Back to Journals » Clinical Pharmacology: Advances and Applications » Volume 7
A clinical evaluation to determine the safety, pharmacokinetics, and pharmacodynamics of an inositol-stabilized arginine silicate dietary supplement in healthy adult males
Authors Kalman D, Feldman S, Samson A, Krieger D
Received 8 March 2015
Accepted for publication 2 June 2015
Published 7 October 2015 Volume 2015:7 Pages 103—109
DOI https://doi.org/10.2147/CPAA.S84206
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Arthur E. Frankel
Douglas S Kalman, Samantha Feldman, Adam Samson, Diane R Krieger
Miami Research Associates, Miami, FL, USA
Purpose: The purpose of this study was to characterize the pharmacokinetics (PKs) and pharmacodynamics (PDs) of an oral inositol-stabilized arginine silicate dietary supplement.
Subjects and methods: Ten healthy males, 26.7±5.4 years, took three 500 mg arginine silicate capsules (active product) for 14 days. The subjects attended test visits on Days 1 and 14. Fasting blood and saliva collections were performed predose and at 0.5 hours, 1 hour, 1.5 hours, 2 hours, 3 hours, 4 hours, 5 hours, and 6 hours postdose for plasma arginine, serum silicon, and salivary nitric oxide (NO) + nitrite.
Results: Day 1 PK parameters (adjusted for body weight) for arginine were peak serum concentration (CMax) 30.06±7.80 µg/mL, time it takes to reach peak serum concentration (tMax) 1.13±0.52 hours, and time required to reach half its original concentration (t1/2) 15.93±9.55 hours and for silicon were CMax 2.99±0.63 µg/mL, tMax 2.44±2.05 hours, and t1/2 34.56±16.56 hours. After Day 1 dose, arginine levels increased at 0.5 hours, 1 hour, 1.5 hours, 2 hours, 3 hours, and 5 hours (P<0.01) and silicon levels increased at 1 hour and 1.5 hours (P<0.05). After Day 14 dose, arginine levels increased at 0.5 hours, 1 hour, and 1.5 hours (P<0.05) and silicon levels increased at 1 hour, 1.5 hours, 2 hours, and 3 hours (P<0.01). After 14 days of use, baseline arginine trended toward being higher than baseline Day 1 (P=0.0645), and 4-hour postdose plasma arginine was significantly higher (P=0.0488) at Day 14 than Day 1. Although not a significant difference, NO, as measured as salivary nitrate, increased in four subjects and stayed the same in six subjects at 0.5 hours after the first dose (P=0.125). After 14 days of use, baseline NO levels increased in six subjects and stayed the same in four subjects; this shift was significant (P=0.031).
Conclusion: The arginine silicate dietary supplement increases blood levels of arginine after a single dose within 30 minutes and blood levels of silicon for up to 1.5 hours. Blood levels of arginine, silicon, and NO (salivary nitrite) were elevated consistently after 14 days of use. The observed increase in baseline salivary nitrite is supporting information that there was some improvement in NO production. Further study on the effect of this supplement on NO production and the resulting physiological effect is warranted. Within the specific protocol of this study, the product was found to be safe.
