Back to Journals » Clinical Ophthalmology » Volume 12
27-gauge needle-assisted externalization and haptic securing technique for sutureless scleral fixation of the intraocular lens – moving toward simplicity
Authors Khatri A , Singh S , Rijal R , Khatri BK , Kharel M
Received 8 April 2018
Accepted for publication 31 May 2018
Published 14 August 2018 Volume 2018:12 Pages 1441—1447
DOI https://doi.org/10.2147/OPTH.S166354
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Anadi Khatri,1 Sweta Singh,1 Roshija Rijal,1 Bal Kumar Khatri,2 Muna Kharel3
1Lumbini Eye Institute, Lumbini, Nepal; 2Birat Eye Hospital, 3Nepalese Army Insitute of Medical Sciences, Kathmandu, Nepal
Purpose: To report a modified technique of sutureless intrascleral fixation of a posterior chamber intraocular lens with use of instruments of anterior segment surgery and its outcomes.
Design: Prospective, noncomparative, interventional case series.
Participants: Ninety-two eyes of 92 patients with aphakia and subluxated lens who underwent surgery were evaluated.
Materials and methods: 27-gauge needles were introduced transclerally and guided by the viscocanula to externalize via the main wound. The haptics were loaded into the lumen and externalized from entry points. The haptics were then fixed in a scleral tunnel made by a 27-gauge needle. The best-corrected visual acuity (BCVA) and complications were determined.
Results: Ninety two eyes which were operated and completed follow-up of 6 weeks were included in the study. The most common indications for scleral-fixated intraocular lens (SFIOL) were subluxated lens – 55 eyes (59%), and surgical aphakia – 31 eyes (34%). Sixty-nine eyes (75.7%) had a postoperative vision of uncorrected visual acuity of 6/18 on day 1. There was an improvement in mean logMAR BCVA (0.086±0.18) at 6 weeks as compared to preoperative visual acuity (p<0.05). BCVA of 6/12 or better was attained in 94% of the cases at 6 weeks. Special mentions need to be made for 6 (7%) of our cases. Three of the patients were cases of fully treated postoperative endophthalmitis who lacked capsular support. Two of the other cases had undergone pars plana vitrectomy for retinal detachment. Both had silicon oil removal done 1 month before the SFIOL procedure. One of the patients had Marfan’s syndrome.
Conclusion: Our procedure is safe, easy, less traumatic, and less resource-demanding with good visual outcomes and can be performed even in low-resource settings of developing countries. It may also be considered in patients who have had posterior segment surgeries previously.
Keywords: scleral fixation, 27-gauge needle-assisted, aphakia
Introduction
Vision restoration with an intraocular lens (IOL) is the ideal choice for cataract surgery. The most anatomically and optically preferred choice is “in the bag.” But in some cases – specifically in a large posterior capsule rupture – IOL implantation in the ciliary sulcus may be mandated.1,2 If the sulcus is unstable and there is a risk of IOL being dislodged into the vitreous, anterior chamber-IOLs (AC-IOLs) are also preferred.3–6 Although they are available in different forms with different mechanisms of support, AC-IOL continues to have a disadvantage. They make the cornea prone to endothelial loss, angles susceptible to trabeculitis, the pupil at risk for occlusion, and uveal tissue in danger of inflammation due to shaffing.7,8
A different surgical technique to avoid all these possible complications due to AC-IOL in an eye where a posterior chamber IOL (PC-IOL) cannot be placed is a transscleral fixation of an IOL.9–14 This technique has gained significant popularity over the past 20 years.15 This has been further facilitated by the improvements in lens designs and implantation techniques.
One of the techniques to fixate a PC-IOL in the absence of sufficient capsular support is transscleral suturing. The other is the sutureless scleral fixation. A number of modifications have also been made for fixation of an IOL to the scleral wall adjacent to the ciliary sulcus or pars plana.7–14 Irrespective of the technique, the primary goal to achieve safe and stable fixation of PC-IOLs with an acceptable level of vision restoration has been achieved – while avoiding most of the complications associated with AC-IOLs.
