Back to Journals » Medical Devices: Evidence and Research » Volume 14
A Biologic Surgical Implant in Complex Abdominal Wall Repair: 3-Year Follow-Up Results of a Multicentric Prospective Study
Authors Gossetti F, Zuegel N , Giordano P, Pullan R, Schuld J, Delrio P, Montorsi M, van Kerschaver O, Lemaitre J, Griffiths B, D'Amore L
Received 14 January 2021
Accepted for publication 29 June 2021
Published 25 August 2021 Volume 2021:14 Pages 257—264
DOI https://doi.org/10.2147/MDER.S297897
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Francesco Gossetti,1 Nikolaus Zuegel,2 Pasquale Giordano,3 Rupert Pullan,4 Jochen Schuld,5 Paolo Delrio,6 Marco Montorsi,7 Olivier van Kerschaver,8 Jean Lemaitre,9 Ben Griffiths,10 Linda D’Amore1
1Department of Abdominal Wall Surgery, University Hospital (C/O Instituto Clinica Chirugica II), Rome, Italy; 2Visceral Surgery, Centre Hospitalier Emile Mayrisch, Esch-sur-Alzette, Luxembourg; 3Department of Colorectal Surgery, Whipps Cross University Hospital, Leytonstone, UK; 4Colorectal Surgery, Torbay Hospital, Torquay, UK; 5Clinic for General and Visceral Surgery, Universitätsklinikum des Saarlandes, Homburg, Germany; 6Department of Abdominal Oncology, Istituto Nazionale Tumori, Napoli, Italy; 7General Surgery, Humanitas University and IRCCS Humanitas Research Hospital, Milano, Italy; 8General Surgery, Algemeen Ziekenhuis Sint LUCAS, Gent, Belgium; 9Thoracic Surgery, CHU Ambroise Pare, Mons, Belgium; 10Colorectal Surgery, Royal Victoria Infirmary, Newcastle Upon Tyne, UK
Correspondence: Francesco Gossetti
Department of Abdominal Wall Surgery, University Hospital (C/O Instituto Clinica Chirurgica II), Policlinico Umberto I, Rome, 00162, Italy
Email [email protected]
Purpose: Despite the advancements in the reinforcement and closure techniques available, complex abdominal wall reconstruction (CAWR) remains a challenging surgical undertaking with considerable risk of postoperative complications. Biological meshes were developed that may help to complement standard closure techniques and offer an alternative to synthetic meshes, which carry significant risks with their use in complex cases.
Patients and Methods: A total of 114 patients underwent surgical treatment for CAWR with a Permacol™ (a biologic surgical implant). The study objective was to evaluate the short-term (6 months), mid-term (12– 24 months), and long-term (36 months) clinical outcomes (through 36 months) associated with the use of a biologic surgical implant in these cases.
Results: The cumulative hernia recurrence rate was 18.7% (17/91) at 24 months and 22.4% (19/85) at 36 months. Twelve (14.1%) subjects required reoperation for hernia repair within 36 months for repair of recurrent hernias. Between 6- and 36-months post-surgery, patients reported improvement in their Carolina comfort scale (CSS) measures of severity of pain, sensation of mesh, and movement limitations.
Conclusion: A biologic surgical implant can provide long-term benefit to complex abdominal wall repairs in patients staged grade III according to the Ventral Hernia Working Group (VHWG).
Keywords: Permacol, biologic surgical implant, complex abdominal wall repair
Introduction
Abdominal wall hernias have been reported to occur in as many as 24% of the patients undergoing abdominal surgery post laparotomy. This number rises when other comorbidities such as prior abdominal surgery, obesity, diabetes, and prior irradiation are factored in.1 In these complex situations, it is necessary to carefully consider the surgical approach to ensure optimum recovery for the patient. Despite the numerous advancements in reinforcement and closure techniques available today, complex abdominal wall reconstruction (CAWR) remains a challenging surgical undertaking with considerable postoperative complications. In obese patients with previous repair failures, recurrence rates at 1 year have been reported to be as high as 50%.1 In these patients, each successive repair poses increasing challenges to the surgeon and risks to the patients.
