT-P13 (biosimilar infliximab) in patients with rheumatoid arthritis: comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension study. Ann Rheum Dis. 2017;76(2):355–363. | ||
Tursi A, Allegretta L, Chiri S, et al. Effectiveness and safety of infliximab biosimilar CT-P13 in treating ulcerative colitis: a real-life experience in IBD primary centers. Minerva Gastroenterol Dietol. 2017;63(4):313–318. | ||
Deiana S, Gabbani T, Annese V. Biosimilars in inflammatory bowel disease: A review of post-marketing experience. World J Gastroenterol. 2017;23(2):197. | ||
Danese S, Fiorino G, Raine T, et al. ECCO Position Statement on the Use of Biosimilars for Inflammatory Bowel Disease-An Update. J Crohns Colitis. 2017;11(1):26–34. | ||
Hernandez LD, Rodriguez Gonzalez GE, Vela Gonzalez M, et al. Efficacy and safety of switching between originator and biosimilar infliximab in patients with inflammatory bowel disease in practical clinic: results to 6 months. European Crohn’s and Colitis Organisation. Clinical: Therapy and Observation. Amsterdam, Netherlands; 2016. Available from: https://www.ecco-ibd.eu/publications/congress-abstract-s/abstracts-2016/item/p449-efficacy-and-safety-of-switching-between-originator-and-biosimilar-infliximab-in-patients-with-inflammatory-bowel-disease-in-practical-clinic-results-to-6x00a0months.html. Accesed July 30, 2018. | ||
Kang YS, Moon HH, Lee SE, Lim YJ, Kang HW. Clinical Experience of the Use of CT-P13, a Biosimilar to Infliximab in Patients with Inflammatory Bowel Disease: A Case Series. Dig Dis Sci. 2015;60(4):951–956. | ||
Smits LJ, Derikx LA, de Jong DJ, et al. Clinical Outcomes Following a Switch from Remicade® to the Biosimilar CT-P13 in Inflammatory Bowel Disease Patients: A Prospective Observational Cohort Study. J Crohns Colitis. 2016;10(11):1287–1293. | ||
Nikiphorou E, Kautiainen H, Hannonen P, et al. Clinical effectiveness of CT-P13 (Infliximab biosimilar) used as a switch from Remicade (infliximab) in patients with established rheumatic disease. Report of clinical experience based on prospective observational data. Expert Opin Biol Ther. 2015;15(12):1677–1683. | ||
Bálint A, Rutka M, Végh Z, et al. Frequency and characteristics of infusion reactions during biosimilar infliximab treatment in inflammatory bowel diseases: results from Central European nationwide cohort. Expert Opin Drug Saf. 2017;16(8):885–890. | ||
Glintborg B, Kringelbach TM, Høgdall E, et al. Non-medical switch from originator to biosimilar infliximab in patients with inflammatory arthritis-impact on s-infliximab and antidrug-antibodies. Results from the Danish Rheumatologic Biobank and the Danbio registry. In: 17th Annual European Congress of Rheumatology; 2016; London, UK. | ||
Baser O, Baser E, Altinbas A, Burkan A. Severity index for rheumatoid arthritis and its association with health care costs and biologic therapy use in Turkey. Health Econ Rev. 2013;3(1):5. | ||
Baser O, Burkan A, Baser E, Koselerli R, Ertugay E, Altinbas A. Direct medical costs associated with rheumatoid arthritis in Turkey: analysis from National Claims Database. Rheumatol Int. 2013;33(10):2577–2584. | ||
Republic of Turkey Prime Ministry Investment Support and Promotion Agency. Available from: http://www.invest.gov.tr/enUS/infocenter/publications/Documents/HEALTHCARE.INDUSTRY.pdf. Accessed January 15, 2018. | ||
Republic of Turkey Statistical Institute. TurkStat. The results of address based population registration system 2011; 2012. Available from: http://www.turkstat.gov.tr/PreHaberBultenleri.do?id=10736. Accessed January 15, 2018. | ||
Seiter A, Celik Y. Turkey: Pharmaceutical Sector Analysis; 2008. Available from: https://www.researchgate.net/publication/242581202_. Accessed January 15, 2018. | ||
Glintborg B, Sørensen IJ, Loft AG, et al. A nationwide non-medical switch from originator infliximab to biosimilar CT-P13 in 802 patients with inflammatory arthritis: 1-year clinical outcomes from the DANBIO registry. Ann Rheum Dis. 2017;76(8):1426–1431. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.