Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
A Clinical Study on the Association of Sodium-Glucose Cotransporter 2 Inhibitors and Acute Kidney Injury Among Diabetic Chinese Population
Authors Shen L, Yang H, Fang X, Huang H, Yao W, Chen D, Shen Y
Received 7 January 2021
Accepted for publication 12 March 2021
Published 13 April 2021 Volume 2021:14 Pages 1621—1630
DOI https://doi.org/10.2147/DMSO.S300494
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Lianglan Shen,* Hongli Yang,* Xingxing Fang, Huaxing Huang, Wubin Yao, Dongmei Chen, Yan Shen
Department of Nephrology, The Second Affiliated Hospital of Nantong University, Nantong, Jiangsu, 226001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yan Shen Tel/Fax +86-0513-85061346
Email [email protected]
Purpose: To investigate the association of Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors and acute kidney injury in comparison to other classes of drugs.
Patients and Methods: A total of 4966 diabetes mellitus patients were investigated for developing Acute Kidney Injury (AKI) who were under prescription with the following class of drugs viz. SGLT2 Inhibitors, Dipeptidyl peptidase-4 (DDP4) inhibitors, Nonsteroidal anti-inflammatory drugs (NSAIDs), first-line drugs and anti-biotics. The primary outcome was based on the hospital encounter and Kidney Disease Improving Global Outcome (KDIGO) threshold values were used to assess the serum creatinine concentration. The secondary outcome was assessed based on the concentration level of serum creatinine after 90 days of hospital admission and evaluation of the KDIGO threshold values.
Results: The study observed that the risk of causing AKI for SGLT2 inhibitors was 5.59% which was comparatively low compared to other class of the investigated drugs (DPP4 inhibitors = 6.47%, antibiotics = 6.30%, first-line drugs = 6.82% and NSAIDs = 10.65%). The multivariate analysis observed that ibuprofen, celecoxib, indomethacin, insulin, cephalexin, and alogliptin were mostly associated with an increased rate of AKI. SGLT2 inhibitors have the lowest risk for developing AKI compared to other drugs and control.
Conclusion: AKI incidence is relatively low after initiation of SLGT2 inhibitors and concludes that regulatory warnings from certain health agencies about its risk for AKI on prescription are unwarranted.
Keywords: SGLT2 inhibitor, DPP4, acute kidney injury, NSAID, type-2 diabetes
Introduction
Acute kidney injury or acute renal failure (ARF) is caused due to the abrupt deterioration in kidney function.1 It is characterized by an increased level of serum creatinine with or without a lesser urine pass.2 The main function of the kidney includes clearing off endogenous waste products, acid-base balance, electrolytes, and endocrine function. The kidneys also regulate the excretion of almost all drugs, which, in turn, may lead to nephropathy.3 Therefore, damaging the kidney may cause various life-threatening complications such as acidosis, body fluid imbalance, and multiple organ failure.4 On the other hand, the mortality rate of patients admitted with AKI requiring dialysis support is more than 50%5 and majority of cases being patients having diabetes mellitus who have infection in their kidney and urinary tract.6 Diabetes mellitus patients also accounts for the majority of end-stage kidney disease. They are also distinguished by severe interstitial inflammation and the majority of the patients are at the risk for developing serious bacterial infections, which may often lead to renal tissue failure and AKI.7
Moreover, the adverse reaction of prescribed drugs for diabetic patients remains an underlying cause for acute kidney injury.8 On the other hand, SGLT2 inhibitors are a class of anti-diabetic drugs that are used to treat type 2 diabetes mellitus.9,10 This class of drugs inhibits the re-absorption of glucose in the kidney and lowers the blood sugar level.11 There are also several prescriptions of such class that have been approved or many of them are presently under clinical trial.12,13 For example, SGLT2 inhibitors such as canagliflozin are reported to enhanced blood sugar control and reduced the body weight and blood pressure of the patient.14 However, there are certain safety warnings issued by Regulatory agencies about SGLT2 inhibitors regarding the risk of AKI after its initiation.15–17 In fact, AKI is generally associated with a certain class of drugs such as NSAIDs and antibiotics. Therefore, our objective was to quantify based on a 90-day risk of AKI in older adults after initiation of SGLT2 inhibitors in routine clinical practice compared to other classes of drugs such as NSAIDs, DPP4 inhibitors, antibiotics, and first-line anti-diabetic drugs.
