Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
A Comparison for Type 2 Cytokines and Lesional Inflammatory Infiltrations in Bullous Pemphigoid and Atopic Dermatitis
Received 5 June 2022
Accepted for publication 5 October 2022
Published 27 October 2022 Volume 2022:15 Pages 2313—2321
DOI https://doi.org/10.2147/CCID.S376845
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Yu-qing Hu, Jian-zhong Zhang
Department of Dermatology, Peking University People’s Hospital, Beijing, People’s Republic China
Correspondence: Jian-zhong Zhang, Tel +86-10-88325472, Fax +86-10-68318386, Email [email protected]
Background: Bullous pemphigoid (BP) and atopic dermatitis (AD) are both type 2 inflammatory skin diseases with similar clinical features. Thymic stromal lymphopoietin (TSLP) is an epithelial-derived cytokine which is upregulated in AD. However, the expression of TSLP in BP and the correlation between TSLP and inflammatory infiltrations have not been fully studied.
Objective: To characterize the serum Th2 cytokines level and Th2 inflammatory cell infiltrations in BP and AD. To study TSLP levels in serum, blister fluids and expression in lesional skin in patients with BP and AD.
Methods: TSLP level in serum and blister fluids was measured by enzyme-linked immunosorbent assay (ELISA). Inflammatory cells (CD4+ T cells, CD8+ T cells, CD1a+ cells, eosinophils and mast cells) were stained immunohistochemically and quantified by image analysis.
Results: TSLP level was significantly increased in blister fluids of BP and was highly expressed in lesional skin of BP and AD. Serum levels of IL-6, IL-4, IL-22, IFN-γ and thymic activation regulates chemokines (TARC) were significantly higher in patients with BP and AD than in healthy controls. CD4+ T cells, CD8+ T cells and CD1a+ cells were significantly more in upper dermis of BP and AD lesions. Eosinophils were found more in BP lesions while mast cells were found more in AD lesions than in healthy controls. A distinct correlation was found between TSLP levels and the intensities of CD4+ T cells, CD1a+ cells infiltrations.
Conclusion: TSLP was significantly higher in blister fluids and skin lesions of BP, suggesting that it might contribute to the pathogenesis of BP. BP exhibited a similar type 2 immune response and a slight difference in cells infiltrations with AD.
Keywords: type 2 immunity, thymic stromal lymphopoietin, bullous pemphigoid, atopic dermatitis, Th2 cytokines, inflammatory infiltrations
Introduction
Type 2 inflammation is characterized by Th2 cells, innate lymphoid cells (ILCs) and type 2 cytokines. Allergens, infection and pollutants can derive type 2 responses and promote secretion of Th2 cytokines.1 A specific type 2 set of cytokines such as IL-4, IL-5, IL-9, IL-13 and TSLP are produced during the induction and maintenance of allergic immune response with the contribution of T cells, innate lymphoid cells (ILC), eosinophils and mast cells (MC).2 Many allergic diseases such as atopic dermatitis (AD) and asthma are characterized by type 2 immune responses.
Bullous pemphigoid (BP) is a common autoimmune blistering disease mostly affecting the elderly. The pathogenesis of BP has not been fully understood. Although BP is classified as a blistering disease and autoantibodies to BP180 and BP230 can be found in patients with BP, the cutaneous manifestations of BP are extremely polymorphic, including urticaria-like and eczema-like lesions.3 Moreover, patients with BP often have severe itching and increased peripheral blood eosinophils and serum IgE level.4 These clinical features can also be found in patients with AD. Atopic dermatitis (AD) is an inflammatory skin disease characterized by chronic recurrent dermatitis with pruritus. Elevated serum IgE levels, eosinophilia and increased expression of thymic stromal lymphopoietin (TSLP) were always found in AD.5
Thymic stromal lymphopoietin (TSLP) is an epithelial derived cytokine. TSLP is expressed primarily by epithelial cells at barrier surfaces such as the skin, gut and lung.6 TSLP is known to have wide-ranging impacts on both hematopoietic and nonhematopoietic cell lineages, including eosinophils, mast cells, T cells, B cells and epithelial cells.7 Recent studies have implicated important role of TSLP in atopic dermatitis.8 However, the role of TSLP in BP has not been studied.
