Back to Journals » Clinical Ophthalmology » Volume 13
360° ab-interno Schlemm’s canal viscodilation in primary open-angle glaucoma
Authors Ondrejka S, Körber N
Received 2 February 2019
Accepted for publication 9 May 2019
Published 15 July 2019 Volume 2019:13 Pages 1235—1246
DOI https://doi.org/10.2147/OPTH.S203917
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Simon Ondrejka,1 Norbert Körber1,2
1Augencentrum Köln, Köln, Germany; 2Eye Clinic, University Eye Hospital, Padova, Italy
Purpose: To evaluate the safety and effectiveness of ab-interno microcatheterization and 360° viscodilation of Schlemm’s canal (SC) using the VISCO360®, Viscosurgical System in treatment of primary open angle glaucoma (POAG).
Setting: Surgical center (Augencentrum Köln, Köln, Germany).
Design: Retrospective analysis of 106 eyes from 71 consecutive patients.
Methods: Ab-interno canal viscodilation (VISCO360®,) with or without cataract extraction was performed in two groups of patients with mild-moderate POAG: Group 1 had a baseline intraocular pressure (IOP) ≥18 mmHg (n=72 eyes) and Group 2 had a baseline IOP <18 mmHg (n=34 eyes). IOP without washout was measured and number of IOP-lowering medications were documented at all visits. Effectiveness was determined by reduction in IOP and reduction in the number of IOP-lowering medications at 12±3 months from baseline. Safety was determined by the rate of adverse events (AEs) and secondary surgical interventions (SSI).
Results: In Group 1, all eyes available at 12±3 months (n=72), had a 41.0% reduction in mean IOP (from 24.6±7.1 mmHg to 14.6±2.8 mmHg), 87% (n=62) of which showed an IOP reduction of ≥20% with no increase in IOP-lowering medications. In Group 2, all eyes (n=34) maintained their baseline IOP at all postoperative visits. In both groups, a significant decrease (>89%) in mean number of IOP-lowering medications was seen at 12 months with 86% of eyes completely off medication with no increase in IOP. The most common AE seen was hyphema (13%) and no eye required SSI during the study period.
Conclusion: Ab-interno SC viscodilation (VISCO360) is safe and effective in lowering IOP and reducing hypotensive medications in patients with OAG.
Keywords: 360-degree trabeculotomy, blebless, MIGS, VISCO360,® POAG, canaloplasty
Introduction
Glaucoma is the leading cause of irreversible blindness and is estimated to affect 79.6 million people worldwide by 2020, 74% of whom will have open angle glaucoma (OAG).1 The traditional treatment algorithm is directed at lowering intraocular pressure (IOP) either medically with ocular hypotensive eye drops or, failing that, through traditional glaucoma filtration surgery. Both of these approaches have associated short-comings; non-compliance with medical regimen and well documented and common postoperative complications for filtration surgery.
There has been a gap in the treatment algorithm for glaucoma between medical therapy and laser on the one hand, and traditional filtration surgery (with the inherent risks mentioned previously) on the other. The last decade has seen the advent and rise of minimally (or micro-) invasive glaucoma surgery (MIGS) providing several different options for meeting this treatment need.2 MIGS procedures and devices share several common features; an ab-interno incisional approach, minimal trauma and disruption to normal anatomy and physiology, a high level of biocompatibility (implants), demonstrable IOP-lowering efficacy, and an extremely high safety profile with rapid post-surgical recovery.3 The patient profile suitable for a MIGS procedure is broad and depends largely on the goals desired. A patient with mild-moderate glaucoma that is above target IOP on maximum tolerated medications may benefit by reaching the target with fewer medications while a more advanced patient who is well controlled may benefit from medication reduction.4 Canaloplasty is one such procedure that is intended to restore the natural aqueous outflow system through microcatheterization and viscodilation of the entire length of the Schlemm’s canal (SC). Canaloplasty offers a comprehensive approach to the natural outflow system and addresses multiple potential sites of resistance by improving the function of the trabecular meshwork (TM), dilating SC, and opening the distal collector channels.5,6 It has a well documented safety and efficacy profile in reducing IOP.7–10 Traditional canaloplasty is performed using an ab-externo approach which is invasive and requires full thickness scleral incisions.11 Advances in medical device technology now allow canaloplasty to be performed ab-interno, allowing for preservation of the conjunctiva.
