Back to Journals » Drug Design, Development and Therapy » Volume 14
A Comprehensive Review on Pharmaceutical Film Coating: Past, Present, and Future
Authors Zaid AN
Received 17 August 2020
Accepted for publication 2 October 2020
Published 29 October 2020 Volume 2020:14 Pages 4613—4623
DOI https://doi.org/10.2147/DDDT.S277439
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Abdel Naser Zaid
Pharmaceutical Chemistry and Technology, Pharmacy Department, Faculty of Medicine & Health Sciences, An-Najah National University, Nablus, West Bank, Palestine
Correspondence: Abdel Naser Zaid
Pharmaceutical Chemistry and Technology, Pharmacy Department, Faculty of Medicine & Health Sciences, An-Najah National University, PO. Box 7, Nablus, West Bank, Palestine
Tel +970 9 234 5113
Email [email protected]
Abstract: Pharmaceutical film coating is considered a key part in the production of solid pharmaceutical dosage forms since it gives superior organoleptic properties products. In addition, it can improve the physical and chemical stability of dosage forms, and modify the release characteristics of the drug. Several troubleshooting problems such as twinning mottling, chipping, etc., may arise during or after or even during the shelf life of the film coated dosage forms. These troubleshooting problems may be due to tablet core faults, coating formulation faults and/or coating process faults. These problems must be overcome to avoid unnecessary product problems. Film coating as well as other parts of the pharmaceutical technology is subjecting to continuous innovation. The innovation may be at different levels including pharmaceutical excipients, processes, software, guidelines and equipment. In fact, of particular note is the growing interest in process analytical technology, quality by design, continuous coating processing and the inclusion of new ready for use coating formulations. In this review, we tried to explore and discuss the status of pharmaceutical film coating, the challenges that face this manufacturing process and the latest technological advances in this important manufacturing process.
Keywords: film coating, troubleshooting, advances, functional
Introduction
Oral solid dosage forms are considered the most convenient dosage forms (DFs) available in the pharmacy. Their production was introduced over centuries ago. These DF have several advantages including their relatively easygoing and convenient manufacture, coupled with high patient compliance.1 Tablets, the most relevant member of this class, have been improved in the last decades by introducing techniques such as tablet coating, double compression, and osmotic systems to achieve controlled and targeted release. Several techniques are available to achieve coating.2 The most common techniques are sugar coating film coating, microencapsulation, and compression coating.3 Sugarcoating is a conventional old method used to coat FDs. In fact, it involves several individual applications of various coating formulations such as sealing of the tablet core (using a thin layer of film coat), sub-coating, smoothing, colouring, polishing, and printing. These steps result in tablet weight gain of about 50 to100% which is considered time-consuming, increases the final coast of the manufactured DF, and negatively impacts its swallowing.4
Compression coating, also known as press coating or dry coating, has been developed to produce tablets containing incompatible drugs and to develop modified-release products. It involves the compaction of the dry coating excipients around tablet cores that have been produced on the same machine. It requires the use of special tableting machines which means further capital investment by the pharmaceutical industry. Therefore, it is considered a complex method and has not been commonly adopted as a method to coat tablets. Accordingly, it is usually exploited when the drug is heat and water sensitive since it eliminates the use of organic or aqueous solvents. Recently compression coating has been found useful to develop and produce novel drug-delivery applications such as controlled release DFs.5
Film coating (FC) is considered the most popular and versatile method. FC is a modern and widely spread process for coating oral solid DFs in the pharmaceutical and food industries. The process of FC involves the spraying of a thin, but uniform polymer-based formulations onto the surface of solid DFs including tablets, capsules, pellets or granules. It can be classified into two specific classes; nonfunctional FC which is used to change tablet appearance, organoleptic properties, swallowing properties, and to protect tablets from the negative effect of the environment such as humidity, oxidation, and light effects. On the other hand, functional FC can be used to modify or delay drug release as well as the aforementioned benefits in the non-functional coating. Microencapsulation is a modified form of FC. In fact, the only difference relay in the size of the particles to be coated and the methods by which the coating is achieved. This rapidly expanding process is based on either mechanical or physicochemical methods or techniques. The mechanical techniques include air-suspension, multi-orifice centrifugal, and modified spray-drying techniques, while the physicochemical methods involve coacervation-phase separation, which needs that the drug to be coated is dispersed in a suitable solution of the polymer6,7 (Figure 1).
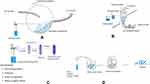 |
Figure 1 Different FC techniques and processes (A) Conventional FC pan, (B) Fluid bed FC, (C) Phases of FC, (D) Phases of microencapsulation. |
In this extensive review, we sought to explore the status of pharmaceutical C, the challenges that face this manufacturing process, and the latest technological advances in this process.
Classification of Film Coating
FC can be classified based on its intend use in the functional and non-functional coating.