Although BP and AD are independent entities, some patients with BP have urticaria-like, eczematous and papular lesions with itching, which was similar to AD.9 Moreover, increased levels of type 2 cytokines (such as IL-4, IL-5, IL-10) and Th22 cytokines have been founded in skin lesions and serum of patients with AD.10 Increased CCL17/TARC (thymic activation regulates chemokines), CCL22/MDC (produced by Langerhans cells), serum total IgE levels and peripheral eosinophils were often found in patients with AD and BP,11 suggesting a close relationship between BP and AD.
In this study, the expression of TSLP in serum, blister fluids and lesional skin were studied in patients with BP and AD. Th2 cytokines and type 2 inflammatory cell infiltrations in BP and AD were also detected.
Methods
Subjects
10 adult patients with BP (7 male and 3 female), 15 adult patients with AD (10 male and 5 female) and 15 age- and sex-matched healthy controls were included in this study. All the AD patients satisfied the criteria of Hanifin and Rajka12 and Chinese criteria.13 Diagnosis of BP was established based on clinical histopathological and direct immunofluorescence findings of BP180. All patients were in active stage of disease. Circulating BP-180 antibody was also measured by indirect immunofluorescence assay. The informed consents were obtained from each patient and healthy control. This study was approved by the Ethics Committee of the Institutional Review Board of Peking University People’s Hospital.
TSLP and Cytokines Measurement
Blood samples were obtained from each patient and healthy control. TSLP, IL-4, IL-6, IL-22, TARC and IFN-γ levels were measured by ELISA (eBioscience, Germany) according to the manufacturer’s instructions. Blister fluids from patients with herpes zoster were used as controls.
Immunohistochemistry
Skin punch biopsies were taken from patients with BP and AD. Control specimens were obtained from the normal skin area of pigmented nevus surgeries excision. The biopsies were formalin fixed and tissue slides were subjected to hematoxylin and eosin (H&E) staining. CD4, CD8 and CD1a were stained by immunohistochemistry. The sliced sections were air dried for 30 min. Then the sections were fixed in cold acetone for 10 min. After blocking 5 min for endogenous peroxidase, using 0.2% sodium azide, they were washed in phosphate buffered saline (PBS) for 10 min. Then samples were incubated by primary antibodies overnight. The following primary antibodies (mouse antihuman) were used: anti-CD4 (no. 11056-2-AP, Proteintech, Beijing, China, 1:200), anti-CD8 (no. 66868-1-Ig, Proteintech, Beijing, China, 1:200), anti-CD1a (no.ab108309, Abcam, Cambridge, UK, 1:500), anti-TSLP (no.ab47943, Abcam, Cambridge, UK, 1:500). Samples were washed in PBS for 15 minutes and repeated for 3 times and incubated by secondary antibody for an hour (biotinylated rabbit anti-mouse IgG, Servicebio, China). A colour deconvolution technique was applied to separate the colour mixture of haematoxylin. The sliced sections were also stained with 1% toluidine blue for detection of mast cells. In toluidine blue staining, mast cell granules are seen purplish red and the nuclei sky blue in color. An image analysis technique was employed for quantitative scoring of the stained slides.
Image Analysis
All slides were read by a pathologist that. Quantification of inflammatory cell was performed at ×200 magnification. At high magnification, CD4, CD8, CD1a and toluidine blue positive cells in upper epidermis and deep epidermis were counted in five randomly selected fields. The eosinophils were counted in H-E staining slices. Quantitative cell counts were expressed in “Positive cells per high power field (HPF)”.2
The images of immunohistochemical staining of TSLP were captured using the image capture system consisting of a light microscope connected to a desktop computer. Epidermal regions were analyzed interactively using Image pro plus (IPP), which allowed for image analysis of densitometry expressed as optical density (OD) over the background.
Statistical Analysis
All the data were analyzed by SPSS 22.0. Qualitative data were presented as the mean ± SEM. Differences between groups were tested using one-way analysis of variance (ANOVA) and unpaired Student’s t-test. Pearson correlation index were applied to describe the correlation between measurement data. P < 0.05 was considered significant.
Results
TSLP Levels in Serum and Blister Fluids
The serum TSLP levels are shown in Figure 1. Serum TSLP levels in BP patients (43.22 pg/μL), AD patients (42.58pgl/μL) were slightly but not significantly higher than in healthy controls (37.67pg/μL, Figure 1A). The TSLP level of BP blister fluids was higher than that from herpes zoster fluids (53.48pg/μL vs 36.11pg/μL, P<0.001, Figure 1B).