Abdominal wall reconstruction procedures pose challenging wound closure decisions for surgeons. Clearly, beyond identifying those in need of special closure techniques, it is also difficult to decide which closure techniques should be used to repair the defects created during surgery. Important considerations regarding the choice for closure techniques for CAWR include the availability of tissue for flap creation as well as the tension at the site of the repair. In cases where there is likely to be insufficient tissue or where there will be a considerable amount of tension, synthetic materials may seem appealing due to their high strength; however, they are considered inappropriate for use in a range of procedures such as those where the mesh may come into contact with the bowel.1–4 Abdominal wall reconstruction with synthetic meshes carries a historical hernia recurrence rate of 11% and overall complication rate of 18–50%, which includes bowel adhesions, fistulization, ulceration, and extrusion, and a 50–90% risk of infection in contaminated cases.3,4 Mathes et al performed a study to compare several reconstructive options for repairing abdominal wall defects which included direct tissue closure, use of synthetic mesh, local advancement or regional flaps, distant flaps, or the combined use of flaps and mesh. Even in smaller defects that were repaired using direct closure, hernia recurrence rates were still 27%.3
To date, biological meshes have been developed that may help to complement standard closure techniques and offer an alternative to synthetic meshes that carry significant risks with their use in complex cases. These biological materials are particularly valuable in situations where synthetic meshes are inappropriate for use due to contamination, existing infections, wound dehiscence, unstable wound coverage, diabetes, obesity, patient smoking history, or immunocompromisation.2,4 The reported incidence of severe biological mesh-related complications is comparable to, or less than, that of synthetic mesh repairs in CAWR, and the long-term success rates of biological vs synthetic meshes appear to be equivalent.2,5–7 Since they can be vascularized rapidly, it is thought that biological meshes could be particularly beneficial where the incidence of fistula and wound infection is high, such as when preoperative chemo-radiation has been used.2,5
The present study was undertaken to evaluate short-term (6 months), mid-term (12–24 months), and long-term (36 months) post-surgical data on Permacol™ (a biologic surgical implant) following complex abdominal wall (CAWR) repair.
Materials and Methods
Between February 2011 and October 2013, patients underwent surgical treatment for complex abdominal wall repair with a biologic surgical implant. The study objective was to evaluate short-term, mid-term, and long-term clinical outcomes associated with the use of a biologic surgical implant in the treatment of complex abdominal wall defects through 36 months (3 years) postoperatively. The study was completed in November 2016 and is registered publicly at ClinicalTrials.gov (NCT01268514).
Test Device
Permacol™ (a biologic surgical implant) is a sheet of acellular porcine dermal collagen. Permacol™ is a patented product that is processed to remove non-collagenous and cellular components and is additionally cross linked to enhance durability.
Participants
Patients ≥18 years of age who required CAWR using a biologic surgical implant were consented if they met eligibility criteria via a screening/baseline visit. A CAWR was defined as infected, contaminated, clean contaminated, or clean with a history of infected or contaminated field, atypical hernia location, loss of domain, fascial dehiscence, and/or requiring abdominal wall mobilization for wound closure. Patients included in the study were 1) subjects who required complex abdominal repair using a biologic surgical implant, 2) were 18 years of age or older, 3) were male or female, and 4) who were willing and able to adhere to protocol requirements and provide written informed consent Exclusion criteria included the following: 1) patients who were pregnant; 2) patients with a medical condition that in the opinion of the investigator may have precluded participation (eg, Ehlers Danlos Syndrome) or interfered with completion of study follow-up; 3) patients could not participate in any other clinical study that clinically interfered with this study while enrolled; 4) concomitant use of a synthetic or another biologic mesh; 5) patients who required use of a biologic surgical implant as only temporary closure with planned reoperation, or a biologic surgical implant used as a temporary dressing in an open abdomen; (6) Patients who had severe systemic sepsis at the time of a biologic surgical implant implantation. Severe sepsis is defined as sepsis with organ dysfunction, hypoperfusion, or hypotension; (7) Patients with ongoing necrotizing pancreatitis; 8) Patients who required use of a biologic surgical implant in parastomal hernia repair alone, where there is no other anterior wall repair; 9) Patients who required prophylactic use of a biologic surgical implant in the formation of stoma with anterior wall repair; and 10) a biologic surgical implant used in pelvic floor reconstruction. The trial protocol was approved at each institution’s ethics committee (see Supplementary Materials) in accordance with the Helsinki declaration, and all patients provided written informed consent to participate.
Trial Design and Endpoints
This was a prospective, observational, multicenter study following CAWR (including abdominal wall defects and fascial dehiscence). The observational design of this study intentionally placed no restrictions on procedural technique to ensure generalization and real-world results. Postoperative, assessments were performed at 1 month, 6 months, 12 months, 24 months, and 36 months.