VISCO360® (VISCO360 Viscosurgical System, Sight Sciences, Inc. Menlo Park, CA, USA) is a single-handed, non-implantable device that facilitates automatic delivery of a predetermined amount of viscoelastic fluid to dilate 360° of SC (Figure 1). The VISCO360 device was CE marked in 2015. In this study we evaluated the safety and effectiveness of ab-interno microcatheterization and viscodilation of SC in lowering IOP and reducing the use of IOP-lowering medications in patients with mild-moderate primary OAG (POAG).
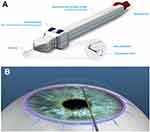 |
Figure 1 VISCO360® Viscosurgical System. (A) Illustration of the device showing a handpiece with a microcatheter, control wheel for advancing and retracting the microcatheter, viscoelastic reservoir/infusion pump, and a locking mechanism. (B) Schematic of circumferential viscodilation of Schlemm’s canal (SC) through clear corneal incision. VISCO360® cannula tip creates an opening into SC. A controlled volume of viscoelastic fluid is delivered as the microcatheter is retracted back. VISCO360 Viscosurgical System. Note: Images are sourced from Sight Sciences, http://sightsciences.com and used with permission. |
Materials and methods
Study design
This single center, consecutive, open-label, retrospective surgical case series evaluated the safety and effectiveness of ab-interno canal viscodilation (VISCO360) in mild-moderate POAG. The study was conducted at Eye Center Cologne in Cologne, Germany between December 2015 and May 2018.
Best corrected visual acuity (BCVA) was assessed at each visit using Snellen charts. IOP (applanation tonometry; without medication washout) was measured, IOP-lowering medications were documented, slit-lamp examination, fundus evaluation including cup to disc ratio (dilated exam at baseline, Month 6 and Month 12), and gonioscopic assessment (all four quadrants) were performed in all patients preoperatively and at all postoperative visits. IOP-lowering medications were continued until the day of surgery and were then discontinued. Patients on anti-coagulation medications were not required to discontinue these medications. Follow-up visits were performed at Day 1, Month 1, Month 3, and then every 3 months thereafter. IOP, IOP-lowering medications, adverse events (AEs), and reinterventions were reported at each follow-up visit. All the patients were seen at 1 week for a safety check (not included in endpoint analysis). Endpoint analysis was performed at 12 months. Results for the 12-month timepoints include data collected at 12 months ±3 months.
This study was performed in accordance with the tenets of the Declaration of Helsinki. The study is considered non-interventional in that it is a retrospective review of patient data accumulated in the normal conduct of standard medical practice; no prospective treatment assignments were made, and all assessments were routine standard of care. The Landesärztekammer Düsseldorf Ethics Committee reviewed the study protocol and determined that approval was not required and waived informed consent in consideration of the non-interventional design. Patient data were deidentified prior to analysis and no patient-level data are reported herein.
Patients
This study explored the effectiveness of canal viscodilation in a clinical practice setting, therefore inclusion and exclusion criteria were selected to model real world experience rather than to narrowly define the population in an artificial way. Key inclusion criteria were adult patients (18 years of age or older) with mild-moderate POAG who had undergone ab-interno canal viscodilation with the VISCO 360 system and had between 9 and 15 months of follow-up. Included patients were either insufficiently controlled on their current medical regimen (Group 1) or, if adequately controlled, in need of reduced medication burden (Group 2). Patients could have undergone canal viscodilation as either a standalone procedure (pseudophakic at the time of surgery) or combined with phacoemulsification cataract extraction (CE). Key exclusion criteria included diagnosis of glaucoma other than POAG, prior glaucoma surgery, terminal stage of OAG (defined as grade 4 in the Aulhorn classification) or central retinal vein occlusion.