Serum Cytokine Levels in Patients with BP and AD
The expression of TARC (Figure 1C), IFN-γ (Figure 1D), IL-6 (Figure 1E), IL-4 (Figure 1F) and IL-22 (Figure 1G) are shown in Figure 1. Serum levels of IL-6, IL-22 and TARC were significantly increased in patients with BP and AD compared with healthy control (P<0.001). Serum IFN-γ in AD patients was significantly higher than healthy control. No difference was found between BP patients and AD patients.
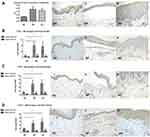
TSLP Expression in Skin Lesions of BP and AD
The expression of TSLP in lesional skin of BP and AD are shown in Figure 2. In normal skin, the TSLP staining was faint in all layers of epidermis (Figure 2Ai). The TSLP expression in lesions of BP (P=0.016) and AD (P=0.045) were significantly higher than healthy controls. In BP lesions, TSLP staining was faint in the granular and upper spinous layers, and became darker towards lower spinous and basal layers (Figure 2Aii). In AD lesions, TSLP staining was uniformly positive in all epidermal layers (Figure 2Aiii).
Inflammatory Infiltrations of BP and AD in Upper Dermis
The inflammatory cell counts in the upper dermis per HPF are shown in Figure 2. CD4+ T cells (Figure 2B), CD8+ T cells (Figure 2C) and CD1a+ cells (Figure 2D) were abundantly present in BP (CD4+ T cells: 39.4±13.7/HPF, CD8+ T cells: 28.2±8.5/HPF, CD1a+ cells: 24.4±13.7/HPF) and AD (CD4+ T cells: 36.9±16.8/HPF, CD8+ T cells: 28.9±11.1/HPF, CD1a+ cells: 28.2±11.0/HPF) than normal skin. These inflammatory cells infiltration were similar between BP and AD (P>0.05).
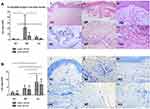
Eosinophils were significantly more observed in BP (21.7±22.5/HPF) than in AD (4.4±2.7/HPF, Figure 3A, P<0.05) and in healthy control (P<0.01). Mast cells were significantly more in AD (8.3±4.4/HPF) than in BP (4.3±2.1/HPF, Figure 3B, P<0.01) and in healthy control (P<0.01).
Inflammatory Infiltrations of BP and AD in Deep Dermis
The inflammatory cell counts in the deep dermis per HPF are shown in Figure 2. CD4+ T cells (Figure 2B), CD8+ T cells (Figure 2C) and CD1a+ cells (Figure 2D) were sporadic in deep dermis of BP lesions (CD4+ T cells: 5.8±6.1/HPF, CD8+ T cells: 4.6±4.0/HPF, CD1a+ cells: 4.0±2.1/HPF) and AD (CD4+ T cells: 3.7±4.1/HPF, CD8+ T cells: 4.8±4.7/HPF, CD1a+ cells: 5.0±3.9/HPF). Eosinophils were more in BP (6.7±6.4/HPF) than in AD (1.7±20.9/HPF, Figure 3A, P<0.05). Mast cells were significantly more in AD (6.6±3.3/HPF) than in BP (3.6±2.6/HPF, Figure 3B, P<0.05).
The Correlation Between TSLP, CD4+ T Cells and CD1a+ Cells
A distinct correlation was found between TSLP expression in epidermis and CD4+ T cells (P=0.012, R=0.514, Figure 4A) and CD1a+ cells (P=0.005, R=0.566, Figure 4C) in BP and AD patients. No correlation was found between TSLP expression and CD8+ T cells (Figure 4B), eosinophils (Figure 4D) or mast cells (Figure 4E).
