Back to Journals » Journal of Inflammation Research » Volume 11
2LARTH®, a micro-immunotherapy medicine, exerts anti-inflammatory effects in vitro and reduces TNF-α and IL-1β secretion
Authors Floris I , Appel K , Rose T, Lejeune B
Received 16 May 2018
Accepted for publication 4 August 2018
Published 29 October 2018 Volume 2018:11 Pages 397—405
DOI https://doi.org/10.2147/JIR.S174326
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Ning Quan
Ilaria Floris,1 Kurt Appel,2 Thorsten Rose,2 Beatrice Lejeune3
1Clinical Affairs, Labo’Life France, Moutiers-Sous-Chantemerle, France; 2VivaCell Biotechnology GmbH, Denzlingen, Germany; 3Clinical Affairs, Labo’Life Belgium, Gembloux, Belgium
Background: Tumor necrosis factor-α (TNF-α) and IL-1β are 2 pro-inflammatory cytokines known to be involved in rheumatic diseases. The therapeutic strategy used in micro-immunotherapy (MI) to reduce chronic inflammation and attenuate pain consists in mainly targeting these 2 cytokines. 2LARTH® is a sublingually administered medicine consisting of lactose-saccharose globules impregnated with ethanolic preparations of immune mediators and nucleic acids at ultra-low doses.
Purpose: The aim of the study is to explore the effect of the MI medicine on TNF-α and IL-1β secretion in human primary enriched monocytes exposed to lipopolysaccharide (LPS).
Materials and methods: Placebo and active globules were diluted in culture medium to test 5 lactose-saccharose globules concentrations (from 1.75 to 22 mM). Freshly isolated enriched monocytes from 6 healthy donors were treated with or without LPS (10 ng/mL), LPS+ placebo, or LPS+ 2LARTH® for 24 hours. IL-1β, TNF-α, and IL-6 release were evaluated by ELISA.
Results: The medicine has significantly decreased the level of IL-1β secretion compared with placebo at these concentrations: 22 mM (P<0.0001), 11 mM (P=0.0086), 5.5 mM (P= 0.0254), and compared with untreated LPS control at these concentrations: 22 mM, 11 mM (P=0.0008), and 5.5 mM (P=0.002). The effect of active globules on the reduction of TNF-α release is significant compared with placebo at these concentrations: 22 mM (P=0.0018), 11 mM (P=0.0005), 5.5 mM (P=0.0136), and compared with untreated LPS control at these concentrations: 22 mM (P=0.0021), 11 mM (P=0.0017), 5.5 mM (P=0.0052) and 2.25 mM (P=0.0196). Besides, IL-6 secretion decreased compared with placebo at 22 mM (P=0.0177) and 11 mM (P=0.0031).
Conclusion: The results indicate that the tested product exerts significant anti-inflammatory effects on human LPS-stimulated monocytes.
Keywords: ultra-low doses, hormesis, chronic inflammation, rheumatic diseases
Introduction
Micro-immunotherapy (MI) is an immunomodulation therapy that uses the same molecular messengers as the immune system, such as cytokines, hormones or growth factors, nucleic acids, and specific nucleic acids (SNAs®), to transmit information throughout the organism and modulate the immune response. SNAs® are single strands of DNA molecules, ranging from 16 to 34 bases, designed to target specifically 1 or more genes (European Patent: EP0670164B1). MI acts to potentiate the body’s defenses in acute and chronic diseases.1,2 MI medicines consist of lactose-saccharose globules for oromucosal administration, impregnated with ethanolic preparations of immune mediators and nucleic acids at ultra-low doses (ULD). ULD of active substances are obtained through the “Sequential Kinetic Process”, consisting of a 1/100 dilution process followed by vertical shaking, reproduced a defined number of times. Medicines composition is expressed as CH (Centesimal Hahnemannian dilutions), and it indicates the number of times by which the 2 proceedings are carried out for each active substance. This manufacturing process is in accordance with the European Pharmacopeia monographs 1,038 and 2,371, current edition.
The medicines are sequentially developed to transmit consecutive information to the body. Indeed, the composition of each capsule is specific, and the intake should follow the ascending order indicated on the blister.