Following ab-interno canal viscodilation, the eyes with high baseline IOP (≥18 mmHg) were expected to show both a reduction in IOP and a reduction in IOP-lowering medications, while the eyes with lower baseline IOP (<18 mmHg) were expected to maintain a low baseline IOP and show a reduction in the number of IOP-lowering medications. Effectiveness was assessed in two cohorts based on the baseline IOP; Group 1 with baseline IOP ≥18 mmHg and Group 2 with baseline IOP <18 mmHg.
The study procedure
Ab-interno 360° microcatheterization and viscodilation of SC using the VISCO360 Viscosurgical System
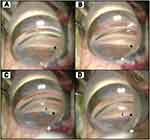
All eyes enrolled in the study received 360° microcatheterization and viscodilation of SC using the VISCO360 Viscosurgical System (Figure 1). For ab-interno canal viscodilation performed in conjunction with CE (94 eyes), the device was introduced into the anterior chamber by way of a single, self-sealing, 2.4 mm clear corneal incision made for CE. In these 94 eyes, ab-interno canal viscodilation was performed after CE was successfully completed. For the eyes that received stand-alone viscodilation treatment (12 eyes), a single, self-sealing, 1.8 mm clear corneal incision was made for introducing the cannula into the anterior chamber. The VISCO360 is designed to advance the microcatheter in two segments of 180° to access the entire circumference of SC. After the cannula was positioned at the desired location (Figure 2A) and an opening into SC was created with the cannula tip (Figure 2B), the finger wheel was used to gently advance the microcatheter out of the cannula and into SC (Figure 2C). The microcatheter was advanced 180° around SC. As the microcatheter was retracted back out of SC and into the cannula, a controlled volume of Healon GV was delivered, thereby dilating SC along its entire length (Figure 2D). This process was then repeated on the other 180° of SC to achieve a complete 360° circumferential canal viscodilation followed by irrigation and aspiration of the anterior chamber.
Post-surgical management
Postoperatively, patients received fixed combination topical tobramycin/dexamethasone (DEXAGENT) five times daily for 1 week followed by dexamethasone drops (DEXAPOS) four times daily the second week, then tapered. All patients were seen at 1 week for a safety check. Follow-up visits were at Day 1, Month 1, Month 3, and then every 3 months thereafter, for evaluation of BCVA, IOP, medications, fundus, the anterior chamber, and the angle.
Outcome measures
Effectiveness was assessed at 12±3 months as compared to baseline. The primary effectiveness outcome measure for Group 1 was change in mean IOP and mean number of IOP-lowering medications at 12 months. The secondary effectiveness measure was proportion of eyes with IOP reduction ≥20% relative to baseline at 12 months without any increase in number of IOP-lowering medications. The primary effectiveness outcome for Group 2 was change in the mean number of IOP-lowering medications. The proportion of eyes achieving medication independence without increase in IOP was also computed for both the groups.
The primary safety outcome was the frequency of any ocular AEs and secondary surgical interventions.
Statistical analysis
Descriptive statistics were used to summarize the data. Ninety-five percent (95%) CIs of the percent per binomial distribution were calculated for change over time in categorical values. Ninety-five percent (95%) CIs of the mean per t-distribution were calculated for change over time in numeric values. A paired t-test along with post hoc Tukey adjustment to account for multiplicity was done to compare IOP and the number of IOP-lowering medications with baseline. P-values <0.05 were considered statistically significant. Effectiveness outcomes were sub-grouped by baseline IOP, with results presented separately for both study groups. Statistical analyses were performed with SAS v9.3 (SAS Institute, Inc., Cary, NC USA).