Keywords: arginine, nitric oxide, silicon, pharmacokinetics, pharmacodynamics, dietary supplements, vasodilation
Introduction
Although nitric oxide (NO) has been shown to be involved in many physiological processes and to affect several organ systems, it is best known for its role in vasodilation and related cardiovascular health.1 Vascular dysfunction and damage have been shown to be associated with impaired endothelial NO metabolism and function. This impairment is considered to be the primary step in the development of metabolic syndrome and atherosclerosis.2–4 Therefore, maintaining NO homeostasis is critical for optimal health and disease prevention. Furthermore, it has been suggested that an increase in NO production may enhance oxygen and nutrient delivery to active muscles, thus offering promise for the potential to improve exercise performance and recovery.5 Supplementation of l-arginine has a vasodilatory effect in systemic and pulmonary vascular beds and can correct abnormal endothelium-dependent vasodilation seen in hypertensive patients.6 NO is synthesized endogenously in various cells from the recirculation of nitrites and from the nonessential amino acid l-arginine.7 Enterosalivary recirculation whereby nitrate from the diet and from endogenous NO production is reduced to nitrite contributes to the maintenance of NO. The recent recognition of this enterosalivary recirculation opens up the potential for using saliva as a biomarker for NO status.8 There has been extensive investigation into the use of supplemental l-arginine to enhance NO production in order to enhance vascular function and prevent associated diseases. There is limited evidence to support the efficacy of supplemental l-arginine in cardiovascular disease.9 It has been suggested that the lack of efficacy may be explained by alterations in bioavailability as a result of the disease state. This was supported by evidence demonstrating that results are more promising when l-arginine is combined with other substances, such as silicon, that enhance absorption and bioavailability.10–14 Silicon is an abundant trace element found in many plant based foods as well as many antacids and analgesics. It plays an important role in skin and bone health.15 Additionally, there is some evidence to suggest that silicon, especially as silicic acid, may protect vascular integrity during age-related vascular diseases.16 It has been demonstrated in rat models that arginine silicate inositol complex is more effective in increasing serum arginine levels than the commonly used arginine hydrochloride.17 Very little is known about the pharmacokinetics (PKs) and dynamics of arginine or its availability relative to an ingested dose, and even less is known about arginine combined with silicon.18 Therefore, it is the purpose of this study to: 1) characterize the short-term (6-hour) single-dose PKs of plasma l-arginine and serum silicon with an oral arginine silicate dietary supplement, before and after 14 days supplementation, based on CMax, tMax, and t1/2 and 2) determine the effects of a single dose and 14 days use of an oral arginine silicate dietary supplement on NO availability/production as measured as salivary nitrite.
Material and methods
Subjects
Ten healthy nonsmoking males of age 18–40 with a body mass index (BMI) between 18 kg/m2 and 29.9 kg/m2 were included in this study. They were recruited from a group of 34 phone-screened subjects from whom 15 were brought in for on-site screening and five did not meet the inclusion criteria. Subjects were eligible if they met the abovementioned criteria and were not taking any supplements at the time of the study. Elevated blood pressure (≥140/90), taking silicate supplements or medications containing silicate, elevated serum creatinine, having a significant medical history, or any medical condition deemed significant by the principal investigator were all exclusion criteria. Written informed consent was obtained from all participants. The study protocol was approved by Aspire Independent Review Board, August 15, 2013.
Study design
This was a prospective, single-product, open-label, PK/pharmacodynamic (PD)/safety clinical trial. Subjects reported to the laboratory for two PK test visits, with visits separated by 14 days ± 2 days. Subjects took three 500 mg (total of 1,500 mg/day) capsules of the arginine silicate dietary supplement, Nitrosigine® (Nutrition 21, LLC, Purchase, NY, USA) – a Food and Drug Administration generally recognized as safe substance, for 14 days between the two PK test visits. The purpose of the 14-day supplementation period was to address concerns that the dose of arginine silicate in a single dose might be too small for the assessment of arginine bioavailability. Prior to each PK test visit, subjects were asked not to consume foods high in arginine or silica for 24 hours, not to exercise for 12 hours, and to fast for at least 10 hours before testing. Upon reporting to the laboratory on Days 1 and 14, subjects received three 500 mg (1,500 mg) capsules of the arginine silicate dietary supplement and had blood collected nine times over a 6-hour test period. Blood collections were drawn prior to taking the test product (for baseline assessment) by subjects and 0.5 hours, 1 hour, 1.5 hours, 2 hours, 3 hours, 4 hours, 5 hours, and 6 hours postproduct administration. All blood samples, for both PK test visits, were analyzed for serum silicon and plasma l-arginine to determine the bioavailability and PK parameters of silicon and arginine. In addition to the blood assessment, saliva samples were analyzed. Saliva samples were collected prior to subjects taking the test product (for baseline assessment) and 0.5 hours, 1 hour, 1.5 hours, 2 hours, 3 hours, 4 hours, 5 hours, and 6 hours postproduct administration for the assessment of salivary nitrite (for both PK test visits). Subjects were not provided standardized meals during the PK test visits but were provided standard quantities of ultra-high purity water for hydration purposes. Safety analysis consisted of adverse events and subjective comments, and none were reported.