Non-Functional FC
FC, along with the tablet shape and size, plays a key role in improving patient compliance since it impacts the final appearance and organoleptic properties of the produced tablets which are considered essential aspects of the brand image.8–10 Moreover, FC plays a very important role in helping elderly patients suffering from dysphagia since swallowing can be facilitated by the presence of a film coat on the DF.11 The US FDA has reported that the presence of a FC can either increase or assist tablet mobility compared with a non-coated tablet of the same shape and size.12 In addition, many APIs have a disagreeable bitter taste, which represents a serious challenge during the development of oral liquid products, particularly for pediatric patients. However, this inconvenience can be overcome by a simple FC of the conventional oral slid DFs. The polymer coat creates a physical barrier between the taste buds and the API, which minimizes the opportunity for the solubilized drug to interact with these buds. However, for chewable tablets, more sophisticated FC approaches may be required, which can include coating the API crystals with the design intent to retard dissolution in the oral cavity without altering the desired dissolution pattern in the gastrointestinal tract (GIT) to avoid any negative effect on drug bioavailability. For example, the API can be coated with suitable polymers or copolymer to form nano or microcapsules, which can be used to form chewable taste‐masked granules (Table 1).13–18
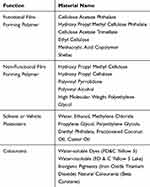 |
Table 1 Some of the Most Used Components in Functional and Non-Functional FC |
Functional FC
As we mentioned earlier in this review, functional FC is mainly used to add a new added value to the produced products. These values may include one or more functions such as improving the stability of the product and modifying its release pattern to produce drug targeting products.
Product Stabilization
Product stability is considered one of the most important goals in pharmaceutical developments. Accordingly, a scrutinized effort should be carried out to achieve stable products for the longest time. This includes using suitable pack design, desiccants, and specialized moisture protective FC polymers (Table 1).19
This step is particularly appropriate to protect the bulk product before its packaging or during transit if the packaging is performed at a remote facility. In addition, it may help the product to withstand the moisture environments after opening the bottle especially when the product is to be repackaged in dose administration aids.20,21
Recently, Burke et al reported that using moisture protection barriers may stabilize a water-sensitive API. Also, the use of such barriers could decrease potential negative interaction with another API in a fixed-dose combination tablet.22 Evidently, FC procedures based on aqueous formulation can still show serious problems and threats toward moisture-sensitive APIs. These challenges and problems could be fixed involving organic solvents in the coating formulation. Nonetheless, the manufacturing process must be conducted in an explosion-proof premises and equipment. In addition, the final coated dosage SF must comply with ICH Q3C (R6) guidelines regarding current residual solvent.23–25
Alternatively, a dry FC technique may be applied to avoid the dissipation of organic solvents in the environment.26 Regarding photo-stabilization of photosensitive API, the use of an opacifying agent, such as titanium dioxide, in the coating formulation would enhance the capacity of the FC to protect the drug from light degradation effect, especially, when the film possesses a contrast ratio values higher than 98%. This could be achieved when the film thickness of the coat is close to 150µm and using a coating suspension containing about 30% TiO2.27 Another study was conducted by Mukharya et al to assess the effect of FC on the photo-stability of highly photo-sensitive antihypertensive products. In this study, the percentage level of FC was optimized by directly exposing core tablets to three levels of FC, 1% w/w, 2% w/w, and 3% w/w. According to the outcome of this study, 2% w/w FC level was found to be appropriate to protect the API in the core tablets after being exposed to a light source as per Option-2 of ICH Q1B.28,29
Modified Release Coating Functionalization
Modifying drug release is a common practice in DF design which can be accomplished using FC. Two types of modified release DFs are described by the USP, those that are enteric-coated and those that are extended-release. Delayed-release products which often designed to prevent drug release in the upper part of the GIT. FDs is designed to produce this type of DF are commonly named enteric coatings.30 On the other hand, FCs that are designed to prolong drug release over a long period or to reduce the drug regimen are commonly named sustained- or extended-release FCs.31
Delayed-Release FC
Delayed or enteric-coated DFs are often achieved using pH-sensitive polymeric coats capable to delay the release of certain APIs, either to protect the drug against the acidic environment in the stomach (ie proton pump inhibitors) or to protect the stomach against the irritant effect of the drug due to its chronic use as non-steroidal anti-inflammatory drugs like diclofenac sodium.32–34 Usually, polymers used to achieve enteric release bear carboxylic moieties on their main chain making them insoluble at pH less than 5 (Table 1). These acid-resistant polymers have been commonly used to forbidden drug release at pH 1.2. On the other hand, they show a significant increase of solubility at a pH higher than 5.5, thereby bypassing the stomach and releasing the drug in the small intestine.35,36 Another type of delayed-release product is used to achieve colon-specific drug delivery. For example, 5-aminosalicylic used to treat irritable bowel disease, has unwanted side effects along with the GIT. This can be achieved by coating the tablets or pellets using polymers soluble at pH higher than 7. Certain brand products use two pH dependant layers, with the first one soluble at pH higher than 5.5 which releases part of the API in the small intestine, while the second layer dissolves at pH higher than 7 in the colon. This design could be realized involving either film coating or by preparing two different film-coated granules as was reported by Howden et.al.37,38 The designed formulation was composed of two