Results
Patient demographics
A total of 106 eyes from 71 (45 females and 26 males) patients that met inclusion criteria were treated with ab-interno canal viscodilation with a mean age of 75.0±7.7 years (range 54–91 years). All patients had mild-moderate POAG. The majority of eyes (94) were phakic and 12 eyes were pseudophakic. Group 1 had 72 eyes with baseline IOP ≥18 mmHg and Group 2 had 34 eyes with baseline IOP <18 mmHg. Twelve eyes (eleven from Group 1 and one from Group 2) received standalone ab-interno canal viscodilation (VISCO360-alone) and 94 eyes (61 from Group 1 and 33 from Group 2) received ab-interno canal viscodilation in conjunction with CE (VISCO360+CE). Patients’ baseline demographics, study eye, and treatment administered are presented in Table 1.
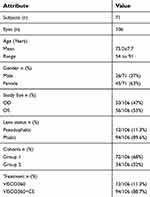 |
Table 1 Patient demographics at baseline |
Across both groups, 105 eyes were available for analysis at 12±3 months. Missing follow-up exam from one eye at 12 months was due to death of the patient.
Group 1 effectiveness outcomes
A total of 72 eyes with baseline IOP ≥18 mmHg were in this group, eleven of which were pseudophakic and received VISCO360-alone and 61 of which were phakic and received VISCO360+CE.
IOP
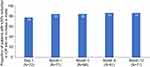
Eyes in Group 1 had a statistically significant reduction in mean IOP at all postoperative visits as compared to baseline (P<0.001), with no significant difference among any postoperative visits (P>0.05). Mean baseline IOP of 24.6±7.1 mmHg was reduced to 14.6±2.8 mmHg at 12 months. Average reduction in IOP at 12 months was 41.0% across all eyes (n=71) (Figure 3). A reduction in IOP ≥20% with the same or fewer medications compared to baseline, was achieved in 87% eyes (CI: 75%–93%) at 12 months (Figure 4).
IOP-lowering medications
Patients were on a mean of 2.1±1.0 IOP-lowering medications at baseline. Postoperatively there was a statistically significant reduction in the mean number of IOP-lowering medications compared to baseline (P<0.001), with no significant differences among any postoperative visits (P>0.05). At 12 months, patients were on a mean of 0.2±0.6 IOP-lowering medications (mean reduction of 1.9±1.1 [CI: −2.0, −1.8]) (Figure 5).
Of the eyes available for analysis at 12 months, 97% (CI: 85–100%) were on either the same or fewer IOP-lowering medications compared to preoperative baseline and 85% eyes (61 out of 72) required no medication to maintain IOP at or below their preoperative baseline IOP (Figure 6).
The small sample size of the VISCO360-alone subgroup (eleven eyes) does not allow valid statistical comparison with the VISCO360+CE subgroup (n=59). Notably, eyes that underwent the VISCO360-alone procedure tended to have a higher baseline IOP (28.8±6.4 vs 23.8±6.9 mmHg) and were using more IOP-lowering medications at baseline (3.0±1.3 vs 1.9±0.8) than eyes that received the VISCO360+CE procedure. This suggests that the VISCO360-alone subgroup was more severely affected at baseline and thus more challenging to treat than the VISCO360+CE group. Nonetheless, the VISCO360-alone subgroup had significant reductions in both IOP and the use of IOP-lowering medications at Day 1 through 12 months (P<0.001) (Table 2).
 |
Table 2 Effectiveness outcomes in VISCO360-alone subgroup (n=11) |
Group 2 effectiveness outcomes
A total of 34 eyes (baseline IOP <18 mmHg) were in this group, 33 of which were treated in conjunction with CE and one eye received VISCO360-alone treatment.
IOP
Eyes in this group had a mean baseline IOP of 14.9±1.8 mmHg. IOP was stable in these eyes over time, with a mean IOP of 13.6±2.3 mmHg at 12 months with no significant difference compared to baseline (P>0.05) (Figure 7).