Biochemical analyses
All analytics for plasma and serum samples were analyzed by a third party laboratory, Keystone Bioanalytical (North Wales, PA, USA). Plasma l-arginine concentrations were determined by liquid chromatography (LC)-tandem mass spectrometry (MS) analysis. Briefly, a 50-μL aliquot of plasma was spiked with stable isotope-labeled l-arginine, which served as internal standards. Protein was precipitated with 100 μL of methanol, filtrated through a 0.22 μm hydrophilic membrane (MultiScreen HTS; EMD Millipore, Billerica, MA, USA) and analyzed by LC-tandem MS. AB/Sciex API5500 was used for detection. Quantification was performed by selected reaction monitoring of the respective daughter ions of analytes and internal standards (Waters, Eschborn, Germany). Quantitative determination of silicon in human plasma was by inductively coupled plasma mass spectrometry (ICP-MS). The sample (0.2 mL) was diluted with 1% nitric acid solution. The diluted solution was then spiked with internal standard solution (vanadium) and pumped to ICP-MS system (Aglient 7500cx) for analysis. 28Si and 51V were monitored. Concentration was calculated based on ratio of Si to V signals with calibration range of 0.1–20 mcg/mL. Helium gas was used as a reaction gas.
Salivary nitrite testing was done via the colorimetric test strip method (Neogenis Labs, Austin, TX, USA).
PK/PD analyses
PK and PD variables consisted of plasma l-arginine, serum silicon, and salivary nitrite at preingestion and 0.5 hours, 1 hour, 1.5 hours, 2 hours, 3 hours, 4 hours, 5 hours, and 6 hours postingestion. PK endpoints consisted of the standard noncompartmental PK parameters (CMax, tMax, and t1/2) for plasma l-arginine and serum silicon. PD endpoints consisted of the changes in salivary nitrite from baseline to 6 hours postproduct administration after a single dose and after 14 days use.
Statistical analyses
All PK endpoints, with and without adjustment for body weight, were evaluated, along with their standard errors and 95% confidence intervals. Changes from preingestion to each postingestion time point and changes between the two testing visits (before and after 14 days of supplementation) were tested for significance by paired Student’s t-test or repeated-measures analysis of variance.
Results
The investigated baseline characteristics of subjects are given in Table 1. All ten participants completed the study and were healthy male nonsmokers, age 18–40, with a BMI between 18 kg/m2 and 29.9 kg/m2. There were no adverse events reported during the study. Therefore, the product was found to be safe within the protocol of this study.
  | Table 1 Baseline characteristics of subjects (N=10) |
PK parameters
Arginine PK parameters are provided in Table 2 and silicon parameters in Table 3. Day 1 PK parameters for arginine were CMax 30.06±7.80 μg/mL, tMax 1.13±0.52 hours, and t1/2 15.93±9.55 hours and for silicon were CMax 2.99±0.63 μg/mL, tMax 2.44±2.05 hours, and t1/2 34.56±16.56 hours. There were no significant changes in PK parameters with 14 days of use (with and without adjustment for body weight). PK data indicate that absorption and active kinetics of the product remain consistent over 14 days of daily use indicating a predictable PK profile.
Plasma arginine
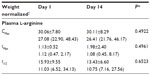
For Day 1 and Day 14, there were statistically significant increases in plasma arginine from baseline at 0.5 hours, 1 hour, and 1.5 hours (P=<0.05). For Day 14, there were also statistically significant increases from baseline at 2 hours and 3 hours (P=<0.05). Baseline plasma arginine was higher at Day 14 than Day 1, and the difference trended toward statistical significance (P=0.0645). Additionally, 4-hour plasma arginine was significantly higher at Day 14 than Day 1 (P=0.0488), Figure 1.
Serum silicon
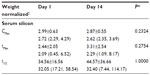
For Day 1 and Day 14, there were statistically significant increases in serum silicon from baseline at 1 hour and 1.5 hours (P<0.05). For Day 14, there were also statistically significant increases from baseline at 2 hours and 3 hours (P<0.01). There were no significant differences in serum silicon levels at any time point over the 6-hour postdose period between Day 1 and Day 14, Figure 2.