proton pump inhibitor granules with the first ingredient released within two hours after dose administration providing day-time therapy for gastroesophageal reflux disease (GORD); while the second ingredient released within 6 hours after dose administration and addressed overnight GORD. Based on the variability of GIT pH recognized within the general population (especially with colonic-pH), the efficiency of colon-specific drug delivery systems involving pH alone has been extensively discussed.39 Accordingly, many alternative approaches have been suggested. Resistant starch or high-amylose maize starch can be mixed with anionic copolymers based on methacrylic acid and methyl methacrylate to promote reproducible colonic-release. This technique depends both on colonic-pH and selective microbial degeneration of the starch in the colon.40 Despite of dietary conditions, this technique exhibited persistent release at the ileocecal junction or within the colon.39 A FC approach employing an outer film of methacrylic acid and methyl methacrylate copolymers and an inner alkaline buffered film was also reported. This approach ensures that the inner alkaline film promotes the dissolution of the polymer which permits targeted release at the ileocecal junction.8,41–44
Controlled Release FC
Sustained-release oral DFs were developed to decrease the number of dosage regimens, especially when the drug requires a reasonable constant blood level over a prolonged period. In addition, it also has been used for those APIs that need to be given in high doses, but at the same time a conventional immediate release is likely to cause undesirable ulceration. This can be accomplished by different techniques such as increasing the particle size of the drug, enclosing the drug in a suitable matrix, complex formations between the API and ion-exchange resins, and coating the API or the DF that contain the API.45 The API dose in a multi-particulate (MP) delivery system is dispersed across the whole GIT. Accordingly, this represents an advantage over the single unit coating since failure of a few units will be significantly less dangerous than the failure of a single-unit tablet or capsule which may cause dose dumping. For this purpose, MPs, nonpareil approach is available. The nonpareil sugar particles which are coated with a FC that contain the AP and then various functional and non-functional film seal coats are applied over the particle to achieve the desired release pattern including; MR release profiles, enteric and/or targeted release, and/or pulsatile release. A recent review summarised the different MP approaches, eg, swelling/rupturing, dissolution and/or erosion, and modification of the intrinsic permeability of the FC.46 Many different polymers have been evaluated to coat MP systems; some of these include starch acetate,46 ethyl cellulose47 and Eudragit RS, RL, andS48. In addition, the impact of the type of FC technique (aqueous or organic) on the performance of the polymer and the release profile of the resultant product was also investigated by Lecomete et al The outcome of this study revealed that FC technique significantly affects the film microstructure and, thus, the release mechanism and profile from pellets coated with polymer blends.49 Another way to control drug delivery and achieving prolonged action is accomplished by the use of a pulsatile delivery system. This control is usually achieved by the layering of nonpareil cores with an active layer, a swelling layer comprising binders, disintegrant and an insoluble, water-permeable polymeric FC.50.47
Dose Dumping
Usually, the majority of modified-release DFs contain higher levels of API than the corresponding immediate-release product. Accordingly, any defect in the FC may cause its rupture causing an instant release of the API with the risk of dose dumping. This troubleshoot is most concerning for APIs with narrow therapeutic indexes. Moreover, dose dumping has also been registered in the concomitant consumption of alcohol. The FDA ordered the withdrawal of hydromorphone, a once-daily modified-release product, as a result of alcohol-induced dose dumping.47 The presence of alcohol may increase the solubility of certain polymers such as Eudragit, which may affect their ability to retard the drug release which may result in dose dumping. On the other hand, the presence of alcohol may retard the swelling of the tablet matrix based on polymers such as hydroxypropyl methylcellulose, and this results in retard of the release-controlling pattern.51,52 Accordingly, in vitro dissolution testing using hydro-alcoholic media for both modified- and delayed-release products is becoming a regulatory requirement.53 Dissolution testing using hydroalcoholic media was conducted on three mesalazine enteric-release/extended-release products to assess any potential dose dumping. Unfortunately, the presence of alcohol negatively affected the integrity of the enteric FC during the acid stage of the dissolution test causing much earlier release of the drug than intended resulting in negating the release of the drug in the distal part of the small intestine and colon segments.54,55.55 Nowadays, many patients have prescribed proton pump inhibitors, which are enteric-coated products. These drugs cause an increase in the gastric pH to values greater than 4.0 for more than 10 hours, which may significantly affect the integrity of the enteric FC, causing degradation of the drug due to its acid sensitivity.56,57
FC Troubleshooting
As well as sugar coating, problems may arise during or following FC process. A comprehensive review of common troubleshooting experienced with FC products has been reported by Porter et.al. Moreover, many manufacturers of coating materials report the most common troubleshooting that may face FC and how formulators can overcome them.58–60 These troubleshoot may be caused by tablet core problems, coating process faults, and/or coating formulation faults. Accordingly, a full understanding of the properties of the various excipients used to produce the cores and to formulate the FC, how these excipients interact with each other and other formulation and manufacturing processes may help the formulator to avoid many of these troubleshoots. Therefore, tablets being FC should first pass the required test to withstand the harsh conditions of the coating processes. For example, it would be very difficult to coat tablets which are friable or not sufficiently hard or have a tendency to