Patients and Methods
Ethical Approval and Standards
All procedures were carried out in accordance with the Declaration of Helsinki 1964 and its later amendments. Written informed consent was provided by all patients and participants. Consent was obtained from each subject regarding their personal information on demographic factors, any other history of medical condition information using a questionnaire. The human subject research was approved by the Institutional Medical Ethics Committee of the Second Affiliated Hospital of Nantong University All protocols and procedures on the animal study were approved by the Hospital Ethical Research Committee.
Study Population and Data Source
In this study, we examined 4966 diabetes mellitus patients enrolled in The Second Affiliated Hospital of Nantong University, Nantong 226,001, Jiangsu, PR China between June 2017 to July 2019. These subjects were prescribed with SGLT Inhibitors, DDP4 inhibitors, NSAIDs, anti-diabetic first-line drugs, and antibiotics. The study includes only patients which are above 55 years and who had been active users of these drugs for at least one year and patients younger than 55 years were excluded from the study.
Main Exposures
Adults aged above 55 years or older who were prescribed with SGLT2 Inhibitors, DDP4 inhibitors, NSAIDs and antibiotics between June 2017 to July 2019 were categorized and investigated for the study. The prescription details such as date, dosage, and duration of prescribed drugs were recorded for each drug. Participants who received more than one SGLT2 inhibitors were excluded from the study. SGLT2 inhibitors’ use was defined as the daily use of the drugs viz. canagliflozin, ipragliflozin, jardiance, and dapagliflozin. DDP4 inhibitors’ use was defined as the daily use of the drugs viz. vildagliptin, alogliptin, and linagliptin. NSAID use was defined as use of the drugs viz. celecoxib, ibuprofen, indomethacin which were received for at least one or 2 doses. Patients who received more than 2 doses of NSAIDs were excluded. Diabetes patients using cephalexin and amoxicillin when required was defined as antibiotic use and the daily use of insulin and metformin was defined as the use of anti-diabetic first-line drug and metformin act as the control drug. All patients were recorded for the history of their drug usage track records and follow-up ended until the completion of the study.
Outcome
The primary outcome of the study was AKI based on the hospital encounter. KDIGO threshold values were used to define the serum creatinine concentration. Whereas the secondary outcome was based on the assessment of the hospital admission after 90 days and serum creatinine concentration was assessed for the KDIGO threshold.
Pharmacokinetics (PK) Study on the Mouse Model
Animal infection models in the pharmacokinetic evaluation serves an important role in preclinical assessments, dosing optimization and setting of confirming susceptibility breakpoints.
Its ultimate goal is to mimic them in humans which allows a robust PK study to discover the optimal drug exposures that lead to therapeutic success thereby minimizing the cost and duration of clinical trials. In this study, animal experiments were carefully performed according to the guidelines of the Care and Use of Laboratory Animals published by the National Institutes of Health18 and was approved by the Institutional Ethical Committee of our Hospital. All animals were kept in a pathogen-free environment on a 12 h light/12 h dark cycle and had access to feed and water ad libitum. For evaluating the pharmacokinetic properties, Male ICR mice (20 ± 2 g, 5–6 weeks) were obtained from Shanghai laboratory animal center (Shanghai, China) and the mice, were kept in plastic pages (25 ± 2°C; 55 ± 5% humidity). They were adapted for 7 days with a free diet and distilled water. The diabetic mice were uniformly grouped based on the blood glucose levels. Pharmacokinetics-based analysis was carried out by administrating the male normal mice with each of the investigated class of drugs viz. SGLT2 inhibitors (canagliflozin, ipragliflozin, jardiance, dapagliflozin), DPP4 (vildagliptin, alogliptin, linagliptin), NSAID (ibuprofen, indomethacin, celecoxib), antibiotics (cephalexin, amoxicillin) and the two First-line drug (insulin and metformin) with a dosage of 3 mg each for 5 days. Drugs were dissolved in water, and administered using a 5-mL syringe with a 2-cm long gavage needle through the mouth to the mouth once daily for 2 weeks. For the pharmacokinetics analysis, 24 h after the last administration of the drugs, the mice were sacrificed and blood was withdrawn from the orbital vein, liver and kidney tissues and they were isolated after dissection for further analysis. The isolated tissues and plasma were initially treated with PBS solution and mixed with 100 mL each of acetonitrile and Methyl tert-butyl ether (MTBE) and centrifuged at 12,000 rpm for 15 mins. The supernatant was taken and collected in a tube and evaporated using a vacuum concentrator and the concentrations of the administered drugs were measured and analyzed with an HPLC equipped with a UV detector. The pharmacokinetics parameters such as Cmax, half-life, and area under the curve (AUC) for 24 hr were calculated.
