Back to Journals » Neuropsychiatric Disease and Treatment » Volume 20
Neuropsychiatric Symptoms Predict Faster Cognitive Decline in Dementia Collaborative Care Than Antipsychotic Use
Authors Chen YJ , Chang MC, Jhang KM, Wang WF, Liao YC
Received 14 December 2023
Accepted for publication 16 March 2024
Published 26 March 2024 Volume 2024:20 Pages 689—696
DOI https://doi.org/10.2147/NDT.S454943
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Yen-Jen Chen,1,2,* Ming-Che Chang,3,* Kai-Ming Jhang,4,* Wen-Fu Wang,4,* Yi-Cheng Liao1
1Department of Psychiatry, Changhua Christian Hospital, Changhua, Taiwan; 2Department of Psychiatry, Yuanlin Christian Hospital, Changhua, Taiwan; 3Department of Nuclear Medicine, Changhua Christian Hospital, Changhua, Taiwan; 4Department of Neurology, Changhua Christian Hospital, Changhua, Taiwan
*These authors contributed equally to this work
Correspondence: Yi-Cheng Liao, Department of Psychiatry, Changhua Christian Hospital, 135, Nan-Xiao St, Changhua, 500, Taiwan, Tel +886 4 7238595 ext. 7160, Fax +886 4-7251004, Email [email protected]
Background: To compare short-term cognitive outcomes among groups with and without neuropsychiatric symptoms (NPSs) or antipsychotic prescription and to determine which disease status or treatment modality is associated with relatively faster cognitive decline.
Methods: We retrospectively analyzed a prospective cohort of patients diagnosed with dementia and mild cognitive impairment. All participants were evaluated using the Cognitive Abilities Screening Instrument (CASI) during their initial clinical assessments and at the annual follow-up. The dependent variable was annual delta CASI. Multivariate linear regression analysis was used to assess the degree of association between NPS, antipsychotic use, and cognitive decline after adjusting for confounding factors. Neuropsychiatric symptoms were examined individually to determine their predictive value for cognitive decline.
Results: A total of 407 (N = 407) patients were included in the study. NPSs, rather than antipsychotic use, led to faster cognitive decline. A higher baseline NPI total score predicted a significantly faster decline in CASI scores (1-year delta CASI = − 0.22, 95% CI = − 0.38~ − 0.05, p = 0.010). Specific items (delusions, agitation, depression, anxiety, euphoria, and apathy) in the NPS significantly increased cognitive decline.
Conclusion: Certain neuropsychiatric symptoms, rather than antipsychotic use, lead to faster cognitive decline in a dementia collaborative care model. Checking for and providing appropriate interventions for NPS in people with dementia and their caregivers are highlighted.
Keywords: neuropsychiatric symptoms, antipsychotics, cognitive decline, cognitive abilities screening instrument, dementia collaborative care
Introduction
Neuropsychiatric symptoms (NPSs) are behavioral (eg aberrant motor behavior) and psychological (eg delusion or hallucination) symptoms. They hold a high prevalence in patients with dementia and occur frequently during the course of cognitive decline. In addition to dementia caused by Alzheimer’s disease (AD), NPSs are common across various types of dementia.1–3
In our previous study on the institutionalization risks for patients with dementia in Taiwan, we found that the presence of NPSs at entry into dementia collaborative care is a significant predictor.4 Institutionalization partially reflects dementia progression5 and provides indirect evidence of the speed of progression. Surveillance of longitudinal changes in dementia severity are essential for providing individualized care plans. A longitudinal survey of neuropsychological functions predicts the prognosis of patients with cognitive impairment.6 Factors associated with faster neurocognitive progression warrant further confirmation and may serve as a pivotal role for continuous care directions.
A case management model in dementia care has been reported to reduce the prevalence of neuropsychiatric symptoms.7,8 The use of psychotropic medications is significantly associated with a high severity of NPS.9 Decreasing the severity of NPS can be achieved with both a cognitive-behavioral approach10 and psychotropic use.11
Increasing evidence has shown that the conversion rate of mild cognitive impairment (MCI) to dementia is higher in patients with more severe NPS.12,13 Certain types of NPSs are predictors of progression to AD.14 The prevalence of NPSs is associated with an increased rate of cognitive decline, even in older adults without dementia.15 Antipsychotic use has been postulated to predict cognitive decline;16 however, confounding effect of the indication should be taken into account.
NPSs are significant factors for families to recognize the progression of dementia.17 NPS severity is associated with caregiver stress and therapeutic outcomes.18 Furthermore, neuropsychiatric symptoms are associated with healthcare utilization, including home personal care and assistive devices.19 Dementia collaborative care has been implemented in our affiliation since 2015 and serves as an appropriate place for investigating patient-family dyads, neuropsychiatric symptoms, and psychotropic prescriptions.
This study aimed to compare short-term cognitive outcomes among groups with and without NPSs or antipsychotic prescription and determine the one associated with faster cognitive decline.
Materials and Methods
Participants
We retrospectively analyzed a prospective cohort diagnosed with dementia and MCI. The diagnosis was based on the Clinical Dementia Rating (CDR) scale20 and confirmed by neurologists and psychiatrists. Their severities ranged from CDR 0.5 to 3. The dementia collaborative care model was established in October 2015. The inclusion criteria for this retrospective cohort were as follows: (1) patients diagnosed with MCI or dementia in an outpatient clinic setting; (2) entry between October 2015 and July 2022; (3) consent from patients and their families to join the care model; and (4) at least two annual neurocognitive tests for comparison: one at entry and the other during 1-year follow-up. The overall cohort was composed of 407 patients including 158 men and 249 women. Their mean age was 78.1 years and were all Taiwanese living in Central Taiwan.