cap or laminate. In addition, FCs are not as thick as sugar and compression coats. Therefore, unlike sugar and compression coats, FCs have the minor capability to cover or hide visible defects that may arise during tablet compression. Therefore, producing tablets with ideal properties, before starting the coating process is considered an essential step to achieving high quality final products, since successive recovery or reworking of tablets may be difficult after a FC has been completed. Another example is mottling, or uneven colour distribution, which may occur due to uneven uniformity of colour in the coating. This troubleshoot is usually caused by the use of soluble dyes in aqueous FC. This would encourage colour migration, either by the evolution of residual solvent in the film or by migration of the plasticizer in which the colorant may be soluble. Accordingly, using water-insoluble colours such as pigments or lakes would hinder the incidence of mottling considerably (Table 1). Moreover, mottling could occur due to inappropriate dispersion of the pigments in the coating solution; therefore, effective mixing and homogenization of all components would be able to overcome this inconvenience (Table 2).
 |
Table 2 Summary of the Various Coating Defects, Their Causes, and Their Remedies |
Recent Advances in Pharmaceutical Coating
Continuous innovation in pharmaceutical processes and equipment allows for continuous innovation in pharmaceutical technology including pharmaceutical coating. The industry now requires technology for coating not only core tablets and pellets, but also other delivery systems such as catheters, ingestible imaging instruments, stents, joint plates etc. Accordingly, with the appearance of such technologies, FC will continue to flourish hand-in-hand. However, such technologies require complicated coating techniques to keep patient safety at the vanguard. In fact, drugs are required to be released at very specific intervals and at very specific sites in the GIT. Accordingly, these requirements push the FC industry toward advanced and novel innovations. This implies pharmaceutical industries to continuously be engaged in process and product improvement and does not limit only to sugar and FC but have also varied into other excipients. Unfortunately, an internal conservatism shown by many pharmaceutical industries towards accepting significant changes in excipients, equipment and processing technologies. Therefore, the intended trend looks to be evolutionary rather that revolutionary. In fact, of particular note is the growing interest in process analytical technology (PAT) which tends to bring several analytical procedures out of the laboratory and closer to the production process with which they may be linked. Therefore, introducing, an in-line control function, specific analytical techniques that can be used to enhance the quality of the final coated products is considered an important innovatory step in this field. For example, near infra-red techniques which can be used to analyse coated product in a manner that, the in process quality tests such as; moisture contents, drug contents, amounts of the applied film coating, and even, to some extent, drug release profile can be predicted before the end of the coating process and before the product is being discharged from the coating pan. This would greatly positively impact the quality and the final cost of the produced coated brand.61,62
Another new trend that is having a significant impact on pharmaceutical FC is the implementation of quality by design (QbD). This would be a huge step to assess the initial formulation and process risks and to shift these risks from red (high) to yellow (low) level. Recently, QbD approaches to optimize polymeric FC has been published. These approaches were of great help to minimize the risk of unwanted defects that might result in the rapture of the film in the unwanted position of the GIT.63–65 For example, three critical formulation and processing parameters, ie plasticizer concentration, polymer ratios and tablet weight gain, coat weight, were statistically assessed to optimize the FC to deliver the desired and predictable release profile.64 Similarly, three comparable parameters, ie, working temperature, coat weight plasticizer, and concentration, were assessed to align the API release profiles with model predictions.65 Another major change that showed a significant impact on pharmaceutical FC is the so-called continuous pharmaceutical coating processes or technology. In the beginning, continuous FC processes were mainly considered as a useful tool to produce large-volume pharmaceutical products. However, in the last decade, the focus has begun to shift toward more common pharmaceutical products, and, in particular, their relevance to in-line continuous pharmaceutical production, where raw ingredients are fed in at one end, and final packaged products come out at the other end. Exactly, such in-line unceasing processes, not only simplify the application of PAT and QbD initiatives but also may result in a significant cost reduction in the manufacturing process and accordingly in the final product.66–69 This process showed many advantages such as a significant increase in the output, significant reduction of the residence time of the process, from several hours to about 15 minutes, where the product is usually exposed to harsh conditions and accordingly better product stability, and finally significant improvement of the uniformity of distribution of FC liquid.68,69 Recently, most FC processes comprise the application of a sprayed liquid coating formulation where solidification of the coating is obtained by drying, and the subsequent distribution of coating formulation is facilitated by keeping constant mixing of tablets being coated. In general, the concept of continuous FC process is based on the use of a stretched side-vented coating pan, where core tablets are fed at one end, passed by a whole bank of pray guns, and emerged fully coated from the other end (Figure 2). However, this FC process is typically employed in a big pharmaceutical industry where huge size production batches are usually produced. Nowadays, due to the increasing interest in complete inline manufacturing processes, where the productivity rate of the coating process requires to be compared to the output of a single tablet press, small volume output continuous FC processes for small pharmaceutical industries are now available.