Discussion
Bullous pemphigoid (BP) is the most common autoimmune bullous disorder which is characterized by intensely pruritic erythematous papules, vesicles or bulla, often accompanied with increased peripheral blood eosinophils and serum IgE.4 These clinical features are quite similar to AD.14 Zhang et al15 reported that dysfunction of BP180 in mouse models can trigger spontaneous AD-like skin inflammation. In this study, increased serum levels of IL-6, IL-4, IFN-γ, IL-22 and TARC were found in both AD and BP patients. It is reported that BP is prominently induced and sustained by a Th2-driven autoimmune response. BP180-reactive T cells produce Th2 cytokines (IL-4, IL-5, IL-6, IL-10 and IL-13), which induce the autoantibody production of the Th2-dependent IgG4 subtype in the active stages.16 TARC/CCL17 is also designated as Th2 type chemokine. TARC has been proven to be increased in AD, especially in moderate to severe patients and in active stage.17 About 50% of BP patients have eosinophilia and about 70% have elevated serum IgE, which was commonly seen in AD.4 In addition, more than 70% patients have serum IgE antibodies against BP180. Degranulation of eosinophils at the BMZ occurs early in BP and is most pronounced in lesions of urticaria and AD. All these indicated a Th2-polarized autoimmune response in BP and AD.18
Recent studies have highlighted TSLP in various inflammatory diseases.6 TSLP-induced Th2 responses are associated with the pathogenesis of allergic inflammatory diseases including AD, asthma and rhinitis.19 Soumelis et al20 reported that TSLP was highly expressed in AD. Cytokines that are found at high levels in lesional skin in AD patients (such as TNF-α, IL-4, and IL-13) can also synergize to induce TSLP expression by keratinocytes.21 Wilson et al22 reported that signaling between epithelial cells and innate immune cells via the TSLP is thought to drive AD and the atopic march. Epithelial cells can directly communicate to cutaneous sensory neurons via TSLP to promote itch, suggesting that TSLP may play an important role in AD. In this study, we found that TSLP levels were significantly increased in lesional skin of BP and AD and in blister fluids of BP, suggesting that it might also play important roles in BP.
In this study, we found that the distributions of CD4+ T cells, CD8+ T cells and CD1a+ cells in upper dermis of BP were significantly increased. AD and BP shared some common histopathological features especially in non-bullous BP, such as spongiosis, papillary dermal edema, and superficial lymphohistiocytic infiltrates without infiltrations in deep perivasculatures. This characteristic is supported by the similarity of the density of CD4+ T cells, CD8+ T cells and CD1a+ cells in deep dermis. Studies have showed a predominance of T cells and Langerhans cells infiltration in skin lesions of BP. It has been postulated that an imbalance of autoreactive Th cells and CD4+ T regulatory cells (Tregs) is critical to BP formation.16 Although CD4+ T lymphocytes are well known to play roles in the development of skin inflammation, CD8+ T lymphocytes were also found in the dermis in both BP and AD lesions. The presence of CD8+ T cells was scarcely reported. In this study, we found that BP and AD also shared a similarity of the densities of CD1a+ dendritic cells. Emtestam et al23 found a redistribution of the dendritic cells towards the basal membrane with increased total number of dendritic cells in BP. CD1a+ cells are predominant high-affinity IgE receptor bearing cells of lesional AD and BP.24 We also identified elevated dendritic cells in dermis of BP and AD.
The impressive predominance of eosinophils in upper and deep dermis in BP lesions was also observed. In AD patients, Th2 cytokines such as IL-4 and IL-5 has been shown to increase the survival of lesional eosinophils, which were often seen in superficial dermis. Eosinophilia and increased eosinophilic cationic protein (ECP) are two characteristic markers for the activity of AD.11 In BP, eosinophils, rather than other inflammatory cells, are the major cells seen in the lesional skin and they are also the most predominant biomarkers in peripheral blood. Early prebullous, urticarial-like lesions may demonstrate eosinophilic spongiosis histologically.25 Th2 cells and cytokines may account for eosinophil recruitment from blood vessels to skin. Eosinophil production of proteolytic enzymes, such as collagenase, plasminogen activators, plasmin and matrix metalloproteinase, lead to breakdown of dermal-epidermal cohesion and further contributes to tissue damage such as blister formation.26
We also found that mast cells were significantly increased in lesional skin of AD and BP. And, mast cell predominance was more obvious in lesional skin of AD compared with BP. Mast cells can be sensitized by IgE via the high-affinity IgE receptor (FcεRI) and release of pruritogenic substances which induces scratching and disruption of the skin barrier.9 Mast cells can produce IL-4, IL-5 and IL-13, thereby playing important roles in skin inflammation. Mast cells were also significantly increased in chronic lesions of AD, especially in areas of lymphocytic infiltration in the papillary dermis.27 Degranulation of mast cells had been proven to be related with inflammatory response of AD. Mast cell degranulation is followed by eosinophil migration and release of mediators, which might contribute to the inflammatory cells recruitment and activation.28 Besides that, although mast cells also play a crucial role in neutrophil recruitment of BP through release of IL-8, the production of matrix metallopeptidase-9 (MMP) by eosinophils rather than mast cells can further contribute to tissue damage and blister formation. That’s the reason why a prominent eosinophil involvement was found in BP contrary to AD.4
In this study, we found a distinct correlation between TSLP expression and the number of infiltrating CD4+ T cells (R=0.514, P=0.012) and CD1a+ cells (R=0.566, P=0.005). TSLP can be produced by a variety of cells including T cells, B cells and CD1a+ dendritic cells (DC).29 TSLP can promote T-cell proliferation and differentiation both in vivo and in vitro. TSLP responsiveness of CD4+ T cells plays a triggering role in the activation, recruitment of IgE-producing B cells, mast cells, and eosinophils. It is a critical feature of allergic inflammation.9 TSLP can also promote naive CD4+ and CD8+ T cells to develop into Th2 cells, because TSLPR (TSLP receptor) activation induces IL-4 gene transcription, which further upregulates TSLPR on CD4+ T cells, resulting in a positive feedback loop.6
We also found correlation between dermal CD1a+ cells and TSLP expression levels (P=0.005). CD1a is a lipid-presenting molecule that is abundantly expressed on epidermal Langerhans cells and dermal dendritic cells. It has been reported that a major TSLP-responsive cellular subset in both human and mice are myeloid-derived dendritic cells.30 Watanabe et al31 reported that co-culture of TSLP-activated DCs with naive syngenic CD4+ T cells led to T cell proliferation but not differentiation, suggesting an important role for DCs and CD4+ T cell homeostasis. These results indicated that TSLP-activated DCs can induce the proliferation of inflammatory Th2 cells. Our results suggested that TSLP might play a crucial role in CD1a+ cell infiltrations of BP and AD.