The primary endpoint was to assess the hernia recurrence rate at 36 months post-surgery. Hernia recurrence is defined as a protrusion through the abdominal repair field following assessment by the operating surgeon or equivalent by training and requiring surgical intervention. Secondary endpoints were 1) to characterize short-term and mid-term outcomes within 24 months post-surgery of the proportion of subjects who undergo reoperation for hernia or hernia recurrence; 2) to assess quality of life by Carolinas Comfort Scale at 6 months, 12 months, 24 months, and 36 months (The Carolina comfort scale (CSS) is a Likert-type questionnaire that measures severity of pain, sensation of mesh, and movement limitations ranging from 0 (no symptoms) to 5 (disabling symptoms)); 3) to assess patient satisfaction questionnaire at 6 months, 12 months, 24 months, and 36 months; and 4) for study personnel to assess subjects incidence of postoperative complications, specifically: wound infection, seroma, hematoma, wound dehiscence, and fistula.
Statistics
Statistical analysis was performed on the per-protocol population (excluding missing data and taking into account patients with at least the 6 months postoperative visit/assessment performed) and consisted of descriptive analysis. Qualitative variables were described by their absolute and relative (%) frequencies of each class or value, and by two-tailed 95% confidence intervals. Quantitative variables were described by their mean, standard deviation (SD), extreme values (minimum and maximum values), and number of missing data. Statistical analysis was performed under SAS Version 9.2 and Minitab Version 15.0 using significance of 5%.
Results
Patient Disposition and Demographics
Between February 2011 and December 2016, 122 patients were consented for the study at 12 sites in 5 European countries. Eight patients were screen failures with a final 114 patients included in the full analysis set. Of these 114 patients, 111 (97.4%) were incisional/abdominal wall cases while 3 (2.6%) were primary hernias. Seventy-three (64.0%) patients completed the study while 41 (36.0%) withdrew for reasons including adverse events, lost to follow-up, death, and withdrawal by patient (Figure 1). Patients had a mean age of 60.8 ± 12.2 [29–87] years and a mean BMI of 31.2 ± 6.0 [18.7–45.4] kg/m2 of which 63 (55.3%) patients had a BMI ≥30 kg/m2 (Table 1). Additionally, 68 (59.7%) patients were current or past smokers.
 |
Table 1 Patient Disposition and Baseline Demographics |
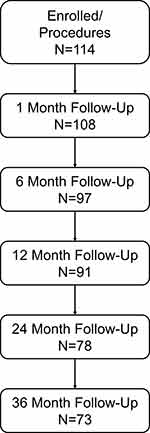 |
Figure 1 Study enrollment and follow-up. |
Surgical Details
Most patients underwent surgery with a primary indication of incisional hernias (92.1%, Table 2). Implant placement techniques and fixation methods varied, but the majority of surgeries used intra peritoneal onlay (n=89, 78.1%), and either non-resorbable sutures (n=50, 43.9%) or slowly resorbable sutures (n=59, 51.8%) (Table 2). Hernias had a mean length of 15.1 ± 8.8cm [2–35 cm] and a mean width of 11.6 ± 6.9cm [3–35 cm]. The majority of procedures used either the 20×30 cm (n=32, 28.1%) or 18×28 cm (n=30, 26.3%) implants for the first use. In addition, six procedures used a second implant of varying sizes and no procedures used a third implant (Table 2). The total mean implant area for the procedures was 460.7 ± 213.7 cm with a minimum of 100 cm and a maximum of 1100 cm. In total, facial closure was achieved on 101 (88.6%) of the patients. No device malfunctions were reported during the study (Table 2). Additionally, although 42 patients had Ventral Hernia Working Group (VHWG) grade III or IV, additional patients with VHWG grade I and II were included due to the presence of various comorbidities.
 |
Table 2 Surgical Details |
Hernia Recurrence and Adverse Events
The cumulative hernia incidence was 18.7% (17/91) at 24 months and 22.4% (19/85) at 36 months (Table 3). Twelve (14.1%) subjects required reoperation within 36 months for repair of recurrent hernias. Total peri- and postoperative complications included 13 (11.3%) wound dehiscences, 11 (9.6%) wound infections, 11 (9.6%) seromas, and 4 (3.5%) hematomas (Table 3). Additionally, a single case (0.9%) of stoma site pain was reported.
 |
Table 3 Hernia Recurrence and Adverse Events |
Quality of Life
Between 6- and 36-months post-surgery, patients showed improvement in all three scores. In particular, significant improvement (p=0.0036) was seen in the reduction of pain at 24 months (Table 4).
 |
Table 4 Carolinas Comfort Scale Scores |
Discussion
The study was initiated in 2011 and completed in 2016. Although the study design may now appear outdated, it was based on criteria and methods adopted in that period and as a result, demographics were reported as precisely as possible. Additionally, while the prospective 3-year follow-up was standard at the time of study design, newer studies now use 5-year follow-up to assess longer-term outcomes and late complications.