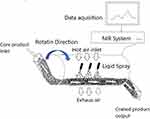 |
Figure 2 Continuous FC process with PAT. |
Recently, Zhu et al have developed a novel method of applying powder coatings using electrostatic charges.70 This process involves sequential spraying of a liquid plasticizer to the tablets being coated. After that, the powder of the remaining coating materials is applied and then completing the formation of the coating using a heat-curing phase. Another work about electrostatic dry powder FC technique was reported by Qiao et al Two immediate release coating liquids were successfully applied using this process. A liquid plasticizer was sprayed onto the surface of the tablet cores to improve the conductivity of these cores. In fact, this would result in enhancement of the deposition of the sprayed particles, reduction of the electrical resistivity, and reduction of the glass transition temperature of the coating polymer in the coating pan. After that, the liquid plasticizer was successfully applied. Then, spraying charged coating particles using an electrostatic charging gun to enhance the uniform deposition on the tablet surface was carried out. This enhanced the coalescence of the coating particles into a thin film by processing it at an acceptable curing temperature. The authors claimed that that the optimized dry powder coating process produced FC tablets with good coating uniformity, smooth surface, and release profiles that are comparable to that of the tablet cores. The data also suggest that this novel electrostatic dry powder FC technique may be used as an alternative approach to aqueous- or solvent-based FC process for solid DFs.71 In addition, this technique showed successful results among a range of existing pharmaceutical coating materials, and has been applied for both immediate-release and modified-release FC. Recently 3D printing is gaining important interest in pharmaceutical technology since it addressed several novel challenges including combinations of several APIs in one DF, on request production at the point of need, personalization of drug release patterns as well as patient-specific solutions. Accordingly, 3D printing may become a novel and promising road to develop and produce drug products, capable to support specific therapies, and improve patient compliance, safety, and efficacy. In 2020, Elini et al, tried to partially coat tablets with a glyceride, namely Precirol ATO 5 using a semi-solids 3D printer as an approach for tuning the release of two APIs, a hydrophilic and lipophilic drug, Melevodopa and Acyclovir respectively. The percentage of the tablet surface coated, the number of coating layers the coated sides of the tablet as well as other manufacturing parameters where adjusted to achieve the desired release profile for both APIs.72 Vacuum film coating follows a novel procedure since it offers explicitly designed pans and offers some aspects of the fluid-bed coating. Precisely, a water jacket is used to keep a constant temperature. In addition, it could be sealed to achieve the desired vacuum level. The core tablets are placed in the sealed pan and nitrogen is used to displace the air in the pan before attaining the desired vacuum state. The heated pan is used to dry and vacuum is used to remove the evaporated liquids. The absence of high-velocity heated air causes an improvement in the efficiency of the process. In addition, there is an energy-saving when compared with the conventional pharmaceutical FC process.73
Conclusion
FC is commonly used in the fields of pharmaceutical, medical devices and food industries. In the pharmaceutical field, especially oral solid DFs, FC is used to address several universal challenges such as poor palatability, dysphagia, and brand image using non-functional FC. Stability of water sensitive APIs can usually be improved by an appropriate selection of a film coat with decreased moisture permeability, while photosensitive drugs can be protected by selecting film coat formulation with opacifying agents. Functional filming can be achieved by pH-sensitive film coats which are often used to delay or modify drug release to facilitate improved patient outcomes. However, the high pH variability observed in certain patient populations may be aggravated by co-administration of certain drugs such as PPIs that increase gastric pH to higher than 4.0, which may compromise the clinical efficacy and safety of these functional film coats. This can be overcome by modifying the permeability of these film coats using additives such as alkalizing agents, super disintegrants, or even microbial sensitive excipients. Moreover, this review highlighted the most common coating problems, their causes, and possible solutions that may face the pharmaceutical manufacturer. In addition, the recent advances that may improve FC technology were also discussed.