Statistical Analyses
SPSS Statistical Software 21.0 (SPSS Inc, USA) was employed for carrying out the statistical analysis. The patient’s characteristics were evaluated based on the categories of the exposed. The characteristics of the patients were assessed based on the drug exposure and the crude incidence rates were calculated by dividing the observed number of outcomes by the sum of all person time within each exposure group. Mean values for continuous variables were calculated based on the frequency of drugs exposed compared using the Student’s t-test. The odds ratios (OR) and 95% confidence intervals (CI) were estimated using multivariable logistic regression to analyze the association of the investigated drugs and acute kidney injury. A drug proportional hazard model was used for estimating the relative risk for AKI. Metformin – a first-line anti-diabetic drug was taken as the reference or the control.
Results
In this study, a total of 4966 diabetes mellitus patients in the age group between 55 and 75 who were prescribed with SGLT2, DDP4, NSAID, first-line drugs, and antibiotics drug were investigated. The mean age was 68.7 years for male and 71.4 years for females. SGLT2 inhibitors account for 32.52%. While other drugs such as DPP4 account for 18.55%, NSAID with 23.08%, antibiotic with 9.38% and first line with 16.47% drug users, respectively. The baseline characteristics of the patients who were exposed to such class of drugs and their usage information are presented in Table 1. One of the first-line drugs (metformin) acts as the control in the present study. At the time of follow-up, there were 1047 patients who were experiencing kidney injury during the first 3 months of the follow-up. However, during the secondary endpoint, 3919 patients were experiencing AKI. Altogether there were 4966 subjects at the end of the study.
 |
Table 1 Characteristics of the Drug Usage of the Type-2 Diabetes Patients |
The incidence rate of AKI at the end of the study varied from 5.38 for canagliflozin (SGLT2 inhibitor) to 11.22 for ibuprofen. Whereas the other SLGT2 inhibitors had an incidence rate of 5.42, 5.58, and 6.0, respectively, for ipragliflozin, jardiance and dapagliflozin. The statistical analysis from Table 2 also observed that NSAID such as ibuprofen, celecoxib, and indomethacin possessed the highest risk for acute kidney injury compared to other classes of drugs. In fact, NSAIDs account for 47.07% of cases of AKI of the total investigated population. The average percentage for the risk of causing AKI for SGLT2 inhibitors was 5.59% compared to 6.47% of DPP4, 6.30% of antibiotics, 6.82% of first-line drugs and 10.65% NSAIDs. The statistical analysis from Table 3 observed that NSAIDs viz. ibuprofen, celecoxib, indomethacin, insulin (first-line drugs), cephalexin (antibiotic), and alogliptin (DPP4) were mostly associated with an increased rate of AKI compared to control drug metformin. Ibuprofen, celecoxib, and indomethacin had a relatively increased rate of 2.41 (95% CI, 1.91–2.54), 2.32 (95% CI, 2.12–2.68), and 2.29 (95% CI, 2.05–2.65) respectively. There was not much difference in risk of AKI among the four SGLT2 inhibitors and linagliptin and vildagliptin (DPP4 inhibitors). In most of the drug users, the association of AKI remained significant even after the adjustment for multivariate analysis. The overall result indicates that NSAIDs drug users possessed a higher chance of developing AKI. While SGLT2 inhibitors have the lowest risk for developing AKI (Table 3). On the other hand, based on the pharmacokinetics analysis, the administration of the investigated drugs (3mg/kg) to the normal mice, the maximum plasma concentration could be reached 1–3 hrs followed by time-dependent elimination (Table 4). In most of the cases, the concentration of drugs peaked at 3h in the kidney and liver which is preceded by time-dependent excretion. The mouse model study also observed that the distribution of all the investigated drugs in the kidney and liver varied widely. The Tmax which was determined from the drug concentration in kidney and plasma was 0.5h for ipragliflozin, vildagliptin, celecoxib and metformin, 1h for canagliflozin, jardiance, dapagliflozin, alogliptin, linagliptin, ibuprofen, indomethacin, insulin, cephalexin, and amoxicillin. The half-life t/2 was the longest for celecoxib (4.5h) in the kidney, and shortest for insulin (1.4 h) in plasma (Table 5).