In 1986, Malbran et al15 first reported trans-scleral sulcus fixation of posterior chamber IOL after intracapsular cataract extraction. They described the use of ab externo suture techniques to guide the sutures through the sclera to fixate the IOL. Since then, there has been a paradigm shift and a huge progress toward sutureless scleral-fixated IOL implantation with intrascleral haptic fixation. Scharioth et al16 reported good results of sutureless surgery, with no complications such as recurrent dislocation or retinal detachment in patients followed up till 7 months. In the glued-IOL technique described by Agarwal et al,13 surgical fibrin glue sealant is used to secure the scleral flap and the externalized haptic to the scleral bed. Gabor and Pavlidis14 have described a technique of sutureless sclera fixation of PC-IOL. In this technique, IOL haptics were introduced and fixated into the prepared scleral tunnels without the use of glue or suture assisted stabilization. Totan and Karadag17 have improved the previously described technique by making scleral tunnels prepared by insertion of the 25-gauge transconjunctival sutureless vitrectomy microcannulas using the trocars. Akimoto et al18 reported using catheters and 30-gauge ultra-thin needles to introduce haptic externally. Yamane et al19 described 27-gauge needle-guided intrascleral PC-IOL implantation technique using needle, forceps, and lamellar scleral dissection. A simpler technique was introduced by Baskaran et al.20 They introduced the use of extraocular needle-guided haptic insertion technique of scleral fixation of IOL – removing the requirement of grasping forceps and bypassing the difficult handshaking technique previously described.
Here, we have improved and improvised on the previously described technique by minimizing the trauma to the tissue, difficulty of the procedure, and requirement of special instruments. We believe our technique can be replicated easily even in low-resource settings with a good outcome. For haptic externalization, we have adopted the use of a needle-guided haptic insertion as described by Baskaran et al20 which omits the use of the grasping type of microforceps, but have improvised the delivery of the needle tip via the main port by the assistance of 23-gauge tip viscocannula instead of the McPherson. This also prevents inadvertent damage to the iris tissue, corneal endothelium, and the anterior and posterior inner lips of the main wound.
We introduce the use of a 27-gauge needle to make the scleral haptic tunnels. The construction of the scleral tunnels has been modified – omitting the use of micro vitreo retinal (MVR) and surgical knives for construction of lamellar scleral dissections and scleral flaps. We report results of 92 patients who underwent this modified procedure and completed follow-up of 6 weeks.
Materials and methods
Patients and methods
An interventional case series was conducted. Aphakic patients with absence of capsular support, subluxated lens (>180°), posteriorly dislocated lens, and posteriorly dislocated IOL were enrolled for the surgery. Surgeries were performed by 3 vitreoretinal surgeons. Patients with hazy cornea or corneal scars, aniridia, macular scar, glaucoma, and patients requiring posterior segment surgery/approach at the same setting were excluded.
Ocular evaluation included uncorrected distance visual acuity, best-corrected distance visual acuity (BCVA), intraocular pressure (IOP) (Goldman Applanation tonometry), slit-lamp biomicroscopy, and dilated fundus examination at the time of surgical treatment and during the follow-ups. Patients who completed follow-up of 6 weeks were included in the study. Intraoperative complications as well as immediate and late postoperative complications were recorded. Further surgical intervention required for any of the complications was recorded.
Statistical analysis
Data were and analyzed using SPSS software (version 20.0; IBM Corporation, Armonk, NY, USA). Continuous variables were expressed as the mean ± SD, and categorical variables were expressed as individual counts. The Snellen visual acuity was converted into logarithm of the minimum angle of resolution (logMAR) units for analysis. Differences were considered statistically significant when the p-value was >0.05.