Acknowledgment
The author would like to thank the Department of Pharmacy, An-Najah National University for the continuous support to the scientific research.
Disclosure
The author declares no conflict of interest.
References
1. Augsburger LL, Hoag SW. Pharmaceutical Dosage Forms-Tablets. CRC press; 2016.
2. Sastry SV, Nyshadham JR, Fix JA. Recent technological advances in oral drug delivery–a review. Pharm Sci Technolo Today. 2000;3(4):138–145.
3. Remington JP. Remington: The Science and Practice of Pharmacy. Vol. 1. Lippincott Williams & Wilkins; 2006.
4. Porter SC. Coating of tablets and multiparticulates. In: Aulton ME, editor. Pharmaceutics. The Design and Manufacture of Medicines.
5. Bose S, Bogner RH. Solventless pharmaceutical coating processes: a review. Pharm Dev Technol. 2007;12(2):115–131. doi:10.1080/10837450701212479
6. Suganya V, Anuradha V. Microencapsulation and nanoencapsulation: a review. Int J Pharm Clin Res. 2017;9(3):233–239. doi:10.25258/ijpcr.v9i3.8324
7. Paulo F, Santos L. Design of experiments for microencapsulation applications: a review. Mater Sci Eng C. 2017;77:1327–1340. doi:10.1016/j.msec.2017.03.219
8. Elder D. Design, formulation and manufacture of film-coated drug products. Eur Pharm Rev. 2017;22(5):37–40.
9. Delwiche J. The impact of perceptual interactions on perceived flavor. Food Qual Prefer. 2004;15(2):137–146. doi:10.1016/S0950-3293(03)00041-7
10. Srivastava R, More AT. Some aesthetic considerations for over the-counter (OTC) pharmaceutical products. Int J Biotechnol. 2010;11(3–4):267–283. doi:10.1504/IJBT.2010.036600
11. Liu F, Ranmal S, Batchelor HK, et al. Patient-centered pharmaceutical design to improve acceptability of medicines: similarities and differences in paediatric and geriatric populations. Drugs. 2014;74(16):1871–1889.
12. Health UD, et al. Size, Shape, and Other Physical Attributes of Generic Tablets and Capsules Guidance for Industry. 2015.
13. Sohi H, Sultana Y, Khar RK. Taste masking technologies in oral pharmaceuticals: recent developments and approaches. Drug Dev Ind Pharm. 2004;30(5):429–448.
14. Akre HS, Mundhada DR, Bhaskaran S, Asghar S, Gandhi GSet al. Dry suspension formulation of taste masked antibiotic drug for pediatric use. J Appl Pharm Sci. 2012;2(7):166.
15. Shen RW, Taste masking of ibuprofen by fluid bed coating. Google Patents 1996.
16. Gergely G, Gergely T, Gergely I. Pharmaceutical Preparation in the form of an effervescent and/or disintegrating tablet or an instant granule and process of producing it. PCT Int Appl. 1993;WO9313760.
17. Roche EJ, Taste masking and sustained release coatings for pharmaceuticals. Google Patents. 1991.
18. Roche EJ, Reo JP, Rotogranulations and taste masking coatings for preparation of chewable pharmaceutical tablets. Google Patents. 1994.
19. Waterman KC, MacDonald BC. Package selection for moisture protection for solid, oral drug products. J Pharm Sci. 2010;99(11):4437–4452. doi:10.1002/jps.22161
20. Bowen L, Mangan M, Haywood A, Glass B. Stability of frusemide tablets repackaged in dose administration aids. J Pharm Pract Res. 2007;37(3):178–181.
21. Raimi‐Abraham BT, et al. Investigating the physical stability of repackaged medicines stored into commercially available multicompartment compliance aids (MCAs). J Pharm Health Serv Res. 2017;8(2):81–89.
22. Burke MD, He X, Cook C, et al. Stability enhancement of drug layered pellets in a fixed dose combination tablet. Aaps Pharmscitech. 2013;14(1):312–320. doi:10.1208/s12249-012-9911-3
23. Modi F, Patel P. Formulation, optimization evaluation of fixed dose combination moisture barrier film coated bilayer tablet of artesunate & amodiaquine hydrochloride. Int J PharmTech. 2011;3:2124–2134.
24. Parmar K, Bhatt NM, Pathak NL, et al. An overview: aqueous film coating technology on tablets. Int J Pharm Chem Sci. 2012;1(3):994–1001.