IOP-lowering medications
Patients in Group 2 were on a mean of 1.8±0.9 IOP-lowering medications at baseline. Postoperatively there was a statistically significant reduction in the mean number of IOP-lowering medications compared to baseline (P<0.001), with no significant difference among any postoperative visits (P>0.05). At 12 months, patients were on a mean of 0.2±0.6 IOP-lowering medications (mean reduction of 1.6±0.9 [CI: −1.6, −0.9]) (Figure 8).
All the eyes available for analysis, at 12 months, had a reduction in number of IOP-lowering medications compared to baseline. In 88% (30 out of 34 eyes) all IOP-lowering medications were discontinued at 12 months with no increase in IOP as compared to baseline (Figure 9).
Safety outcomes (both groups)
In the 106 eyes treated, there were 14 cases of postoperative hyphema (13%), the majority (11) of which were <1 mm and three of which were between 1–3 mm. All cases of hyphema resolved within seven days without the need for additional intervention. No patients required postoperative paracentesis. One eye (0.9%) was reported to have fibrin in the pupillary plane after VISCO360+CE procedure which resolved in one day.
Surgical interventions unrelated to ab-interno canal viscodilation were required in two patients: one patient underwent vitrectomy for epiretinal gliosis nine months postoperatively and one received anti-VEGF treatment for myopic choroidal neovascularization.
There was one intraoperative event in which a portion of the VISCO360 catheter had detached from the handle and cannula and had to be retrieved with vitrectomy forceps. The patient suffered no adverse sequelae from this event. There was no loss in BCVA at any postoperative visit compared to baseline in either group. The incidence of ocular AEs is presented in Table 3.
 |
Table 3 Ocular adverse events |
Discussion
Ab-interno microcatheterization and 360° viscodilation of SC, with or without CE, was effective in lowering IOP and medication use in eyes with an elevated, medicated baseline IOP ≥18 mmHg (Group 1) up to 12 months. In eyes with a medicated baseline IOP <18 mmHg (Group 2), ab-interno microcatheterization and 360° viscodilation of SC in conjunction with CE, was effective in maintaining the baseline IOP while decreasing the need for medications for up to 12 months. These results are consistent with published data demonstrating successful outcomes for other canaloplasty procedures including both ab-interno and ab-externo approaches.12–14 In a large retrospective cohort study of 602 eyes treated ab-externo with the ABiC/iTrack system (Ellex Medical), over 80% of eyes undergoing canaloplasty combined with cataract surgery were judged “complete successes” (achieved target IOP at 12 months on zero medications) while approximately 70% of eyes met this criteria when canaloplasty was performed as a standalone procedure.
The AEs that were observed in this case series are common for MIGS.15 The rate of elevated IOP after 1 month (≥10 mmHg than preoperative baseline) was 1.0%. In this study the frequency and severity of these events are comparable to or lower than AEs reported in metanalysis of other MIGS studies.16 While several cases of hyphema were observed in this study, the majority were small (<1 mm) and all resolved within 1 week without the need for additional intervention. There was no loss in BCVA at any postoperative visit compared to baseline.