Salivary nitrite
Although not a significant difference, salivary nitrite increased in four subjects and stayed the same in six subjects at 0.5 hours after the first dose (P=0.125). After 14 days of use, baseline salivary nitrite levels increased in six subjects and stayed the same in four subjects; this shift was significant (P=0.031).
Discussion
It has been suggested that adding substances shown to enhance arginine absorption, such as silicon, may be an effective method for addressing issues with arginine bioavailability.9–14 Therefore, in this study, we tested an arginine silicate supplement to determine if it increased plasma l-arginine and serum silicon as well as NO production. Our results indicate that an arginine silicate dietary supplement increased blood levels of arginine and silicon in both an acute and chronic dosing regimen. After a single 1,500 mg dose of an arginine silicate supplement, levels of arginine increased significantly for up to 5 hours and levels of silicon for up to 1.5 hours. Similarly, a study by Proctor et al demonstrated in rat models that arginine silicate inositol complex is more effective in increasing serum arginine levels than the commonly used arginine hydrochloride product.17 This was supported by evidence demonstrating that results in clinical populations are more promising when l-arginine is combined with other substances such as silicon that enhance absorption and bioavailability.10–14 The increase observed in the current study indicates that the product we tested does increase levels of both arginine and silicon.
The peak concentration of plasma arginine (CMax) was 30.06±7.80 μg/mL, ~1 hour (tMax) after an oral dose of 1,500 mg of the arginine silicate dietary supplement and t1/2 15.93±9.55 hours. These results indicate that the product was well absorbed. There is very little reported in the literature regarding the PK parameters for arginine.19,20 Tangphao et al reported a peak concentration (CMax) for arginine of 50±13.4 μg/mL 1 hour after oral administration of 10 g l-arginine.20 Schwedhelm et al reported peak concentrations of 49.0±6 μg/mL 3.7 hours after 7 days of a 3.2 g daily oral dose of slow release l-arginine and 84.0±9.7 hours after a 3 g daily dose of immediate release arginine.21 The higher peak values measured in the Tangphao et al and Schwedhelm et al studies could be explained by the higher doses (10 g and 3 g over 7 days vs 681 mg) or by individual differences influencing a small subject population. For example, Schwedhelm et al demonstrated that plasma arginine and the associated NO-dependent signaling is increased in a dose-dependent manner when they compared the PK parameters of different doses of l-arginine, indicating the dose does influence peak serum values. Tangphao et al measured plasma arginine in 20 subjects consuming standardized meals over 8 hours and reported significant individual variations. The authors suggested that individual differences in bioavailability and clearance make clarifying PK parameters for arginine challenging.20 It is promising to note that although our peak values were slightly lower than those seen in the other studies, we reported similar tMax values. Furthermore, the peak serum arginine was only slightly lower considering the much lower dose used in our study, which was 50% of the dose used in the Schwedhelm et al study and 15% of the dose used in the Tangphao et al study. This is possibly explained by the addition of silicon in the product used in our study.
In our study, the peak concentration (CMax) for silicon was 2.99±0.63 μg/mL approximately 2 hours and 44 minutes after the 1,500 mg dose of the product and t1/2 34.56±16.56 hours. Defining the PK parameters for silicon is difficult due to the variation in absorption rates for different forms of silicon.22,23 A study by Sripanyakorn et al22 attempted to define the silicon absorption of various foods and common dietary supplemental sources. Sripanyakorn et al reported peak serum silicon concentrations after ingestion of various common supplemental forms of silicon ranging from 30 minutes to 4 hours, with peak concentrations dependent on the source of silicon.