In the context of rheumatic diseases (RDs), including arthritis, MI strategy aims at reducing the chronic inflammation acting mainly on 2 pro-inflammatory cytokines, tumor necrosis factor-α (TNF-α) and IL-1β, known to be involved in pain, stiffness, swelling, and reduced mobility, the major clinical signs of RD.3–5 Once activated, both proteins serve as potent pro-inflammatory cytokines at the local level, triggering vasodilatation and attracting monocytes and neutrophils to sites of tissue damage and stress. These cytokines are crucial for the induction of matrix enzymes and act as potent mediators of tissue damage, altering cartilage, and bone homeostasis. Furthermore, both cytokines trigger the production of “classical pain mediators”, such as prostaglandins, activating and/or sensitizing nociceptive neurons, through the induction of cyclooxygenase-2.4,5 Pharmaceutical agents, especially paracetamol and non-steroidal anti-inflammatory drugs, already play a key role in symptoms control;6 however, managing chronic pain still remains clinically challenging.7 In addition, long-term use of anti-inflammatory medicines is associated with several side effects.8,9 The association of 2 or more analgesic agents should be carefully evaluated because, although they can have synergistic effects and increase therapeutic efficacy, their combination may also imply more important side effects.
In MI, the active substances are ultra-diluted to avoid toxicity and side effects, therefore, MI medicines can be used alone or in association with other therapies to provide clinical benefits and synergistic effects.10
2LARTH® is an MI medicine developed to attenuate signs and symptoms of RD, as well as arthritis. The formula is thought to counteract the overexpression of TNF-α and IL-1β once the inflammatory cascade is activated and the chronic inflammatory status is established. The medicine is notified as a homeopathic medicine under notification number 1507CH36 F1 by the Federal Agency for Medicines and Health Products in Belgium.
To investigate the efficacy of the MI medicine, the authors have chosen the recognized scientific approach and an in vitro model of inflammation widely used for testing anti-inflammatory compounds and drugs. Such study wanted also to provide the basis of important understandings into the mode of action of ULD and MI medicines.
Materials and methods
Materials
The tested MI medicine is a sequential drug. Capsules in blister are numbered from 1 to 10 and the intake should respect a sequential order. The tested capsule contains lactose-saccharose globules impregnated with ULD of 3 human recombinant pro-inflammatory cytokines (IL-1β, TNF-α, and IL-2), and ULD of SNA® targeting genomic regions and transcripts of human leukocyte antigen (HLA) class I and II molecules and human IL-2. The composition of the tested capsule is as follows: IL-1β at 17 CH, TNF-α at 17 CH, and IL-2 at 10 CH, SNA-HLA-I at 10 CH, SNA-HLA-II at 10 CH, and SNA-ARTH at 10 CH. The placebo capsules, which were used as controls contain lactose-saccharose globules impregnated with the only ethanolic preparation, without any active substance.
Isolation of human peripheral enriched monocytes
The whole blood samples of 6 healthy volunteers were collected at the University Medical Center of Freiburg (Germany). Written informed consent was obtained from all donors. The study has been approved by the Ethics Committee of Albert Ludwigs University of Freiburg.
Monocytes were extracted following a standardized protocol using completely endotoxin-free cultivation.11,12 Using 50 mL tubes, 25 mL Ficoll was loaded with 25 mL of blood (buffy coats). The gradient was established by centrifugation at 1,800 rpm, 20°C for 40 minutes. with slow acceleration and deceleration. Peripheral blood mononuclear cells in the interphase were carefully removed and re-suspended in 50 mL pre-warmed PBS (Pan Biotech, Aidenbach, Germany), followed by centrifugation for 10 minutes at 1,600 rpm and 20°C. The supernatant was discarded and the pellet washed in 50 mL PBS and centrifuged as aforementioned. The pellet was then re-suspended in 50 mL Roswell Park Memorial Institute (RPMI)-1640 low-endotoxin medium supplemented with 10% human serum (Hexcell, Berlin, Germany) and cells incubated at 37°C with 5% CO2. The day after, medium and the non-adherent cells (mostly lymphocytes) were removed and fresh RPMI-1640 medium containing 1% human serum was added.
AlamarBlue assay
After counting the number of cells in a particle counter (Euro Diagnostics, Krefeld, Germany), human primary enriched monocytes from 1 donor were seeded in 96-well plates, at a density of 220,000 cells/well and incubated at 37°C with 5% CO2.
