Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
A Case Report of Possibly Related Acute Generalized Exanthematous Pustulosis with Staphylococcus pettenkoferi
Received 6 January 2023
Accepted for publication 5 March 2023
Published 17 March 2023 Volume 2023:16 Pages 673—676
DOI https://doi.org/10.2147/CCID.S399138
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Su Wang, Juan Bai, Jianjun Qiao
Department of Dermatology, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, 310003, People’s Republic of China
Correspondence: Jianjun Qiao; Juan Bai, Email [email protected]; [email protected]
Abstract: Acute generalized exanthematous pustulosis (AGEP), an uncommon severe cutaneous adverse reaction, is believed to be a T cell-mediated hypersensitivity reaction, of which the most common cause is medication. However, infections have also been reported to be associated with AGEP. Here, we present a case of AGEP possibly related with Staphylococcus pettenkoferi.
Keywords: acute generalized exanthematous pustulosis, Staphylococcus pettenkoferi
Introduction
Acute generalized exanthematous pustulosis (AGEP), a severe cutaneous adverse reaction, was first described by Baker and Ryan in 1968 and named by Beylot et al in 19801,2 AGEP is characterized by an acute onset of mainly small non-follicular pustules on an erythematous base and accompanied by fever (>38°C). Increased neutrophil count and high sensitivity C-reactive protein (CRP) are also common in AGEP.3 The incidence of AGEP is 1 to 5 per million patients a year,4 while the mortality rate is estimated to be around 5%.5
Ninety percent of AGEP are drug-induced.6 A small number cases are associated with infections.7,8 Here we report a case of typical AGEP associated with S. pettenkoferi, a relatively rare coagulase Negative Staphylococcus (CoNS).
Case Report
A 17-year-old boy with a 6-day history of skin rash and a 4-day history of high fever sought medical advice in our department of dermatology. The highest temperature was 40.5°C. The patient experienced sore throats and knee pain during movement. He did not recall any history of obvious inducement, especially suspect drugs. After empirical ceftriaxone sodium for injection anti-infection and loratadine tablets anti-allergy treatment for two days, his symptoms did not improve significantly. Physical examination revealed generalized small pustules on an erythematous base over the trunk, extremities, and flexures (Figure 1A–D). There were no lesions on the oral mucosa and genital skin. The patient was otherwise healthy and denied a family history of psoriasis. Laboratory tests showed increased white blood cells of 17.76×109/L (normal range 4.0–10.0×109/L); neutrophilia of 15.81×109/L (normal range 2.0–7.0×109/L); high C-reactive protein of 108.81 mg/L (normal range 0–8.0mg/L). Biochemical examination demonstrated that alanine aminotransferase was 100 U/L (normal range 7–40 U/L); total bilirubin was 44.9umol/L (normal range 0–21 U/L); direct bilirubin was 32.8 mol/L (normal range 0–8 U/L); glutamyl transpeptidase was 156U/L (normal range 7–45 U/L). Procalcitonin level was 1.13ng/mL(0.00–0.50). Metagenomic examination of pathogens in peripheral blood indicated the sequence number of S. pettenkoferi was 1330. Blood bacterial culture did not growth. The throat swab culture was negative. CT scan of the lungs showed that there were a few exudative changes with interstitial changes in both lungs and pleural effusion. Histopathology of a skin biopsy specimen showed subcorneal neutrophilic pustule and edema, as well as peripheral infiltration of neutrophils and lymphocytes in the superficial dermis (Figure 2). A diagnosis of acute generalized exanthematous pustulosis (AGEP) was considered based on rash morphology, clinical progress and cutaneous histopathology. Then, we used methylprednisolone 40mg per day to control the inflammation, and linezolid injection 0.2 q12h for anti-infection treatment. After 4 days, the pustules subsided and the erythema became dull in color.
 |
Figure 1 (A–D) Generalized small pustules on an erythematous base were present over the trunk, extremities, and flexures. |
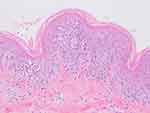 |
Figure 2 Subcorneal neutrophilic pustule and edema, as well as peripheral infiltration of neutrophils and lymphocytes in the superficial dermis. |
Discussion
S. pettenkoferi, a relatively new CoNS, was first identified and described in 2002 by Trulzsch et al.9 The pathogene usually cause opportunistic infections (eg, peripheral or central catheter artificial heart valves) and nosocomial infections, or influence people with compromised immune systems. The virulence of S. pettenkoferi was confirmed by using a zebrafish model.10 S. pettenkoferi can persist in the human body using macrophages as bacterial reservoirs.10 To the best of our knowledge, AGEP associated with S. pettenkoferi has not been reported yet.