l-Arginine is oxidized to synthesize NO. Therefore, it can be hypothesized that increased serum levels of l-arginine can increase NO production and under healthy normal conditions, NO production typically proceeds quite efficiently.24 The recognition of an enterosalivary cycle whereby nitrate and nitrite are reduced to NO makes it reasonable to use saliva as a potential biomarker for NO status.8 Therefore, we used salivary nitrite as a biomarker for NO production. Although not a significant difference, salivary nitrite increased in four subjects and stayed the same in six subjects at 30 minutes after the first dose. After 14 days of use, baseline salivary nitrite levels increased in six of the ten subjects. This is supporting evidence that there was some improvement in NO production after ingesting the dietary arginine silicate supplement. Similarly, Schwedhelm et al showed evidence of increased NO production after supplementation of 3.2 g daily of l-arginine for 1 week as measured by the ratio of plasma l-arginine over asymmetric dimethylarginine, an endogenous inhibitor of NO synthase (arginine/asymmetric dimethylarginine ratio). Schwedhelm et al did not report any effect from lower doses of l-arginine. Our study used a lower dose and saw evidence suggesting an increase in NO production most likely due to the addition of silicate in the product tested, whereas in the Schwedhelm et al study, arginine HCl was tested.
While the use of salivary nitrite as a biomarker for NO production offers an inexpensive noninvasive method for measuring NO production, its limitations need to be considered. Individuals do not have the same communities of oral bacteria that are responsible for reducing salivary nitrate to nitrite. It can be altered by the use of antiseptic mouthwash, overuse of antibiotics, reduced gastric acidity, decreased swallowing, and by different degrees of oral hygiene. All efforts were made to standardize salivary collections to minimize chances of contamination or nonoptimal sample collection. Second, steady-state concentrations of nitrite in the saliva are dependent upon salivary flow rates and production. Therefore, patients with various clinical conditions and/or surgeries will potentially have a breakdown in this pathway making accurate assessment challenging.8
The administration of performance-enhancing supplements is often rationalized on the basis of a chain of plausibility arguments. Arginine has been studied extensively for 15 years as a natural metabolic donor of NO; so arginine-containing products should increase circulating arginine levels, which should increase (or at least maintain) relatively high NO levels, which should promote relaxation of smooth muscle in blood vessels, which should increase blood flow to the muscles, which should lead to improved muscle function and stamina, and which should show up as improved athletic performance. The results of this study support two links in the above chain: the first link, the arginine silicate product increased circulating arginine levels, and the second link, this increase in circulating arginine was associated with a tendency of baseline salivary nitrite to increase which could suggest increased NO levels. Studies exploring the remaining links of this plausibility chain are warranted as the potential for the arginine silicate product to enhance athletic performance is promising.
Conclusion
The arginine silicate dietary supplement increases blood levels of arginine after a single dose within 30 minutes for up to 5 hours and increases blood levels of silicon for up to 1.5 hours. This result is reproducible as evident by the 14-day PK data. Blood levels of arginine silicon and NO (salivary nitrite) were elevated consistently after 14 days of daily use. PK data indicate that absorption and active kinetics of the product remain consistent over 14 days of daily use, indicating a predictable PK profile. The observed increase in baseline salivary nitrite is supporting information that there was some improvement in NO production. The product was found to be safe within the protocol of this study. Further study on the effect of this supplement on NO production and the resulting physiological effect is warranted.
Acknowledgment
The authors wish to acknowledge that professional medical writing was provided by Susan J Hewlings, PhD. The abstract of this paper was presented at the 2014 Experimental Biology Conference as a poster presentation with interim findings. The poster’s abstract was published in Poster Abstracts in The FASEB Journal (The Journal of Federation of American Societies for Experimental Biology), April 2014, vol 28, no 1, Supplement LB418 (http://www.fasebj.org/content/28/1_Supplement/LB418.abstract). The actual paper, however, has never been published.
Disclosure
The authors report no conflicts of interest in this work.