The globules of placebo or 2LARTH® were dissolved in cell culture media at different lactose-saccharose concentrations (from 2.75 to 22 mM). 1 µL of the stock solution or the dilutions were added per well (100 µL). Cells were incubated with sodium fluoride, NaF (250 µg/mL), to induce cell death and have a positive control, or only with complete media. The experiment was run in quadruplicates.
After 24 hours of incubation, 10 µL AlamarBlue® (Thermo Fisher Scientific, Waltham, MA, USA) was added to each well. After 2 hours, the fluorescence was measured with a fluorescence spectrophotometer, using 544EX nm/590EM nm filter settings. The amount of fluorescence is proportional to the number of living cells and corresponds to the cells metabolic activity. Damaged and nonviable cells have lower innate metabolic activity and thus generate a proportionally lower signal than healthy cells.
The active ingredient of AlamarBlue® (resazurin) is a nontoxic, cell permeable compound that is blue in color and virtually nonfluorescent. Upon entering cells, resazurin is reduced to resorufin, which produces very bright red fluorescence. Viable cells continuously convert resazurin to resorufin, thereby generating a quantitative measure of viability and cytotoxicity.
Determination of inflammatory parameters after treatment
Cells were seeded in 24-well plates (2.2 million cells/well) and incubated at 37°C with 5% CO2.
The cells, 30 minutes prior to stimulation with lipopolysaccharide (LPS 10 ng/mL from Salmonella enterica serotype typhimurium, Sigma, Taufkirchen, Germany) were incubated with active lactose-saccharose globules contained in the capsule of the medicine or with placebo globules, dissolved in culture media, at different lactose-saccharose concentrations (22, 11, 5.5, 2.75, and 1.37 mM). After incubation at 37°C and 5% CO2 for 24 hours, the supernatants were removed, centrifuged, and investigated for IL-1β, TNF-α, and IL-6 concentrations (pg/mL) using commercially available single analyte ELISA kits (Immunotools, Friesoythe, Germany). The protocol used has followed the manufacturer’s instruction.
The experiments (2 independent in 2 experimental days) were performed in duplicates.
Statistical analysis
Cytokines levels, released by placebo- or active-treated cells, were normalized to levels released by LPS-control cells. A total of 100% is the normalized value of each donor response to LPS; indeed, each treated sample was compared with its own normalized control. The responses of the six donors were individually evaluated and the average of each duplicate was considered for statistical analysis. Statistical analysis was performed using GraphPad Prism version 7.0 for Windows (GraphPad Software, La Jolla, CA, USA). Levels of IL-1β, TNF-α, and IL-6 secreted by active MI-treated cells were compared with the proper controls (placebo-treated cells and LPS-control cells) using paired t tests.
Results
Cell viability test
Cultured human enriched monocytes from 1 donor were treated for 24 hours with: i) complete medium (control), ii) NaF (250 µg/mL), and iii) four different concentrations (2.75, 5.5, 11, and 22 mM) of active or placebo lactose-saccharose globules. Cell viability was evaluated by AlamarBlue®.
All tested concentrations of active and placebo globules have not affected the viability of human primary enriched monocytes (Figure 1).
Inducible pro-inflammatory cytokines secretion in human monocytes
LPS from Gram-negative bacteria is one of the most potent innate immune-activating stimuli. Human monocytes are very sensitive to LPS and respond by expressing many inflammatory cytokines.13,14 We tested the secretion of IL-1β, TNF-α, and IL-6 on primary enriched monocytes after LPS (10 ng/mL) stimulation, and we confirmed the increased secretion of pro-inflammatory cytokines. IL-1β secretion has increased by about 15 times in stimulated cells compared with unstimulated cells, and TNF-α secretion by about 40 times (Figure 2). IL-6, absent in supernatant of unstimulated cells, has reached levels comparable with TNF-α levels in LPS-inflamed cells (11,000 pg/mL and 11,400 pg/mL).