25. Guideline IHT. Impurities: guideline for residual solvents Q3C (R5). Current Step. 2005;4:1–25.
26. Obara S, Maruyama N, Nishiyama Y, et al. Dry coating: an innovative enteric coating method using a cellulose derivative. Eur J Pharm Biopharm. 1999;47(1):51–59. doi:10.1016/S0939-6411(98)00087-3
27. Bechard S, Quraishi O, Kwong E. Film coating: effect of titanium dioxide concentration and film thickness on the photostability of nifedipine. Int J Pharm. 1992;87(1–3):133–139. doi:10.1016/0378-5173(92)90236-U
28. Baertschi SW, Alsante KM, Tønnesen HH. A critical assessment of the ICH guideline on photostability testing of new drug substances and products (Q1B): recommendation for revision. J Pharm Sci. 2010;99(7):2934–2940. doi:10.1002/jps.22076
29. Mukharya A, Patel PU, Chaudhary S. Effect assessment of “film coating and packaging” on the photo-stability of highly photo-labile antihypertensive products. Int J Pharm Investig. 2013;3(2):77. doi:10.4103/2230-973X.114903
30. Felton LA, Porter SC. An update on pharmaceutical film coating for drug delivery. Expert Opin Drug Deliv. 2013;10(4):421–435.
31. Ashton P, Chen J, Guo H, Polymer-based, sustained release drug delivery system. Google Patents. 2009.
32. Zaid AN, Qaddomi A. Development and stability evaluation of enteric coated Diclofenac sodium tablets using Sureteric. Pak J Pharm Sci. 2012;25:1.
33. Zaid AN, Natour S, Ghoush A, et al. Formulation and in vitro and in vivo evaluation of film-coated montelukast sodium tablets using Opadry® yellow 20A82938 on an industrial scale. Drug Des Devel Ther. 2013;7:83. doi:10.2147/DDDT.S37369
34. Zaid A, Fadda AM, Nator S, et al. Development and stability evaluation of enteric coated diclofenac sodium tablets using AquaPolish E. J Pharm Invest. 2011;41(4):211–215. doi:10.4333/KPS.2011.41.4.211
35. Flößer A, Kolter K, Reich H-B, et al. Variation of composition of an enteric formulation based on Kollicoat MAE 30 D. Drug Dev Ind Pharm. 2000;26(2):177–187. doi:10.1081/DDC-100100342
36. Felton L, Haase MM, Shah NH, et al. Physical and enteric properties of soft gelatin capsules coated with eudragit ® L 30 D-55. Int J Pharm. 1995;113(1):17–24. doi:10.1016/0378-5173(94)00169-6
37. Park HJ, Jung HJ, Ho MJ, et al. Colon-targeted delivery of solubilized bisacodyl by doubly enteric-coated multiple-unit tablet. Eur J Pharm Sci. 2017;102:172–179. doi:10.1016/j.ejps.2017.03.006
38. Howden CW. Update on dual delayed-release PPI formulations. Gastroenterol Hepatol (N Y). 2010;6(7):417.
39. Maroni A, Moutaharrik S, Zema L, Gazzaniga A. Enteric Coatings for Colonic Drug Delivery: State of the Art. Taylor & Francis; 2017.
40. Ibekwe V, Khela MK, Evans DF, Basit AW. A new concept in colonic drug targeting: a combined pH‐responsive and bacterially‐triggered drug delivery technology. 2008;28(7):911–916.
41. Liu F, Moreno P, Basit AW. A novel double-coating approach for improved pH-triggered delivery to the ileo-colonic region of the gastrointestinal tract. Eur J Pharm Biopharm. 2010;74(2):311–315. doi:10.1016/j.ejpb.2009.11.008
42. Varum F, Hatton GB, Freire AC, et al. A novel coating concept for ileo-colonic drug targeting: proof of concept in humans using scintigraphy. Eur J Pharm Biopharm. 2013;84(3):573–577. doi:10.1016/j.ejpb.2013.01.002
43. Schellekens R, Stellaard F, Mitrovic D, et al. Pulsatile drug delivery to ileo-colonic segments by structured incorporation of disintegrants in pH-responsive polymer coatings. J Controlled Release. 2008;132(2):91–98.
44. Schellekens R, Stellaard F, et al. Oral ileocolonic drug delivery by the colopulse-system: a bioavailability study in healthy volunteers. J Controlled Release. 2010;146(3):334–340.
45. Tang ES, Chan L, P Heng. Coating of Multiparticulates for Sustained Release. Am J Drug Delivery. 2005;3(1):17–28.
46. Roy P, Shahiwala A. Multiparticulate formulation approach to pulsatile drug delivery: current perspectives. 2009;134(2):74–80.
47. Siepmann F, et al. How to adjust desired drug release patterns from ethylcellulose-coated dosage forms. 2007;119(2):182–189.
48. Lotlikar V, Kedar U, Shidhaye S, Kadam V. pH-responsive dual pulse multiparticulate dosage form for treatment of rheumatoid arthritis. Drug Devel Ind Pharm. 2010;36(11):1295–1302.
49. Lecomte F, Siepmann J, Walther M, MacRae RJ, Bodmeier R. Polymer blends used for the coating of multiparticulates: comparison of aqueous and organic coating techniques. 2004;21(5):882–890.