The hernia recurrence rate identified as the primary endpoint of this study appears to be aligned with other literature.3,4 However, although recurrence remains a main problem in abdominal wall repair, it is no longer the only aspect to be considered. Many other features have been demonstrated to be critical when a biological implant could be used, mainly indications and costs. In 2015, the Canadian Agency for Drug and Technologies in Health asked four questions to consider about this issue: 1) What is the clinical effectiveness of biological implants? 2) What is their cost-effectiveness? 3) What are the evidence-based guidelines regarding appropriate clinical indications? And 4) What are the evidence-based guidelines regarding their use?
These questions are still largely unresolved and there is insufficient high-quality evidence to suggest the optimal indications for the use of biological meshes in CAWR as confirmed in a recent review performed by Kockerling.8
In the present study, the majority of patients had a VHWG grade I and II (72/114) and this contrasts with current indications that restrict the use of biologics to VHWG grade III. It is well known that the VHWG grade represents an independent risk factor for recurrence and complications. In fact, according to Slater, the number of comorbidities seems to be strictly related to postoperative adverse events.9 As a result, the best results should be achieved with a low-grade VHWG. In the present study, however, it was observed that recurrence rates reported by sites using a biologic surgical implant in grade I and II cases were higher than expected, while those sites utilizing the implant in grade III cases were lower than expected (Table 5). This higher recurrence rate in grade I and II can be explained by the inappropriate techniques used, including fixation and limited mesh overlap. This seems to confirm that the implant of a biologic surgical implant in potentially contaminated fields is a correct indication which can lead to satisfying results. In grade IV, the use of any prosthetic material is contraindicated and a down-staging to grade III, according to accepted medical practice, eventually using negative pressure wound therapy (NPWT) is advisable. In grade IV cases, a biologic surgical implant cross-linked implant presents a high risk of infection that may lead to weakening or breakdown and the possibility of the mesh to be removed, according to data from literature.10 In the same situation, non-cross-linked implants do not need to be removed because they are destroyed by infection leading to subsequent high risk of hernia recurrence.
 |
Table 5 Hernia Recurrences by VHWG Wound Grade |
The high number of sites participating in the study reflects the relatively low number of subjects enrolled by each individual site (range 2–27). Moreover, numerous surgeons with different degrees of skill and experience in abdominal wall repair took part in the study, so that a suitable number of cases could be reached. Indications, operative techniques, and mesh implant sites varied across clinical sites and likely impacted patient outcomes, especially concerning operative technique and mesh site of implant, that play a primary role in the outcome of the patient. A comment by Negro et al regarding the publication by Abdelfatah et al, underlined the key role of correct indications and, overall, on a proper surgical technique for successful CAWR with a biologic surgical implant, after Abdelfatah’s series reported a recurrence rate of 13% versus 66% with similar incidence of surgical site infection (SSI).10,11
With regards to the surgical procedures, the technique of placement of the implant seems to be correct in 94.8% of cases, with 78.1% of intraperitoneal “bridge” and 16.7% of retromuscular sublay placement. Although closing the defect where possible is highly recommended, in cases when closure is not achievable, it is correct, according to Italian Guidelines and the Italian Biological Prosthesis Working Group, to use a cross-linked implant in a “bridge” position while a non-cross-linked implant would likely assure failure.12 Alternatively, in 5.2% of the cases, an incorrect technique with onlay or inlay positioning is reported. Both these techniques, in fact, had been progressively dismissed due to the high recurrence rate and the high risk of SSO and SSI, confirmed by our personal experience also. In the site with the highest recurrence rate, many different techniques were performed, emphasizing how an unstandardized procedure leads to disappointing results; however, these results may more accurately mimic what health care providers see in real-world cases.
Regarding mesh fixation and size of implant, 46.6% of surgeons used non-resorbable fixations (43.9%, non-resorbable sutures, 0.9% non-resorbable tacks, 1.8% glue) while 51.8% utilized unsuitable fixation methods, as slowly resorbable sutures (PDS, Maxon), resorbable tacks and glue, that reabsorb before the remodeling of the cross-linked implants has been completed. Additionally, in six patients, two implants were sutured together. This increases the risk of recurrence but underlines the need of larger prostheses. Of note, in 19.3% of the cases, the biological implant measured only 150 cm2. Given the relatively small size of these meshes, it should be meaningful to understand if the extent of the mesh was suitable to properly cover the defect. Taking into account hernia recurrence and follow-up, the overall trend is aligned with previous data from literature.12
Results from the present study are limited by the relatively low number of patients enrolled by each site, the variety of indications for the use of biological implants, and the lack of standardization of operative techniques across sites. Moreover, although the 3-year follow-up could be considered adequate at the time the study was initiated, an extension to at least 5 years would have been preferable.