We acknowledge a few inherent limitations of this study. It is often difficult to separate cells, especially when the immunohistochemical staining is suboptimal. Furthermore, our study is also restricted by its small sample size. More large-scale studies for verification are needed. In conclusion, our study defines the similarities and differences in type 2 inflammatory response between BP and AD. TSLP is of great significance in diagnosis of Th2 inflammatory skin diseases such as BP, which might point to a future role for biological therapy.
Ethics Approval Statement
The study design was approved by the Institutional Ethics Committee. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by National Natural Science Foundation of China (No. 82103711) and Peking University People’s Hospital Research and Development Foundation (RDY2021-18).
Disclosure
The authors have no conflicts of interest to declare.
References
1. Akdis CA, Arkwright PD, Bruggen MC, et al. Type 2 immunity in the skin and lungs. Allergy. 2020;75(7):1582–1605. doi:10.1111/all.14318
2. Garcovich S, Maurelli M, Gisondi P, Peris K, Yosipovitch G, Girolomoni G. Pruritus as a distinctive feature of type 2 inflammation. Vaccines-Basel. 2021;9(3). doi:10.3390/vaccines9030303
3. Borradori L, Van Beek N, Feliciani C, et al. Updated S2 K guidelines for the management of bullous pemphigoid initiated by the European Academy of Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol. 2022;36(10):1689–1704. doi:10.1111/jdv.18220
4. Miyamoto D, Santi CG, Aoki V, Maruta CW. Bullous pemphigoid. An Bras Dermatol. 2019;94(2):133–146. doi:10.1590/abd1806-4841.20199007
5. Wollenberg A, Oranje A, Deleuran M, et al. ETFAD/EADV Eczema task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patients. J Eur Acad Dermatol Venereol. 2016;30(5):729–747. doi:10.1111/jdv.13599
6. Cianferoni A, Spergel J. The importance of TSLP in allergic disease and its role as a potential therapeutic target. Expert Rev Clin Immunol. 2014;10(11):1463–1474. doi:10.1586/1744666X.2014.967684
7. Ziegler SF. Thymic stromal lymphopoietin and allergic disease. J Allergy Clin Immunol. 2012;130(4):845–852. doi:10.1016/j.jaci.2012.07.010
8. Heo WI, Park KY, Lee MK, Moon NJ, Seo SJ. TSLP polymorphisms in atopic dermatitis and atopic march in Koreans. Ann Dermatol. 2018;30(5):529–535. doi:10.5021/ad.2018.30.5.529
9. Li SZ, Jin XX, Ge XL, Zuo YG, Jin HZ. Thymic stromal lymphopoietin is implicated in the pathogenesis of bullous pemphigoid by dendritic cells. J Immunol Res. 2020;4594630. doi:10.1155/2020/4594630
10. Lo Schiavo A, Ruocco E, Brancaccio G, Caccavale S, Ruocco V, Wolf R. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol. 2013;31(4):391–399. doi:10.1016/j.clindermatol.2013.01.006
11. Furue M, Chiba T, Tsuji G, et al. Atopic dermatitis: immune deviation, barrier dysfunction, IgE autoreactivity and new therapies. Allergol Int. 2017;66(3):398–403. doi:10.1016/j.alit.2016.12.002
12. Hanifin JM. Diagnostic criteria for atopic dermatitis: consider the context. Arch Dermatol. 1999;135(12):1551. doi:10.1001/archderm.135.12.1551
13. Liu P, Zhao Y, Mu ZL, et al. Clinical features of adult/adolescent atopic dermatitis and Chinese criteria for atopic dermatitis. Chin Med J. 2016;129(7):757–762. doi:10.4103/0366-6999.178960