The IOP reduction and reduction in medication usage observed for Group 1 (baseline IOP ≥18 mmHg) was similar to a comparable group treated with the more invasive ab-externo canaloplasty procedure using the iTrack microcatheter (Table 4).11 In the iTrack pivotal study, ab-externo canaloplasty was performed either as a standalone procedure or in conjunction with CE in mild-moderate POAG with baseline IOP ≥16 mmHg on maximal medications. Results from the iStent (gen 1) study are also provided for reference (Table 4).17 Gallardo et al compared results for ab-interno and ab-externo canaloplasty in a small paired-eye study in which each patient received treatment with both methodologies. They found no significant difference between the two groups at 12 months in either IOP reduction or medication usage.13
 |
Table 4 Comparison of VISCO360 results for Group 1 (baseline-IOP ≥18 mmHg) to iTrack11 and iStent17 studies |
Most of the eyes (90.5%, 94 of 105) included in this study underwent ab-interno canal viscodilation in conjunction with CE. Cataract is the most common comorbidity present in patients with glaucoma and accounts for 33% of visual disability worldwide.18 When a patient with glaucoma also requires surgical intervention for cataract(s), ophthalmologists often perform simultaneous cataract and glaucoma surgery. Cataract extraction is known to enhance the effect of glaucoma interventions on IOP and reduce the need for IOP-lowering medications.19–21 Interestingly, in our small cohort of patients that underwent a standalone canaloplasty, the average percent reduction in IOP and the average change from baseline in IOP at 12 months was greater than for the CE combined patients (50% reduction, −14.3 mmHg standalone; 39% reduction, -9.2 mmHg combined with CE). In contrast, the results of a large study utilizing the ab-externo approach that compared 262 standalone cases and 322 combined, showed overall better effectiveness when combined with phaco.12 The current results should be viewed considering the small number of patients in the standalone cohort and also noting that these patients required greater average adjunctive medication at 12 months than the combined group. A review of recent literature reveals that cataract surgery in glaucoma patients (not involving washout of IOP-lowering medications) was associated with a reduction in IOP ranging between 0.6–2.5 mmHg at 1 year.22–24
The 360° ab-interno canal viscodilation efficiently meets all the cardinal features of MIGS3 it is a safe, ab-interno, micro-incisional approach to lowering IOP and medication use with minimal anatomical and physiological disruption. In this case series, ab-interno canal viscodilation demonstrated reliable IOP lowering and medication reduction with a good safety profile. There was a rapid postoperative patient recovery and minimal need for postoperative medications. Also, this study demonstrates that ab-interno canal viscodilation can safely and effectively be performed in combination with CE or as a standalone procedure for patients with POAG.
Elevation of IOP in OAG is thought to be attributable to the increased outflow resistance due to pressure-dependent structural changes to the TM and collapse of SC.25,26 A decrease in effective filtration area for aqueous humor and changes to the tissue architecture (collapse of SC and TM herniations in collector channels) are believed to contribute to increase in outflow resistance.27,28 The ab-interno microcatheterization and viscodilation of SC (VISCO360) is designed to take a comprehensive approach to treat all sites of outflow resistance by a) dilating and breaking microadhesions in SC, b) improving the function of the TM, c) opening the distal collector channels, and d) treating all 360° of SC thus restoring physiological aqueous outflow throughout the entire trabeculocanalicular outflow pathway.2 Compared to invasive ab-externo canaloplasty, the minimally invasive ab-interno approach with VISCO360 offers ease in accessing, catheterizing, and viscodilating SC through a single clear corneal incision. The ab-interno approach does not require any conjunctival or scleral dissection and spares the risk of unintentional subconjunctival bleb formation during postoperative recovery as seen in ab-externo canaloplasty.11
Study limitation
This was a single center, retrospective case series with a relatively small sample size. Both eyes from a patient were included in the analysis in instances where both eyes met eligibility criteria, therefore not all data points are independent, statistically speaking. This might result in tighter CIs than for a sample including only one eye per patient and should therefore be considered when interpreting the results. The standalone (VISCO360-alone) subgroup was too small to allow a valid statistical comparison with VISCO360+CE and limited presentation of standalone results to descriptive statistics. Eligibility criteria were purposely broad. While this introduces additional variability, it also more closely replicates “real-world” performance. A control or a comparative arm would have increased the reliability of the results of this study by eliminating alternate explanations of effectiveness of ab-interno microcatheterization and viscodilation of SC in lowering IOP and need for medications. Follow-up in this study was limited to between 9 and 15 months and long-term effectiveness beyond approximately 1 year was not demonstrated, however the mean IOP reduction was stable between Month 3 and Month 12 without the need for IOP-lowering medications for most patients. Despite these limitations, we believe that this study provides important new insights into novel, less invasive canal reconstruction approaches to treat POAG.