Conclusion
The hernia recurrence rate of 22.4% found in this 3-year follow-up study is similar to that found in current literature and suggests that the use of a biologic surgical implant can provide long-term benefit to CAWR in patients staged grade III according to VHWG.
Acknowledgments
This study was sponsored by Medtronic. Nicholas Paquette PhD (Medtronic) provided medical writing assistance under the authors’ direction and based on content and conclusions developed by the authors. The patient datasets generated and analyzed during the current study are not publicly available due to restrictions that apply to the availability of these data. Data are however available from F. Gossetti ([email protected]) upon reasonable request and with permission of Medtronic.
Disclosure
Mr Pasquale Giordano reports personal fees from Medtronic, outside the submitted work. Professor Marco Montorsi reports personal fees from Baxter, outside the submitted work. Dr Ben Griffiths reports non-financial support and personal fees from Medtronic. The authors report no other conflicts of interest in this work.
References
1. Davison SP, Parikh PM, Jacobson JM, Iorio ML, Kalan M. A “buttressed mesh” technique for fascial closure in complex abdominal wall reconstruction. Ann Plast Surg. 2009;62(3):284–289. PubMed PMID: 19240526. doi:10.1097/SAP.0b013e31817e9c6d
2. Baillie DR, Stawicki P, Eustance N, Warsaw D, Desai D. Use of human and porcine dermal-derived bioprostheses in complex abdominal wall reconstructions: a literature review and case. Ostomy Wound Manag. 2007;53(5):30–37.
3. Mathes SJ, Steinwald PM, Foster RD, Hoffman WY, Anthony JP. Complex abdominal wall reconstruction: a comparison of flap and mesh closure. Ann Surg. 2000;232(4):586–596. PubMed PMID: 10998657; PubMed Central PMCID: PMCPMC1421191. doi:10.1097/00000658-200010000-00014
4. Rodriguez ED, Bluebond-Langner R, Silverman RP, et al. Abdominal wall reconstruction following severe loss of domain: the R Adams Cowley Shock Trauma Center algorithm. Plast Reconstr Surg. 2007;120(3):669–680. PubMed PMID: 17700118. doi:10.1097/01.prs.0000270303.44219.76
5. Butler CE, Langstein HN, Kronowitz SJ. Pelvic, abdominal, and chest wall reconstruction with AlloDerm in patients at increased risk for mesh-related complications. Plast Reconstr Surg. 2005;116(5):
6. Catena F, Ansaloni L, Gazzotti F, et al. Use of porcine dermal collagen graft (Permacol) for hernia repair in contaminated fields. Hernia. 2007;11(1):57–60. doi:10.1007/s10029-006-0171-6
7. Franklin ME, Gonzalez JJ, Glass JL. Use of porcine small intestinal submucosa as a prosthetic device for laparoscopic repair of hernias in contaminated fields: 2-year follow-up. Hernia. 2004;8(3):186–189. doi:10.1007/s10029-004-0208-7
8. Köckerling F, Alam NN, Antoniou SA, et al. What is the evidence for the use of biologic or biosynthetic meshes in abdominal wall reconstruction? Hernia. 2018;22(2):249–269. doi:10.1007/s10029-018-1735-y
9. Slater NJ, Montgomery A, Berrevoet F, et al. Criteria for definition of a complex abdominal wall hernia. Hernia. 2014;18(1):7–17. PubMed PMID: 24150721. doi:10.1007/s10029-013-1168-6
10. Abdelfatah MM, Rostambeigi N, Podgaetz E, Sarr MG. Long-term outcomes (>5-year follow-up) with porcine acellular dermal matrix (Permacol) in incisional hernias at risk for infection. Hernia. 2015;19(1):135–140. PubMed PMID: 24129420. doi:10.1007/s10029-013-1165-9
11. Negro P, D’Amore L, Ceci F, Gossetti F. Comment to “Long-term outcomes (>5 year follow-up) with porcine acellular dermal matrix (Permacol) in incisional hernias at risk for infection” by Abdelfatah MM, Rostambeigi N, Podgaetz E, Sarr MG (DOI 10.1007/s10029-013-1165-9). Hernia. 2015;19(6):1023–1024. PubMed PMID: 26169535. doi:10.1007/s10029-015-1406-1
12. Flum DR, Horvath K, Koepsell T. Have outcomes of incisional hernia repair improved with time? A population-based analysis. Ann Surg. 2003;237(1):129–135. PubMed PMID: 12496540. doi:10.1097/00000658-200301000-00018
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