AGEP is classified as T cell-associated sterile neutrophil inflammatory response (IV type).11 T cells and cytotoxic proteins such as granase B perforin induce apoptosis of the keratinocytes and the formation of subcorneal vesicles.12 Specific T cells overproduce chemokine (C-X-C motif) ligand 8 (CXCL8)/IL-8 in AGEP patients, which is a powerful neutrophil chemokine and plays a key role in pustular formation.13 Mutations in the IL-36RN gene, encoding the interleukin-36 receptor antagonist (IL-36RA), appear to be more associated with drug-induce AGEP.14–16 IL-17, IL-22 and GM-CSF are also involved in maintaining strong neutrophil activity in AGEP,17 which are novel potential therapeutic targets for AGEP.
The mechanism of infection as a cause of AGEP is not completely clear, it is generally believed that changes in cytokine storms caused by infection in many ways are similar to those caused by drugs.8 For example, in a case of AGEP associated with Coccidioides infection, it was proposed that both Coccidioides infection and AGEP showed similar changes in cytokines dominated by Th1 inflammation.18 S. pettenkoferi is a relatively rare CoNS, the clinical significance and epidemiology of S. pettenkoferi are still not sufficient. Studies of other CoNS showed that they can induce the expression of IL-6, IL-8 and other cytokines in animals.19,20 Infection-mediated transient immune changes set the stage for loss of antigen tolerance and the development of reversible delayed hypersensitivity reactions.21
AGEP is generally self-limiting with a favorable prognosis. Treatment for drug-induced AGEP is based on removal of the triggering agents, topical steroid administration and/or systemic corticosteroids administration in severe cases.3 The significance of systemic corticosteroid administration in shortening the course of the disease is controversial.22 Supportive care, specifically infection prevention, is crucial when pustules begin to coalesce and large sheets of skin desquamate. In some cases of infection-associated AGEP with clear laboratory evidence, aggressive anti-infective therapy is considered necessary.18 Over the past few years, it was highlighted the prominent role of IL-17 in the pathogenesis of AGEP.17,23 Secukinumab has promising outcome for the AGEP patients resistant to hormone therapy.24 It was reported that IL22 has been reported to play a major role in AGEP, providing a new perspective for further exploration of its mechanism and new therapies.25
Conclusions
In this report, we presented a case of AGEP associated with S. pettenkoferi. The characteristics, etiological mechanism and therapy of AGEP are briefly reviewed.
Ethical Concerns
The patient’s mother signed the informed consent. They agreed to publish the details of this case. Institutional approval has been obtained.
Disclosure
The authors do not have any conflicts of interest to declare in this work.
References
1. Baker H, Ryan TJ. Generalized pustular psoriasis. A clinical and epidemiological study of 104 cases. Br J Dermatol. 1968;80(12):771–793. doi:10.1111/j.1365-2133.1968.tb11947.x
2. Beylot C, Bioulac P, Doutre MS. Pustuloses exanthématiques aiguës généralisées, à propos de 4 cas [Acute generalized exanthematic pustuloses (four cases) (author’s transl)]. Ann Dermatol Venereol. 1980;107(1–2):37–48. French.
3. Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73(5):843–848. doi:10.1016/j.jaad.2015.07.017
4. Sussman M, Napodano A, Huang S, et al. Pustular psoriasis and acute generalized exanthematous pustulosis. Medicina. 2021;57. doi:10.3390/medicina57101004
5. Sidoroff A. Acute generalized exanthematous pustulosis. Chem Immunol Allergy. 2012;97:139–148. doi:10.1159/000335625
6. Chang SL, Huang YH, Yang CH, Hu S, Hong HS. Clinical manifestations and characteristics of patients with acute generalized exanthematous pustulosis in Asia. Acta Derm Venereol. 2008;88:363–365. doi:10.2340/00015555-0438
7. Taguchi K, Oka M, Bito T, Nishigori C. Acute generalized exanthematous pustulosis induced by Mycoplasma pneumoniae infection. J Dermatol. 2016;43(1):113–114. doi:10.1111/1346-8138.13151
8. Ayatollahi A, Robati RM, Kamyab K, Firooz A. Late-onset AGEP-like skin pustular eruption following COVID-19: a possible association. Dermatol Ther. 2020;33:e14275. doi:10.1111/dth.14275
9. Trulzsch K, Rinder H, Trček J, et al. ”Staphylococcus pettenkoferi”, a novel staphylococcal species isolated from clinical specimens. Diagn Microbiol Infect Dis. 2002;43(3):175–182. doi:10.1016/s0732-8893(02)00399-1
10. Ahmad-Mansour N, Plumet L, Huc-Brandt S, et al. Investigating pathogenicity and virulence of Staphylococcus pettenkoferi: an emerging pathogen. Int J Mol Sci. 2021;22:13614. doi:10.3390/ijms222413614
11. Schmid S, Kuechler PC, Britschgi M, et al. Acute generalized exanthematous pustulosis: role of cytotoxic T cells in pustule formation. Am J Pathol. 2002;161(6):2079–2086. doi:10.1016/S0002-9440(10)64486-0
12. Schlapbach C, Zawodniak A, Irla N, et al. NKp46+ cells express granulysin in multiple cutaneous adverse drug reactions. Allergy. 2011;66(11):1469–1476. doi:10.1111/j.1398-9995.2011.02677.x
13. Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17(8):1214. doi:10.3390/ijms17081214
14. Gabay C, Towne JE. Regulation and function of interleukin-36 cytokines in homeostasis and pathological conditions. J Leukoc Biol. 2015;97(4):645–652. doi:10.1189/jlb.3RI1014-495R
15. Navarini AA, Valeyrie-Allanore L, Setta-Kaffetzi N, et al. Rare variations in IL36RN in severe adverse drug reactions manifesting as acute generalized exanthematous pustulosis. J Invest Dermatol. 2013;133(7):1904–1907. doi:10.1038/jid.2013.44
16. Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365(7):620–628. doi:10.1056/NEJMoa1013068
17. Kabashima R, Sugita K, Sawada Y, et al. Increased circulating Th17 frequencies and serum IL-22 levels in patients with acute generalized exanthematous pustulosis. J Eur Acad Dermatol Venereol. 2011;25(4):485–488. doi:10.1111/j.1468-3083.2010.03771.x
18. McBride MO, Davis MS, Casey MA, Lam TS. Acute generalized exanthematous pustulosis associated with coccidiomycosis infection. JAAD Case Rep. 2021;9:36–38. doi:10.1016/j.jdcr.2020.12.026
19. Lu Y, Lu Q, Cheng Y, et al. High concentration of coagulase-negative staphylococci carriage among bioaerosols of henhouses in central China. BMC Microbiol. 2020;20(1):21. doi:10.1186/s12866-020-1709-y
20. Kawecka-Grochocka E, Zalewska M, Rzewuska M, et al. Expression of cytokines in dairy cattle mammary gland parenchyma during chronic staphylococcal infection. Vet Res. 2021;52:132. doi:10.1186/s13567-021-01003-y
21. Pezzarossa E, Ungari M, Caresana G, et al. Acute Generalized Exanthematous Pustulosis (AGEP) in 12 patients treated for SARS-CoV-2 positive pneumonia. Am J Dermatopathol. 2021;43(5):342–348. doi:10.1097/DAD.0000000000001819
22. Nili A, Zarei E, Ghamari A, et al. Acute generalized exanthematous pustulosis with a focus on hydroxychloroquine: a 10-year experience in a skin hospital. Int Immunopharmacol. 2020;89:107093. doi:10.1016/j.intimp.2020.107093
23. Kakeda M, Schlapbach C, Danelon G, et al. Innate immune cells express IL-17A/F in acute generalized exanthematous pustulosis and generalized pustular psoriasis. Arch Dermatol Res. 2014;306(10):933–938. doi:10.1007/s00403-014-1488-0
24. Gualtieri B, Solimani F, Hertl M, et al. Interleukin 17 as a therapeutic target of acute generalized exanthematous pustulosis (AGEP). J Allergy Clin Immunol Pract. 2020;8(6):2081–2084 e2082. doi:10.1016/j.jaip.2020.01.045
25. Klaewsongkram J, Buranapraditkun S, Thantiworasit P, et al. The role of in vitro detection of drug-specific mediator-releasing cells to diagnose different phenotypes of severe cutaneous adverse reactions. Allergy Asthma Immunol Res. 2021;13(6):896–907. doi:10.4168/aair.2021.13.6.896
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