References
Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol. 2006;147(Suppl 1):S193–S201. | |
O’Brien SF, Russell JC. Insulin resistance and vascular wall function: lessons from animal models (review). Endocrinol Metab. 1997;4: 155–162. | |
Radomski MW, Salas E. Nitric oxide – biological mediator, modulator and factor of injury: its role in the pathogenesis of atherosclerosis. Atherosclerosis. 1995;118:S69–S80. | |
Napoli C, de Nigris F, Williams-Ignarro S, Pignalosa O, Sica V, Ignarro LJ. Nitric oxide and atherosclerosis: an update. Nitric Oxide. 2006;15:265–279. | |
Bescós R, Sureda A, Tur J, Pons A. The effect of nitric-oxide-related supplements on human performance. Sports Med. 2012;42(2):99–117. | |
Mehta S, Stewart DJ, Langleben D, Levy RD. Short-term pulmonary vasodilation with l-arginine in pulmonary hypertension. Circulation. 1995;92(6):1539–1545. | |
Parthasarathy DK, Bryan NS. Sodium nitrite: the “cure” for nitric oxide insufficiency. Meat Sci. 2012;92:274–279. | |
Bryan NS, Loscalzo J, editors. Nitrite and nitrate in human health and disease. In: Nutrition and Health, (Bendich A, series editor). New York: Humana Press; 2011. | |
Loscalzo J. l-Arginine and atherothrombosis. J Nutr. 2004;134: 2798S–2800S. | |
Proctor SD, Kelly SE, Russell JC. A novel complex of arginine-silicate improves micro- and macrovascular function and inhibits glomerular sclerosis in insulin-resistant JCR:LA-cp rats. Diabetologia. 2005;48: 1925–1932. | |
Russell JC, Kelly SE, Proctor SD. The JCR:LA-cp rat: animal model of the metabolic syndrome exhibiting micro- and macro-vascular disease. In: Shafrir E, editor. Animal Models of Diabetes. Boca Raton, FLA: CRC Press; 2007:157–180. | |
O’Brien SF, Russell JC, Davidge ST. Vascular wall dysfunction inJCR:LA-cp rats: effects of age and insulin resistance. Am J Physiol. 1999;277:C987–C993. | |
Juturu V, Komorowski JR, Gudi R. Orally administered arginine silicate inositol complex is not clastogenic in Chinese hamster ovary cells. FASEB J. 2004;18:A869. [Abstract]. | |
Komorowski JR, Juturu V, Gudi R. Arginine silicate inositol complex is not toxic in mouse micronucleus assay. FASEB J. 2004;18:A869. | |
Jugdaohsingh R. Silicon and bone health. J Nutr Health Aging. 2007;11: 99–110. | |
Buffoli B, Foglio E, Borsani E, Exley C, Rezzani R, Rodella LF. Silicic acid in drinking water prevents age-related alterations in the endothelium-dependent vascular relaxation modulating eNOS and AQP1 expression in experimental mice: an immunohistochemical study. Acta Histochem. 2013;115(5):418–424. | |
Proctor SD, Kelly SE, Vine DF, Russell JC. Metabolic effects of a novel silicate inositol complex of the nitric oxide precursor arginine in the obese insulin-resistant JCR:LA-cp rat. Metabolism. 2007;52: 1318–1325. | |
Cynober L. Pharmacokinetics of arginine and related amino acids. J Nutr. 2007;137:1646S–1649S. | |
Bode-Böger SM, Böger RH, Galland A, Tsikas D, Förlich JC. l-arginine-induced vasodilation in healthy humans: pharmacokinetic- pharmacodynamic relationship. Br J Clin Pharmacol. 1998;46(5):489–497. | |
Tangphao O, Grossmann M, Chalon S, Hoffman B, Blaschke T. Pharmacokinetics of intravenous and oral l-arginine in normal volunteers. Br J Clin Pharmacol. 1999;47(3):226–261. | |
Schwedhelm E, Maas R, Freese R, et al. Pharmacokinetic and pharmacodynamic properties of oral l-citrulline and l-arginine: impact on nitric oxide metabolism. Br J Clin Pharmacol. 2008;65(1):51–59. | |
Sripanyakorn S, Jugdaohsingh R, Dissayabutr W, Anderson SH, Thompson RP, Powell JJ. The comparative absorption of silicon from different foods and food supplements. Br J Nutr. 2009;102(6):825–834. | |
Jugdaohsingh R, Sripanyakorn S, Powell JJ. Silicon absorption and excretion is independent of age and sex in adults. Br J Nut. 2013;110: 1024–1030. | |
Bryan N. Application of nitric oxide in drug discovery and development. Expert Opin Drug Discov. 2011;11:1139–1154. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.