Operative procedure
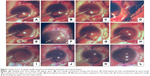
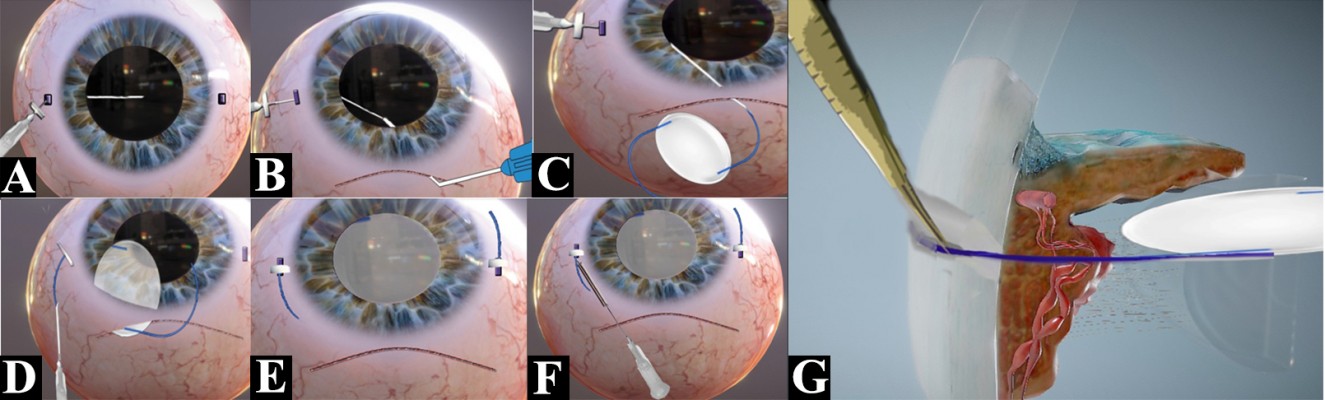
All cases were performed under peribulbar anesthesia. A fornix-based peritomy is made from 8 o’ clock to 3 o’clock. The tips of the Castroveijo’s caliper are marked with tissue pen and reading is set at approximately 12–13 mm. The peripheral cornea or the limbus is marked with the caliper to set 2 points 180° apart (Figure 1A). We used the 2–8 o’clock axis in all of our cases, but this can be modified as per surgeon’s preference and ease (Figure 1B).
The 3 and 9 o’clock positions are best avoided to prevent damage to the long ciliary nerves. Using the caliper again, a 2 mm reading is set and the sclera is marked from the limbus (Figure 1B). These are the reference points from where the haptics are externalized (Figure 2A).
A standard 6 mm small incision cataract surgery incision is made superiorly, and the AC is entered into in a controlled fashion (Figure 1B). At this stage, the surgeon has the choice to perform anterior vitrectomy or to use an AC maintainer to maintain the structural integrity of the globe. If the surgeon feels there is no requirement for both, the surgeon can proceed to next step.
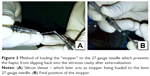
Two 27-gauge needles along with 1 mL syringe are prepared in such a way that the needle makes an angulation of roughly 60°–70° (Figure 3A). Then a rubber/plastic sleeve – usually cut from the intravenous tubing – is cut to an approximate size of 3×3 mm2 and is loaded to the needle up to the hub. This also omits the use of silicon sleeves previously mentioned in different techniques20 which are usually not available in the operating room of the anterior segment setup. This will act as a “stopper” to prevent haptic from slipping into the vitreous cavity (Figure 3B).
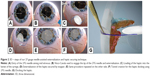
The needle is entered aiming at the mid-vitreous until the tip is seen from the pupillary edge (Figures 1B and 2A). Then, the needle is turned in the direction of the main wound at 12 o’clock and advanced until it reaches near the pupillary margin superiorly. A syringe fit with viscocannula tip is advanced from the tunnel end and the tip of the 27-gauge is engaged and slowly externalized with the bevel end up (Figures 1C and 2B).
The 3 piece PMMA (modified “C” loops with 10° angulation, 13.5 mm length, optic diameter 6 mm) IOL is oriented in a similar fashion as it would be placed in the bag. The leading haptic is grasped 1–2 mm posteriorly from the tip and advanced inside the needle from the bevel. Approximately 1/3rd of the haptic length needs to be loaded for safe delivery (Figures 1D and 2C) and externalization (Figures 1E and 2D). Once the haptic is externalized, the stopper is placed as close to the sclera as possible. It must be kept in mind at this stage that some length of haptic is still reserved intraocularly so the lens does not resist movement while loading the other haptic.
Using the same technique, the procedure is repeated on the contralateral side to load, deliver, and externalize the trailing haptic. The needle should pass behind the optic while trying to externalize it from the main wound (Figures 1F–H and 2E).