14. Kamiya K, Aoyama Y, Nishio E, Horio A, Tokura Y. Management of erythematous skin lesions in bullous pemphigoid associated with atopic dermatitis. J Dermatol. 2016;43(9):1102–1103. doi:10.1111/1346-8138.13330
15. Zhang Y, Hwang B-J, Liu Z, et al. BP180 dysfunction triggers spontaneous skin inflammation in mice. Proc Natl Acad Sci U S A. 2018;115(25):6434–6439. doi:10.1073/pnas.1721805115
16. Giusti D, Le Jan S, Gatouillat G, Bernard P, Pham BN, Antonicelli F. Biomarkers related to bullous pemphigoid activity and outcome. Exp Dermatol. 2017;26(12):1240–1247. doi:10.1111/exd.13459
17. Uysal P, Birtekocak F, Karul AB. The relationship between serum TARC, TSLP and POSTN Levels and childhood atopic dermatitis. Clin Lab. 2017;63(7):1071–1077. doi:10.7754/Clin.Lab.2017.161107
18. Hammers CM, Stanley JR. Mechanisms of disease: pemphigus and bullous pemphigoid. Annu Rev Pathol. 2016;11:175–197. doi:10.1146/annurev-pathol-012615-044313
19. Zhang Y, Zhou B. Functions of thymic stromal lymphopoietin in immunity and disease. Immunol Res. 2012;52(3):211–223. doi:10.1007/s12026-012-8264-z
20. Soumelis V, Reche PA, Kanzler H, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3(7):673–680. doi:10.1038/ni805
21. Debinska A. New treatments for atopic dermatitis targeting skin barrier repair via the regulation of FLG expression. J Clin Med. 2021;10(11):2506. doi:10.3390/jcm10112506
22. Wilson SR, The L, Batia LM, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. 2013;155(2):285–295. doi:10.1016/j.cell.2013.08.057
23. Emtestam L, Hovmark A, Lindberg M, Asbrink E. Human epidermal Langerhans’ cells in bullous pemphigoid. Acta Derm Venereol. 1987;67(6):529–532.
24. Kwiek B, Lesniewska A, Kowalewski C, Wozniak K. Langerhans cells are predominant high affinity immunoglobulin E receptor bearing cells in the epidermis of bullous pemphigoid skin. J Dermatol Sci. 2017;85(1):60–63. doi:10.1016/j.jdermsci.2016.09.012
25. Kasperkiewicz M, Zillikens D. The pathophysiology of bullous pemphigoid. Clin Rev Allergy Immunol. 2007;33(1–2):67–77. doi:10.1007/s12016-007-0030-y
26. Grando SA. Pemphigus autoimmunity: hypotheses and realities. Autoimmunity. 2012;45(1):7–35. doi:10.3109/08916934.2011.606444
27. Liu FT, Goodarzi H, Chen HY. IgE, mast cells, and eosinophils in atopic dermatitis. Clin Rev Allergy Immunol. 2011;41(3):298–310. doi:10.1007/s12016-011-8252-4
28. van Beek N, Schulze FS, Zillikens D, Schmidt E. IgE-mediated mechanisms in bullous pemphigoid and other autoimmune bullous diseases. Expert Rev Clin Immunol. 2016;12(3):267–277. doi:10.1586/1744666X.2016.1123092
29. Soumelis V, Liu YJ. The discovery of human TSLP as a critical epithelial cytokine in type 2 immunity and allergic disease. Nat Immunol. 2020;21(12):1471–1473. doi:10.1038/s41590-020-0720-7
30. Kitajima M, Lee HC, Nakayama T, Ziegler SF. TSLP enhances the function of helper type 2 cells. Eur J Immunol. 2011;41(7):1862–1871. doi:10.1002/eji.201041195
31. Watanabe N, Hanabuchi S, Soumelis V, et al. Human thymic stromal lymphopoietin promotes dendritic cell-mediated CD4+ T cell homeostatic expansion. Nat Immunol. 2004;5(4):426–434. doi:10.1038/ni1048
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.