Conclusion
This is the first published report of the safety and IOP-lowering effectiveness of ab-interno 360° canaloplasty using the VISCO360 surgical system. Analysis of this single center case series demonstrates that microcatheterization and viscodilation of SC with the VISCO360 Viscosurgical System with or without CE can be safely and consistently performed in patients with mild-moderate POAG. Significant improvements in IOP and reductions in the need for IOP-lowering medications observed through 12 months were similar to other established MIGS procedures. In addition, eyes treated with the VISCO360 Viscosurgical System experienced few AEs, none of which were serious device-related events, and the need for secondary surgical intervention was rare.
Acknowledgments
We are grateful to Julie Crider, PhD (Collaborative Medical Writing, LLC) for medical writing services that included discussing the context, background, and importance of the work with the authors and preparing a draft manuscript based on this discussion. The original draft has been revised extensively. We thank Dr Barbara A Smit, MD, PhD for her editorial assistance, and Kavita Dhamdhere, MD PhD and Jaime E Dickerson, PhD for assistance in revising the manuscript. The authors appreciate help provided by Yi-Jing Duh, PhD (StatServ Consulting Inc) with statistical analysis. The manufacturer of the VISCO360 device, Sight Sciences, provided financial assistance to support writing, editorial, and statistical analysis. This work was otherwise self-funded.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors have no commercial or proprietary interest in any of the materials discussed in this article. The authors report no conflicts of interest in this work.
References
1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi:10.1136/bjo.2005.081224
2. Grieshaber MC, Pienaar A, Olivier J, Stegmann R. Canaloplasty for primary open-angle glaucoma: long-term outcome. Br J Ophthalmol. 2010;94(11):1478–1482. doi:10.1136/bjo.2009.163170
3. Saheb H, Ahmed II. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23(2):96–104. doi:10.1097/ICU.0b013e32834ff1e7
4. Ahmed K
5. Stegmann R. Visco-canalostomy: a new surgical technique for open angle glaucoma. Ann de Istituto Barraquer. 1995;25:229–232.
6. Stegmann R, Pienaar A, Grieshaber MC. Schlemm’s canal surgery: restoring physiological aqueous outflow. In: Grieshaber MC, Orgul S, Flammer J, editors. Glaucoma Therapy—State of the Art. Basel, Switzerland: Association for Continuing Education in Ophthalmology; 2009:113–120.
7. Lewis RA, von Wolff K, Tetz M, et al. Canaloplasty: three-year results of circumferential viscodilation and tensioning of Schlemm’s canal using a microcatheter to treat open-angle glaucoma. J Cataract Refract Surg. 2011;37(4):682–690. doi:10.1016/j.jcrs.2010.10.055
8. Brüggemann A, Despouy JT, Wegent A, Müller M. Intraindividual comparison of canaloplasty versus trabeculectomy with mitomycin C in a single-surgeon series. J Glaucoma. 2013;22(7):577–583. doi:10.1097/IJG.0b013e318255bb30
9. Klink T, Sauer J, Körber NJ, et al. Quality of life following glaucoma surgery: canaloplasty versus trabeculectomy. Clin Ophthalmol. 2014;18(9):7–16. doi:10.2147/OPTH.S72357
10. Bull H, von Wolff K, Körber NJ, Tetz M. Three-year canaloplasty outcomes for the treatment of open-angle glaucoma: European study results. Graefes Arch Clin Exp Ophthalmol. 2011;249(10):1537–1545. doi:10.1007/s00417-011-1728-3
11. Lewis RA, von Wolff K, Tetz M, et al. Canaloplasty: circumferential viscodilation and tensioning of Schlemm’s canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults: interim clinical study analysis. J Cataract Refract Surg. 2007;33(7):1217–1226. doi:10.1016/j.jcrs.2007.03.051