Now from the point of haptic externalization, 4 mm reading is set in the calipers and then the paralimbal sclera is marked parallel to the limbus. This denotes where we will introduce the 27-gauge needle – with bevel up – in a lamellar fashion and externalize it as close as possible to the point where the haptic is externalized (Figures 1I and 2F).
The tunnel is tented slightly upwards using the 27-gauge needle. This opens the whole length of the tunnel and the haptic is introduced into which the 27-gauge needle is withdrawn back simultaneously. The haptic gets secured in the track left behind by the needle (Figures 1J and 2G).
The same procedure is repeated on the other side (Figure 1K and L).
The peritomy is then closed using either 8-O vicryl sutures or ballooned using a subconjunctival mixture of antibiotics with steroids. From the 1st operative day, steroid–antibiotic adjunct topical drops are started 1 hourly for a day 1, 2 hourly for 1 week, and then 4 times a day for 5 weeks.
Ethical clearance
The research has been approved by the ethics committee and the institutional review board of Lumbini Eye Institute, Lumbini, Nepal, and has adhered to the tenets of the declaration of Helsinki. Written informed consent has been provided by the patients, or their parents or legal guardian if under the age of 18.
Consent
Written informed consent has been provided by the patient’s parent to have the case details and any accompanying images published.
Results
Ninety two eyes which were operated using this procedure and completed follow-up of 6 weeks were included in the study. About 61 (66%) of the patients were male and 31 (34%) were female. The median age of the patients was 51 years (17–80 years). About 50 (54%) right and 42 (44%) left eyes underwent the surgery (Table 1).
The most common indications for SFIOL were subluxated lens – 55 eyes (59%), surgical aphakia due to unstable sulcus due to zonular dialysis or capsular bag – 31 eyes (34%). Fifty-six eyes (61%) underwent primary SFIOL implantation and 36 eyes (39%) underwent secondary SFIOL implantation (Table 1).
Summary of vision status at the time of presentation, day 1 following the procedure, and at 6 weeks is illustrated in Figure 4.
  | Figure 4 The pattern of preoperative, postoperative, and 6th week postoperative change in visual acuity. |
Uncorrected visual acuity improved significantly after surgery with improvement in mean logMAR BCVA (0.086±0.18) at 6 weeks as compared to preoperative VA (p<0.05) (Figure 4). BCVA of 6/12 or better was attained in 94% of the cases at 6 weeks. None of the eyes reported a decrease in the visual acuity when followed up to 6 weeks.
Special mentions need to be made of 6 (7%) of our cases. Three of the patients undergoing SFIOL were cases of fully treated postoperative endophthalmitis who had previously undergone intraocular lens explantation, capsule removal, and vitrectomy (mean duration – 9 months). Two of the other cases had undergone pars plana vitrectomy for retinal detachment (mean duration – 7 months). Both had undergone silicon oil removal done 1 month before the SFIOL procedure. One of the patients had Marfan’s syndrome. The details of their visual acuity at presentation and BCVA at the 6th week of follow-up are illustrated in Table 2.
No intraoperative complications like iridodialysis, cyclodialysis, hyphema, and IOL drop were encountered. We also did not have any complications like vitreous hemorrhage or retinal/choroidal detachment. Raised IOP was the most common immediate postoperative complication seen in 4 (4.5%) of the cases. All of them had a traumatic subluxation of the lens preoperatively. Antiglaucoma agents were not required beyond 2 weeks from the date of surgery. IOP was within normal range without medications by the 6th week. There was 1 case of haptic breakage/disinsertion from the optic–haptic junction intraoperatively for which the IOL was exchanged. There was also 1 case of haptic extrusion from the opposite end of the tunnel on the 1st postoperative day in an eye which was repositioned and trimmed on the same day. The most common complaint at 1st follow-up on day 7 postoperatively was photophobia (14%), followed by foreign body sensation (6%). At 6 weeks, none of the patients had any ocular issues or complaints.