 |
Table 2 AKI Outcome During the Primary and Secondary Endpoints Caused by Different Types of the Investigated Drugs |
 |
Table 3 Associations Between AKI and the Investigated Class of Drugs |
 |
Table 4 Pharmacokinetics Analysis of the Investigated Drugs in Normal Mice and Their Time Course Changes in Plasma, Kidney and Liver |
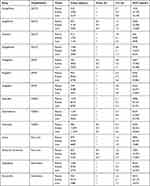 |
Table 5 Pharmacokinetics Analysis of the Investigated Drugs in Mouse Model Showing the Cmax, Tmax and Half Life |
Discussion
In the present population-based study of type-2 diabetes patients, it is observed that patients with SGLT2 inhibitor were associated with a lower risk for AKI compared with other diabetic drugs such as DPP4 inhibitors, first-line drugs, NSAIDs, and antibiotics. The study provides assurance about the safety of SGLT2 inhibitors as currently prescribed in routine diabetic care for patients. In fact, SGLT2 inhibitors were once thought to be very effective for the treatment of type-2 diabetes mellitus that usually target the SGLT2 transporter in the proximal convoluted tubule and avert the reabsorption of filtered glucose which results in glucosuria.19,20 In this study, 4966 (between 55 and 75 years old) patients were sorted out based on the drug users of SGLT2, DDP4, NSAID, first-line drugs, and antibiotics. The study also consists of a comprehensive investigation of the overview of these drugs that may be associated with acute kidney injury. During the first 90 days or 3 months of the hospital admission, 1047 patients were experiencing AKI and 3919 patients were experiencing AKI at the end of the study. The investigation noticed that the risk of developing AKI differs among the investigated patients and the NSAIDs drug users possessed the highest risk for developing AKI followed by anti-biotic users. Ibuprofen, celecoxib indomethacin, insulin, and cephalexin possessed a higher risk for AKI compared to control drug (metformin.) Therefore, in this study, it is observed that SGLT 2 inhibitor was associated with a lower 90-day risk of a hospital encounter with AKI compared to other drugs dispensed with first-line anti-diabetic drugs, DPP4 inhibitor, NSAIDs, and antibiotics. Apart from glucose control, the patients associated with SGLT2 inhibitor were observed with various clinical benefits such as reduced in blood pressure, increased calorie loss, body-weight reduction, cardio-vascular benefits and albuminuria. Similar demonstrations and benefits on reduction of stroke, heart failure, and cardiovascular disease have been reported by Rabizadeh et al based on a meta-analysis of several clinical trials.21 SGLT2 inhibitors are also known to be a good agent which bring various benefits to the kidney which includes a reduction in the progression of end-stage kidney disease, reduced worsening of glomerular filtration rate and reduced progression of chronic kidney disease, especially in type-2 diabetes mellitus patients.22 Because of the success and beneficial effect among type-2 diabetic patients and its distinctive mode of action, SGLT2 inhibitors are also currently undergoing investigation for use in type 1 diabetic patients.23 Clinical trials such as EMPEROR-Reduced and EMPEROR-Preserved and EMPA-Kidney also proposed the potential benefit and use of SGLT2 inhibitors. This is because various cardiologists and nephrologists also highly recommended the use of SGLT2 inhibitors because of its potential benefit.24 In spite of such beneficial effects, there are certain reports on AKI which include the need for dialysis, limb amputation, etc that have raised concern to the FDA requiring warning announcement.25 A possible mechanism for AKI among the type-2 diabetic patients is that the SGLT2 inhibitors may interfere with the uptake of glucose and sodium in the proximal nephron thereby increasing the delivery of sodium to the distal nephron. This process may cause afferent arteriole vasoconstriction and reduction in the estimated glomerular filtration rate (eGFR).26 However, recent clinical studies reported either no increase or a decrease in AKI risk after initiation of SGLT2 