12. Vastardis I, Fili S, Gatzioufis Z, Kohlhaas M. Ab externo canaloplasty results and efficacy: a retrospective cohort study with a 12-month follow-up. Eye Vis (Lond). 2019;6(1):9. doi:10.1186/s40662-019-0134-5
13. Gallardo MJ, Supnet RA, Ahmed IIK. Circumferential viscodilation of Schlemm’s canal for open-angle glaucoma: ab-interno vs ab externo canaloplasty with tensioning suture. Clin Ophthalmol. 2018;12:2493–2498. doi:10.2147/OPTH.S178962
14. Körber N. Ab interno canaloplasty for the treatment of glaucoma: a case series study. Specktrum Augenheilkd. 2018;32:223–227. doi:10.1007/s00717-018-0416-7
15. Yook E, Vinod K, Panarelli JF. Complications of micro-invasive glaucoma surgery. Curr Opin Ophthalmol. 2018;29(2):147–154. doi:10.1097/ICU.0000000000000457
16. Lavia C, Dallorto L, Maule M, Ceccarelli M, Fea AM, Virgili G. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta-analysis. PLoS One. 2017;12(8):e0183142. doi:10.1371/journal.pone.0183142
17. Craven ER, Katz J, Wells J, Giamporcaro JE, iStent Study Group. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012;38(8):1339–1345. doi:10.1016/j.jcrs.2012.03.025
18. Bourne RR, Stevens GA, White RA, et al. Causes of vision loss worldwide, 1990-2010: a systematic analysis. Lancet Glob Health. 2013;1(6):339–349. doi:10.1016/S2214-109X(13)70113-X
19. Samuelson TW, Chang DF, Marquis R, et al. A Schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: the HORIZON study. Ophthalmology. 2019;126(1):29–37. doi:10.1016/j.ophtha.2018.05.012
20. Zhang ML, Hirunyachote P, Jampel H. Combined surgery versus cataract surgery alone for eyes with cataract and glaucoma. Cochrane Database Syst Rev. 2015;7:CD008671.
21. Vold S, Ahmed II, Craven ER, et al. Two-year COMPASS trial results: supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataracts. Ophthalmology. 2016;123(10):2103–2112. doi:10.1016/j.ophtha.2016.06.032
22. Francis BA, Berke SJ, Dustin L, Noecker R. Endoscopic cyclophotocoagulation combined with phacoemulsification versus phacoemulsification alone in medically controlled glaucoma. J Cataract Refract Surg. 2014;40(8):1313–1321. doi:10.1016/j.jcrs.2014.06.021
23. Arthur SN, Cantor LB, WuDunn D, et al. Efficacy, safety, and survival rates of IOP-lowering effect of phacoemulsification alone or combined with canaloplasty in glaucoma patients. J Glaucoma. 2014;23(5):316–320. doi:10.1097/IJG.0b013e3182741ca9
24. Armstrong JJ, Wasiuta T, Kiatos E, Malvankar-Mehta M, Hutnik CML. The effects of phacoemulsification on intraocular pressure and topical medication use in patients with glaucoma: a systematic review and meta-analysis of 3-year data. J Glaucoma. 2017;26(6):511–522. doi:10.1097/IJG.0000000000000643
25. Moses RA. The effect of intraocular pressure on resistance to outflow. Surv Ophthalmol. 1977;22(2):88–100.
26. Brubaker RF. The effect of intraocular pressure on conventional outflow resistance in the enucleated human eye. Invest Ophthalmol. 1975;14(4):286–292.
27. Lu Z, Overby DR, Scott PA, Freddo TF, Gong H. The mechanism of increasing outflow facility by rho-kinase inhibition with Y-27632 in bovine eyes. Exp Eye Res. 2008;86(2):271–281. doi:10.1016/j.exer.2007.10.018
28. Battista SA, Lu Z, Hofmann S, Freddo T, Overby DR, Gong H. Reduction of the available area for aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes. Invest Ophthalmol Vis Sci. 2008;49(12):5346–5352. doi:10.1167/iovs.08-1707
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.