Discussion
The concept of sutureless scleral fixation of IOL in eyes with absent capsular support is gaining special interest in recent years. Our technique is unique in the sense that we have modified the existing techniques and also added new methods for scleral fixation. We have used 3 piece PMMA IOL, which are loaded and introduced into position with use of a 27-gauge needle. We have advocated the use of a simple yet very effective and safe 23G viscocannula to engage the sharp end of the needle and guide it exteriorly. This cannula is easily available, safe in terms that it protects the ocular structures from the sharp end of the 27-gauge needle, and removes the requirement of special instruments. We have also used a 27-gauge needle to create scleral tunnels/tracks for securing the haptics. This method is safe, less traumatic, and also provides an added stability to the lens due to the size of the wound. The use of special or more traumatic instruments – such as MVR – is omitted. The whole surgery can be performed using less expensive and basic instruments that are readily available for a regular anterior segment surgery. This also makes the technique of SFIOL easy to be adopted in low-resource eye centers – specially developing nations around the world.
The results of our technique have been encouraging, with 94% of the cases attaining a stable BCVA of 6/12 or better at 6 weeks. Agarwal et al21 have reported 72% of their patients undergoing SFIOL of having vision better than or equal to 6/18 in a similar setup but with a different technique. They have also reported that there was no drop in BCVA at 1-month follow-up. Kumar et al22 have reported a decrease in BCVA in 11% of and Luk et al23 reported 29% of their patients having drop in BCVA eyes undergoing surgery in their techniques.
Most authors have reported a low IOP in their cases following surgery in the immediate postoperative period.21,24 In our study, postoperative hypotony was not present in any of the cases. Instead, raised IOP was the most common immediate postoperative complication seen in 4 (4.5%) of the cases.
We have also reported the outcomes of the SFIOL surgery done by our technique in special aphakic cases such as those who were treated for and had resolved endophthalmitis, or in patients post retinal detachment surgery, and even in a patient with Marfan’s syndrome. The results are equally promising in these patients, showing improvement from mean logMAR BCVA of 1.67 (SD 0.24) to mean logMAR BCVA of 0.36 (SD 0.03). None of the cases reported with any anterior or posterior segment complications till 6 weeks of follow-up.
Conclusion
With various techniques or approach currently available for performing SFIOL, we have improvised a few steps and added new ones such as the use of a 27-gauge needle. Our technique removes the need for special or expensive instruments. It is also less traumatic, safe, and easy. It can be performed with basic instruments available for an anterior segment surgery. This, we believe, is specially going to benefit many institutes or eye hospitals around the world in developing countries where the resources are limited. Our procedure may also be considered in relatively challenging and complicated aphakic eyes, although a study of this technique in more number of such cases is required to evaluate for its safety standards and outcome.
Disclosure
The authors report no conflicts of interest in this work.
References
Chang DF, Masket S, Miller KM, et al; ASCRS Cataract Clinical Committee. Complications of sulcus placement of single-piece acrylic intraocular lenses: recommendations for backup IOL implantation following posterior capsule rupture. J Cataract Refract Surg. 2009;35(8):1445–1458. | ||
Oetting TA. When and how should I implant an intraocular lens in the ciliary sulcus? In: Chang DF, editor. Curbside Consultation in Cataract Surgery: 49 Clinical Questions. Chapter 33, 1st ed. Thorofare, NJ, USA: SLACK; 2007:161–166. | ||
Wagoner MD, Cox TA, Ariyasu RG, Jacobs DS, Karp CL, American Academy of Ophthalmology. Intraocular lens implantation in the absence of capsular support: a report by the American Academy of Ophthalmology. Ophthalmology. 2003;110(4):840–859. | ||