50. Dashevsky A, Mohamad P. Development of pulsatile multiparticulate drug delivery system coated with aqueous dispersion Aquacoat®. ECD. 2006;318(1–2):124–131.
51. Levina M, Vuong H, Rajabi-Siahboomi AR. The influence of hydro-alcoholic media on hypromellose matrix systems. Drug Dev Ind Pharm. 2007;33(10):1125–1134. doi:10.1080/03639040701377862
52. Avachat AM, Nandare DS. Effect of alcohol on drug release kinetics from HPMC-based matrix tablets using model drugs. Dissolut Technol. 2014;21:11–17.
53. Ann TPFFA, Hebestreit GKHJ-P, Faham YRMYA, Regulatory Considerations for Alcohol-Induced Dose Dumping of Oral Modified-Release Formulations. 2015.
54. Fadda HM, Mohamed MA, Basit AW. Impairment of the in vitro drug release behaviour of oral modified release preparations in the presence of alcohol. Int J Pharm. 2008;360(1–2):171–176. doi:10.1016/j.ijpharm.2008.04.035
55. D’Souza S, Mayock S, Salt A. A review of in vivo and in vitro aspects of alcohol-induced dose dumping. AAPS Open. 2017;3(1):1–20. doi:10.1186/s41120-017-0014-9
56. Miner P
57. Thakral S, Thakral NK, Majumdar DK. Eudragit®: a technology evaluation. Expert Opin Drug Deliv. 2013;10(1):131–149.
58. D’Souza S, Mayock S, Salt A. A review of in vivo and in vitro aspects of alcohol-induced dose dumping. AAPS Open. 2017;3(1):1–20.
59. Porter S, Sackett G, Liu L. Development, Optimization, and Scale-Up of Process Parameters: Pan Coating, in Developing Solid Oral Dosage Forms. Elsevier; 2017:953–996.
60. Porter SC, Preventing Film Coating Problems by Design. 2016.
61. May RK, Evans MJ, Zhong S, et al. Terahertz in-line sensor for direct coating thickness measurement of individual tablets during film coating in real-time. J Pharm Sci. 2011;100(4):1535–1544. doi:10.1002/jps.22359
62. Korasa K, Hudovornik G, Vrečer F. Applicability of near-infrared spectroscopy in the monitoring of film coating and curing process of the prolonged release coated pellets. Eur J Pharm Sci. 2016;93:484–492.
63. Li M, Sander S, Duan J, et al. Scientific and regulatory considerations in solid oral modified release drug product development. AAPS J. 2016;18(6):1406–1417. doi:10.1208/s12248-016-9974-2
64. Kramar A, Turk S, Vrečer F. Statistical optimisation of diclofenac sustained release pellets coated with polymethacrylic films. Int J Pharm. 2003;256(1–2):43–52.
65. Nutan MT, Soliman MS, Taha EI, et al. Optimization and characterization of controlled release multi-particulate beads coated with starch acetate. Int J Pharm. 2005;294(1–2):89–101. doi:10.1016/j.ijpharm.2005.01.013
66. Ferreira AP, Tobyn M. Multivariate analysis in the pharmaceutical industry: enabling process understanding and improvement in the PAT and QbD era. Pharm Dev Technol. 2015;20(5):513–527. doi:10.3109/10837450.2014.898656
67. Perez-Ramos JD. Monitoring and Modeling of Film Coating in a Side Vented Pan Coater Using Near-Infrared Reflectance Spectroscopy, Digital Video Imaging and Computational Methods. Purdue University; 2007.
68. Cunningham C, Hansell J, Nuneviller III F, et al. Evaluation of recent advances in continuous film coating processes. Drug Dev Ind Pharm. 2010;36(2):227–233. doi:10.3109/03639040903410326
69. Wirges M, Funke A, Serno P, et al. Development and in-line validation of a process analytical technology to facilitate the scale up of coating processes. J Pharm Biomed Anal. 2013;78:57–64. doi:10.1016/j.jpba.2013.01.037
70. Prasad LK, McGinity JW, Williams RO. III, Electrostatic powder coating: principles and pharmaceutical applications. Int J Pharm. 2016;505(1–2):289–302. doi:10.1016/j.ijpharm.2016.04.016
71. Qiao M, Zhang L, Ma Y, Zhu J, Chow K. A novel electrostatic dry powder coating process for pharmaceutical dosage forms: immediate release coatings for tablets. Eur J Pharm Biopharm. 2010;76(2):304–310.
72. Tsintavi E, Rekkas DM, Bettini P, Partial tablet coating by 3D printing. 2020: 119298.
73. Kapoor D, et al. Coating Technologies in Pharmaceutical Product Development, in Drug Delivery Systems. Vol. 2020. Elsevier: 665–719.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