inhibitors.27 Moreover, patients undergoing SGLT2 inhibitor treatment during routine clinical practice should include proper counseling and monitoring not to take SGLT2 inhibitors during any acute illness. But this is not the case in many clinical practices since they are not monitored properly and many patients have a higher comorbidity rate compared to clinical trial study,28 thus resulting in a false observation for certain safety issues and adverse side effects based on certain observational studies of SGLT2 inhibitors.29 On the other hand, the pharmacokinetic study on mouse model experiments which was determined from the drug concentrations in plasma, kidney, and liver observed that ipragliflozin (SGLT2 inhibitor) vildagliptin (DPP4 inhibitor), metformin (First-line drug), and celecoxib (NSAIDs) have the shortest Tmax with 0.5 h. Celecoxib has the longest t1/2 value in the kidney (4.5 h) while insulin has the shortest t1/2 value in plasma (1.4 h). The kidney/plasma AUC ratio was highest for celecoxib in the kidney (46,325) and lowest for jardiance (SGLT2 inhibitor) in plasma. Thus, it indicates that the investigated drugs showed diverse distribution in plasma, kidney, and liver which maybe because of the chemical nature and structure of the compound. Therefore, the pharmacokinetic study observed that the SLGT2 inhibitors are mostly intermediate-acting drugs and the chances of their interference and causing acute injury are very less compared to other drugs. The study has also certain limitations such as patients with drinking and smoking status were not taken into account and only the Chinese population was considered in this study.
Conclusions
The study concludes that incidence of AKI is comparatively low after initiation of SLGT2 inhibitors and concludes that regulatory warnings from various health agencies about its higher risk for AKI on its prescription are unwarranted.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
All procedures were carried out in accordance with the Declaration of Helsinki 1964 and its later amendments. Written informed consent was provided by all patients and participants. Consent was obtained from each subject regarding their personal information on demographic factors, any other history of medical condition information using a questionnaire. All protocols and procedures including the animal study were approved by the Institutional Medical Ethics Committee of the Second Affiliated Hospital of Nantong University (Approval Grant No. SAH/NU/Neph/2016/N-21D).
Acknowledgments
The authors acknowledged the Health and Family Planning Commission of Nantong City, Jiangsu, China for finding this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was supported by the Health and Family Planning Commission of Nantong City, Jiangsu, China (No: MB2019009).
Disclosure
The authors declare that they have no competing interests.
References
1. Makris K, Spanou L. Acute kidney injury: definition, pathophysiology and clinical phenotypes. Clin Biochem Rev. 2016;37(2):85–98.
2. Kellum JA, Sileanu FE, Murugan R, et al. Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol. 2015;26(9):2231–2238. doi:10.1681/ASN.2014070724
3. Podkowińska A, Formanowicz D. Chronic kidney disease as oxidative stress- and inflammatory-mediated cardiovascular disease. Antioxidants. 2020;9(8):752–780. doi:10.3390/antiox9080752
4. Karasu E, Nilsson B, Köhl J, et al. Targeting complement pathways in polytrauma- and sepsis-induced multiple-organ dysfunction. Front Immunol. 2019;10:543–551. doi:10.3389/fimmu.2019.00543
5. Oliveros H, Buitrago G. Effect of renal support therapy on 5-year survival in patients discharged from the intensive care unit. J Intensive Care. 2020;8(1):63–75. doi:10.1186/s40560-020-00481-0
6. La Vignera S, Condorelli RA, Cannarella R, et al. Urogenital infections in patients with diabetes mellitus: beyond the conventional aspects. Int J Immunopathol Pharmacol. 2019;33:2058–2062. doi:10.1177/2058738419866582
7. Su H, Wan C, Song A, et al. Oxidative stress and renal fibrosis: mechanisms and therapies. Adv Exp Med Biol. 2019;1165:585–604.
8. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669–2701. doi:10.2337/dci18-0033
9. Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7(11):845–854. doi:10.1016/S2213-8587(19)30256-6
10. Menne J, Dumann E, Haller H, et al. Acute kidney injury and adverse renal events in patients receiving SGLT2-inhibitors: a systematic review and meta-analysis. PLoS Med. 2019;16(12):e1002983. doi:10.1371/journal.pmed.1002983
11. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–39. doi:10.1016/S0140-6736(18)32590-X
12. Buse JB, Wexler DJ, Tsapas A, et al. Correction to: 2019 update to: management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of diabetes (EASD). Diabetologia. 2020;63(8):1667. doi:10.1007/s00125-020-05151-2
13. Fernandez-Fernandez B, D’Marco L, Górriz JL, et al. Exploring Sodium Glucose Co-Transporter-2 (SGLT2) inhibitors for organ protection in COVID-19. J Clin Med. 2020;9(7):2030. doi:10.3390/jcm9072030
14. Mosley JF, Smith L, Everton E, et al. Sodium-Glucose Linked Transporter 2 (SGLT2) inhibitors in the management of type-2 diabetes: a drug class overview. PT. 2015;40(7):451–462.
15. Baker ML, Perazella MA. SGLT2 inhibitor therapy in patients with type-2 diabetes mellitus: is acute kidney injury a concern. J Nephrol. 2020;33(5):985–994. doi:10.1007/s40620-020-00712-5
16. Scheen AJ. An update on the safety of SGLT2 inhibitors. Expert Opin Drug Saf. 2019;18(4):295–311. doi:10.1080/14740338.2019.1602116
17. McGill JB, Subramanian S. Safety of sodium-glucose co-transporter 2 inhibitors. Am J Cardiol. 2019;124(Suppl 1):S45–S52. doi:10.1016/j.amjcard.2019.10.029
18. National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals.
19. Hsia DS, Grove O, Cefalu WT. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73–79. doi:10.1097/MED.0000000000000311
20. van Raalte DH, Bjornstad P. Role of sodium-glucose cotransporter 2 inhibition to mitigate diabetic kidney disease risk in type 1 diabetes. Nephrol Dial Transplant. 2020;35(Suppl 1):i24–i32. doi:10.1093/ndt/gfz228
21. Rabizadeh S, Nakhjavani M, Esteghamati A. Cardiovascular and renal benefits of SGLT2 inhibitors: a narrative review. Int J Endocrinol Metab. 2019;17(2):e84353. doi:10.5812/ijem.84353
22. Ninčević V, Omanović Kolarić T, Roguljić H, et al. Renal benefits of SGLT 2 inhibitors and GLP-1 receptor agonists: evidence supporting a paradigm shift in the medical management of type 2 diabetes. Int J Mol Sci. 2019;20(23):5831. doi:10.3390/ijms20235831
23. Pradhan A, Vohra S, Vishwakarma P, et al. Review on sodium-glucose cotransporter 2 inhibitor (SGLT2i) in diabetes mellitus and heart failure. J Family Med Prim Care. 2019;8(6):1855–1862. doi:10.4103/jfmpc.jfmpc_232_19
24. Williams DM, Evans M. Are SGLT-2 inhibitors the future of heart failure treatment? The EMPEROR-Preserved and EMPEROR-Reduced trials. Diabetes Ther. 2020;11(9):1925–1934. doi:10.1007/s13300-020-00889-9
25. Nadkarni GN, Ferrandino R, Chang A, et al. Acute kidney injury in patients on SGLT2 inhibitors: a propensity-matched analysis. Diabetes Care. 2017;40(11):1479–1485. doi:10.2337/dc17-1011
26. Iskander C, Cherney DZ, Clemens KK, et al. Use of sodium-glucose cotransporter-2 inhibitors and risk of acute kidney injury in older adults with diabetes: a population-based cohort study. CMAJ. 2020;192(14):E351–E360. doi:10.1503/cmaj.191283
27. Novikov A, Vallon V. Sodium glucose cotransporter 2 inhibition in the diabetic kidney: an update. Curr Opin Nephrol Hypertens. 2016;25(1):50–58. doi:10.1097/MNH.0000000000000187
28. Kalra S, Baruah MP, Sahay R. Medication counselling with sodium glucose transporter 2 inhibitor therapy. Indian J Endocrinol Metab. 2014;18(5):597–599. doi:10.4103/2230-8210.139206
29. Raschi E, Poluzzi E, Fadini GP, et al. Observational research on sodium glucose co-transporter-2 inhibitors: a real breakthrough? Diabetes Obes Metab. 2018;20(12):2711–2723. doi:10.1111/dom.13468
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