The effect of MI medicine on pro-inflammatory cytokines secretion
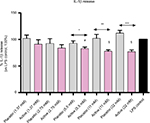
Cultured human enriched monocytes from 6 donors were exposed to 10 ng/mL of LPS for 24 hours to activate the expression and the secretion of pro-inflammatory cytokines. The treatment with placebo, or active lactose-saccharose globules, at 5 different concentrations (from 1.37 to 22 mM) started 30 minutes. earlier than the stimulation with LPS, then protein levels in cell medium were evaluated by ELISA. Graph in Figure 3 shows that, when monocytes were treated with active globules, IL-1β secretion significantly decreased compared with placebo at these concentrations: 22 mM (P<0.0001), 11 mM (P=0.0086), and 5.5 mM (P=0.0254), and it decreased compared with untreated LPS control at these concentrations: 22 and 11 mM (P=0.0008), and 5.5 mM (P=0.002).
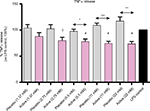
Also, TNF-α results were in line with the assumption, and IL-1β results (Figure 4). The effect on TNF-α secretion is significant at these concentrations: i) vs placebo, 22 mM (P=0.0018), 11 mM (P=0.0005), and 5.5 mM (P=0.0136); ii) vs LPS control, 22 mM (P=0.0021), 11 mM (P=0.0017), 5.5 mM (P=0.0052), and 2.75 mM (P=0.0196).
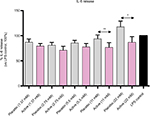
The effect of active globules on IL-6 secretion (Figure 5), while less pronounced, is consistent with IL-1β and TNF-α results: the treatment reduced IL-6 release compared with placebo at 2 concentrations: 22 mM (P=0.0177) and 11 mM (P=0.0031).
In Figure 6, we show the percentage of IL-1β, TNF-α, and IL-6 release of each donor at the 2 higher globules concentrations (11 and 22 mM). In spite of the intra-individual variability, all donors responded to the active globules in the same manner.
Discussion and conclusion
To ensure that placebo and active lactose-saccharose globules are not cytotoxic at the tested concentrations, human enriched monocytes from 1 donor were exposed to 2.75, 5.5, 11, and 22 mM of placebo or active globules, or cell medium (control). NaF (250 µg/mL) was used to induce cell death in positive control cells. The results show that both active and placebo globules, at all tested sugar concentrations, did not impact the cell viability (Figure 1). Because lactose and saccharose are not hydrolyzed in mammalian cells, we decided to stay in a “safe range”, below the used concentrations to induce autophagy and osmotic stress.15–17
The in vitro tested MI medicine was formulated to reduce the pro-inflammatory responses and, more specifically, to modulate toward an inhibition, the altered levels of IL-1β and TNF-α.
To examine the effects of the medicine on the pro-inflammatory cytokines secretion, we used a human in vitro model of inflammation: enriched monocytes, isolated from 6 healthy donors, stimulated with the bacterial antigen LPS for 24 hours to induce an inflammatory status.13,14 LPS causes an initial increase in IL-1β secretion, which, in turn, induces further IL-1β production and secretion, an amplification system that is called “autoinduction”.18 Furthermore, IL-1β induces the expression and the secretion of TNF-α and IL-6, too.19 On the other side, via the TNFR1 receptor, LPS also induces the secretion of TNF-α leading to the establishment of an autocrine positive loop that sustains the upregulation of pro-inflammatory cytokines.20 Indeed, we observed the IL-1β induction and the remarkable increase of TNF-α and IL-6 secretion in LPS-stimulated monocytes compared with unstimulated cells (Figure 2).
Once verified the in vitro inflammatory model, we tested the efficacy of the active capsule compared with placebo, by dissolving the lactose-saccharose globules in cell medium at 5 concentrations (from 1.37 to 22 mM). The graphs in Figures 3 and 4 show that the active globules, at 3 tested lactose-saccharose concentrations (5.5, 11, and 22 mM), exert significant anti-inflammatory effects compared with placebo and untreated LPS control on primary LPS-stimulated enriched monocytes reducing IL-1β and TNF-α release of about 10%–30%. The observed effect on IL-6 release is less pronounced (Figure 5); also, we noticed a higher intra-individual variability between donors (Figure 6). In the meantime, the graphs in Figure 6 show that all primary cells responded to the treatment secreting lower cytokines levels compared with placebo.