Fasih U, Ahmed I, Shaikh A, Fahmi MS. Comparison of complications after primary and secondary anterior chamber comparison of complications after intraocular lens implantation. Pak J Ophthalmol. 2010;26(2):57–64. | ||
Natchairg, Kar D. Complications of intraocular lens implantation. In: Dutta LC, Dutta NK, editors. Modern Ophthalmology. 3rd ed. Vol 1. New Delhi: Jaypee Publications; 2005:464–467. | ||
Sawada T, Kimura W, Kimura T, et al. Long-term follow-up of primary anterior chamber intraocular lens implantation. J Cataract Refract Surg. 1998;24(11):1515–1520. | ||
Kwong YYY, Yuen HKL, Lam RF, Lee VYW, Rao SK, Lam DSC. Comparison of Outcomes of Primary Scleral-Fixated versus Primary Anterior Chamber Intraocular Lens Implantation in Complicated Cataract Surgeries. Ophthalmology. 2007;114(1):80–5. | ||
Belluci R, Pucci V, Morselli S, Bonomi L. Secondary implantation of angle supported anterior chamber and sclera fixated posterior chamber intraocular lenses. J Cataract Refract Surg. 1996;22(2):247–252. | ||
Por YM, Lavin MJ. Techniques of intraocular lens suspension in the absence of capsular/zonular support. Surv Ophthalmol. 2005;50(5):429–462. | ||
Mittelviefhaus H. A modified technique of transscleral suture fixation of posterior chamber lenses. Ophthalmic Surg. 1992;23(7):496–498. | ||
Schmidt JC, Nietgen GW, Freisberg L, Neisskenwirth NN. Modified transscleral suture for sulcus fixation of posterior chamber lenses. J Cataract Refract Surg. 2002;28(1):15–17. | ||
Smiddy WE, Sawusch MR, O’Brien TP, Scott DR, Huang SS. Implantation of scleral-fixated posterior chamber intraocular lenses. J Cataract Refract Surg. 1990;16(6):691–696. | ||
Agarwal A, Kumar DA, Jacob S, Baid C, Agarwal A, Srinivasan S. Fibrin glue-assisted sutureless posterior chamber intraocular lens implantation in eyes with deficient posterior capsules. J Cataract Refract Surg. 2008;34:1433–1438. | ||
Gabor SG, Pavlidis MM. Sutureless intrascleral posterior chamber intraocular lens fixation. J Cataract Refract Surg. 2007;33(11):1851–1854. | ||
Malbran ES, Malbran E Jr, Negri I. Lens guide suture for transport and fixation in secondary IOL implantation after intracapsular extraction. Int Ophthalmol. 1986;9(2–3):151–160. | ||
Scharioth GB, Prasad S, Georgalas I, Tataru C, Pavlidis M. Intermediate results of sutureless intrascleral posterior chamber intraocular lens fixation. J Cataract Refract Surg. 2010;36(2):254–259. | ||
Totan Y, Karadag R. Trocar-assisted sutureless intrascleral posterior chamber foldable intra-ocular lens fixation. Eye. 2012;26(6):788–791. | ||
Akimoto M, Taguchi H, Takayama K, Nakagawa S, Hiroi K. Intrascleral fixation technique using catheter needles and 30-gauge ultrathin needles: lock-and-lead technique. J Cataract Refract Surg. 2015;41(2):257–261. | ||
Yamane S, Inoue M, Arakawa A, Kadonosono K. Sutureless 27-gauge needle-guided intrascleral intraocular lens implantation with lamellar scleral dissection. Ophthalmology. 2014;121(1):61–66. | ||
Baskaran P, Ganne P, Bhandari S, Ramakrishnan S, Venkatesh R, Gireesh P. Extraocular needle-guided haptic insertion technique of scleral fixation intraocular lens surgeries (X-NIT). Indian J Ophthalmol. 2017;65(8):747–750. | ||
Agarwal L, Agarwal N, Gurung RL, Chaubey R, Jha BK, Chaudhary BP. Visual outcome and early complications of sutureless and glueless scleral fixated intraocular lens. Nepal J Ophthalmol. 2016;8(15):41–46. | ||
Kumar DA, Agarwal A, Prakash G, Jacob S, Saravanan Y, Agarwal A. Glued posterior chamber IOL in eyes with deficient capsular support: a retrospective analysis of 1-year post-operative outcomes. Eye. 2010;24(7):1143–1148. | ||
Luk ASW, Young AL, Cheng LL. Long-term outcome of scleral-fixated intraocular lens implantation. Br J Ophthalmol. 2013;97(10):1308–1311. | ||
Nadal J, Kudsieh B, Casaroli-Marano RP. Scleral fixation of posteriorly dislocated intraocular lenses by 23-gauge vitrectomy without anterior segment approach. J Ophthalmol. 2015;2015:391619. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.