ULD of the pro-inflammatory cytokines IL-1β and TNF-α have reduced the secretion of both cytokines in human LPS-activated monocytes, an in vitro inflammatory model. These results might astonish. In addition, the formula contains ULD of IL-2, a powerful activator of human monocyte, too; it induces increased secretion of IL-1β, TNF-α, and IL-6.21–23 However, we have observed that its use at ULD, together with ULD of IL-1β and TNF-α, is associated with an opposite effect: human LPS-inflamed monocytes reacted by secreting lower levels of pro-inflammatory cytokines.
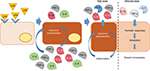
The mode of action of ULD is unknown. We hypothesize that the anti-inflammatory effect of the tested MI medicine is attributed to ULD of IL-1β, TNF-α, and IL-2, and that it is mediated by hormetic responses that can drive to a state of “adaptation” that tends toward the homeostasis (Figure 7).
The “hormesis” concept describes a phenomenon first observed by the pharmacologist and toxicologist Schulz, during his multiple chemical tests on yeast,2 and further studied by Calabrese. It consists of cellular biphasic dose–response curve induced by a substance, or a molecule, and can be graphically represented as an inverted U-shaped or J-shaped dose–response curve.24,25
Hormesis can have a wide range of biological implications and it has been applied to immune-related responses, hence, it could be exploited to improve therapeutic strategies and clinical outcomes.25,26
At the molecular level, hormesis might be explained with the existence of receptors having different ligand affinity for the same substance. More recently, hormesis has been associated with the murburn concept, which consists of a “reaction outside the active site”, induced by a diffusible reactive species, and produced, stabilized or modulated by an enzyme.27 We are aware that many questions are still unanswered, that more studies and a systematic approach are needed to confirm our hypothesis. Here, we are describing preliminary data to pose the basis into the mode of action of ULD and MI medicines.
The present manuscript reports a beneficial effect of the tested MI medicine in an in vitro model of inflammation: human primary enriched monocytes (n=6) that responded to LPS inducing IL-1β release and, in turn, the release of the other pro-inflammatory cytokines involved in the inflammatory amplification system, TNF-α, and IL-6. The active globules have reverted the tendency, causing a~10%–30% reduction of IL-1β and TNF-α secretion, “disturbing” the positive loop that establishes and maintains the chronic inflammation.
Upcoming in vitro experiments might investigate whether longer exposure to 2LARTH® can enhance that anti-inflammatory effect, or not. Further studies are necessary to explore more deeply the mode of action of pro-inflammatory cytokines at ULD, and to know which pathways are involved. In addition, subsequent clinical trials can be planned to demonstrate its efficacy to treat patients suffering from RD.
Acknowledgments
The authors would like to thank Anne Naedts for preparing and providing the placebo globules and Alain Lejeune for providing and sending the medicine to VivaCell Biotechnology GmbH. This research study was funded by Labo’Life France.
Author contributions
IF and BL conceived the experiments; KA designed the experiments; TR performed the experiments; TR, KA, and IF analyzed the data; and IF wrote the paper and prepared the figures. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
IF works for Labo’Life France, the company service provider for Labo’Life, specializing in preclinical, clinical, and regulatory affairs. BL is employed by Labo’Life Belgium, the pharmaceutical company that commercializes the micro-immunotherapy medicine tested in the study. IF and BL declare that their professional relationships with Labo’Life France and Labo’Life Belgium do not imply any misconduct in this study. KA and TR work for VivaCell Biotechnology GmbH, a contract research organization that specializes in preclinical research. The authors report no other conflicts of interest in this work.
References
Thomas G, Cluzel H, Lafon J, et al. Efficacy of 2LPAPI®, a Micro-Immunotherapy Drug, in Patients with High-Risk Papillomavirus Genital Infection. Advances in Infectious Diseases. 2016;6:7. | ||
Floris I, Lechner J, Lejeune B. Follow-up of patients with systemic immunological diseases undergoing fatty-degenerative osteolysis of the jawbone surgery and treated with RANTES 27CH. J Biol Regul Homeost Agents. 2018;32(1):37–45. | ||
Schett G, Dayer JM, Manger B. Interleukin-1 function and role in rheumatic disease. Nat Rev Rheumatol. 2016;12(1):14–24. | ||
Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361(1-3):184–187. | ||
Schaible HG. Nociceptive neurons detect cytokines in arthritis. Arthritis Res Ther. 2014;16(5):470. | ||
NICE CG177. Osteoarthritis Care and Management in Adults. London: National Institute for Health and Care Excellence; 2014. | ||
van Laar M, Pergolizzi JV, Mellinghoff HU, et al. Pain treatment in arthritis-related pain: beyond NSAIDs. Open Rheumatol J. 2012;6:320–330. | ||
Marcum ZA, Hanlon JT. Recognizing the risks of chronic nonsteroidal anti-inflammatory drug use in older adults. Ann Longterm Care. 2010;18(9):24. | ||
O’Neil CK, Hanlon JT, Marcum ZA. Adverse effects of analgesics commonly used by older adults with osteoarthritis: focus on non-opioid and opioid analgesics. Am J Geriatr Pharmacother. 2012;10(6):331–342. | ||
Schwartz A. Ozone Therapy in Papillomavirus Infection-HPV. Revista Española de Ozonoterapia. 2017;7:17–28. | ||
English D, Andersen BR. Single-step separation of red blood cells. Granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll-Hypaque. J Immunol Methods. 1974;5(3):249–252. | ||
Noble PB, Cutts JH, Carroll KK. Ficoll flotation for the separation of blood leukocyte types. Blood. 1968;31(1):66–73. | ||
Rossol M, Heine H, Meusch U, et al. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol. 2011;31(5):379–446. | ||
Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13(2):85–94. | ||
Higuchi T, Nishikawa J, Inoue H. Sucrose induces vesicle accumulation and autophagy. J Cell Biochem. 2015;116(4):609–617. | ||
Chen X, Li M, Li L, et al. Trehalose, sucrose and raffinose are novel activators of autophagy in human keratinocytes through an mTOR-independent pathway. Sci Rep. 2016;6:28423. | ||
Decourcy K, Storrie B. Osmotic swelling of endocytic compartments induced by internalized sucrose is restricted to mature lysosomes in cultured mammalian cells. Exp Cell Res. 1991;192(1):52–60. | ||
Dinarello CA, Ikejima T, Warner SJ, et al. Interleukin 1 induces interleukin 1. I. Induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol. 1987;139(6):1902–1910. | ||
Tosato G, Jones KD. Interleukin-1 induces interleukin-6 production in peripheral blood monocytes. Blood. 1990;75(6):1305–1310. | ||
Gane JM, Stockley RA, Sapey E. TNF-α autocrine feedback loops in human monocytes: the pro- and anti-inflammatory roles of the tnf-α receptors support the concept of selective tnfr1 blockade in vivo. J Immunol Res. 2016;2016:1079851. | ||
Weis S, Rubio I, Ludwig K, Weigel C, Jentho E. Hormesis and defense of infectious disease. Int J Mol Sci. 2017;18(6):1273. | ||
Kovacs EJ, Brock B, Varesio L, Young HA. IL-2 induction of IL-1 beta mRNA expression in monocytes. Regulation by agents that block second messenger pathways. J Immunol. 1989;143(11):3532–3537. | ||
Strieter RM, Remick DG, Lynch JP, Spengler RN, Kunkel SL. Interleukin-2-induced tumor necrosis factor-alpha (TNF-alpha) gene expression in human alveolar macrophages and blood monocytes. Am Rev Respir Dis. 1989;139(2):335–342. | ||
Calabrese EJ, Baldwin LA. Hormesis: U-shaped dose responses and their centrality in toxicology. Trends Pharmacol Sci. 2001;22(6):285–291. | ||
Calabrese EJ, Mattson MP. How does hormesis impact biology, toxicology, and medicine? NPJ Aging Mech Dis. 2017;3:13. | ||
Calabrese EJ. Hormetic dose-response relationships in immunology: occurrence, quantitative features of the dose response, mechanistic foundations, and clinical implications. Crit Rev Toxicol. 2005;35(2–3):89–295. | ||
Parashar A, Gideon DA, Manoj KM. Murburn Concept: A molecular explanation for hormetic and idiosyncratic dose responses. Dose Response. 2018;16(2):1559325818774421. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.