After the diagnosis of dementia or MCI, patients and caregivers received an initial assessment, including functional and living status and NPSs by evaluating the Neuropsychiatric Inventory (NPI),21,22 nutritional status, financial support, and medication reviews to determine their unmet needs.23,24 Medication reviews by pharmacists were obtained from cloud drug lists registered at the National Health Insurance system. The antipsychotics included quetiapine, aripiprazole, risperidone, olanzapine, amisulpride, lurasidone, brexpiprazole, clozapine, paliperidone, zotepine, ziprasidone, haloperidol, and clotiapine. Risk-benefit documentation for antipsychotic use was obtained from the patients receiving antipsychotic treatment. Due to different receptor profiles and relevant potential adverse reactions, the above antipsychotics were also grouped as first-generation antipsychotic (FGA) and second-generation antipsychotic (SGA) for further analysis.
All data required for the present study were extracted from electronic charts after deleting personal information. This clinical study was approved by the Institutional Review Board of Changhua Christian Hospital (CCH IRB 220920). The requirement for informed consent from each participant was waived by the institutional review board because of the retrospective chart review design. Attentions were paid on minimal risk of patient data confidentiality and the study was in compliance with the Declaration of Helsinki.
Outcomes and Variables
The outcome measure of the cognitive screening test used in this study was the Cognitive Ability Screening Instrument (CASI).25 The CASI evaluates memory, executive function, orientation, visuospatial function, and language cognitive domains, with a range of 0–100. Higher scores indicated better cognitive function. The dependent variable was annual delta CASI. All participants were evaluated using the CASI during their initial clinical assessment and at the subsequent annual follow-ups (baseline and 12 months). Higher decrease in CASI scores during the observed time period was regarded as faster cognitive decline.
Specific neuropsychiatric symptoms are associated with fast dementia progression; however, previous studies have reported heterogeneous results.9,26 Each item of the NPI was examined individually to determine its distribution within the cohort.
Statistical Analysis
Data were analyzed using R software (R Foundation for Statistical Computing). For baseline demographics and clinical information, categorical variables are presented as numbers (percentages) and continuous variables as means (standard deviations). The variables were checked for deviations from normal distribution. Nominal variables among patients with or without antipsychotic use were compared using Pearson’s chi-square test or Fisher’s exact test, whereas continuous variables were compared using analysis of variance or the Kruskal–Wallis rank sum test.
Potential predictors of dementia progression, including age at dementia/MCI diagnosis, years of education, sex, dementia type, history of CVA, and walking capability,27–31 were analyzed.
We categorized the patients into AD and non-AD groups according to the dementia type. Walking capability was categorized as fully ambulatory (free), ambulatory with assistance or holding a cane (needing assistance), or wheelchair bound (wheel-bound).
Multivariate linear regression analysis was used to assess the degree of association between NPS, antipsychotic use, and cognitive decline after adjusting for confounding factors. Variation inflation factors (VIFs) of the included variables were calculated for examination of collinearity. Since antipsychotic use were possibly in relation to severe NPSs, a mediation analysis with removing these two factors individually were performed.
Results
Table 1 shows the demographic information of our study cohort (N = 407) of patients with and without antipsychotic use at entry into dementia collaborative care. Significant differences were found between the groups in terms of age, walking capability, and NPI (in total, presence of delusions or hallucinations, and moderate-severe psychosis clusters). Psychosis was defined as the NPI severity score multiplied by the frequency score of either delusion or hallucination more than 0. Moderate-severe psychosis was defined as NPI severity score multiply by frequency score of either delusion or hallucination more or equal to 4. The mean CASI interval was 1.11 (0.53) years. The primary outcomes (annual delta CASI) for the two groups were −6.30 and −8.34 (p = 0.371, not significant).
 |
Table 1 Comparison of Covariates Among Groups with and without Antipsychotic Use |
Table 2 provides a multivariate linear regression model of cognitive decline using delta CASI. VIFs of the included variables ranged from 1.106 to 1.291. Minimal collinearity was considered. After adjusting for other confounders (age at dementia diagnosis, sex, education, dementia type, history of CVA, and walking capability), a higher baseline NPI total score predicted a significantly faster decline in CASI scores. The 1-year delta CASI was −0.22 with 95% confidence interval of −0.38 to −0.05 (p = 0.010).
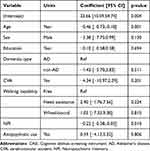 |
Table 2 Multivariate Linear Regression of Dementia Progression Using Delta CASI |
As for the mediation analysis regarding NPSs and antipsychotic use, the NPI independently predicted fast cognitive decline in multivariate analysis, regardless of antipsychotic use putting into the model or not. In other words, NPSs, rather than antipsychotic use, leads to a faster cognitive decline. These findings are summarized in Table 3. Subgroup analysis according to antipsychotic drug classes (FGA and SGA) showed similar outcome and the results were presented in Supplemental Table 1.
 |
Table 3 Multi-Variate Linear Regression of Dementia Progression Using Delta CASI Focusing on Antipsychotic Use and NPI |
A comparison of dementia progression using delta CASI among each item of the NPI (sub-score of each NPS in substitution of NPI total score) showed that specific items (delusion, agitation, depression, anxiety, euphoria, and apathy) of the NPS significantly increased the cognitive decline of the participants, as presented in Table 4. The presence of psychosis (delusions or hallucinations) or moderate-to-severe psychosis was also associated with faster cognitive decline.
 |
Table 4 Comparison of Dementia Progression Using Delta CASI Among Each Item of NPI |
Discussion
This study shows that neuropsychiatric symptoms at entry to dementia collaborative care predicted faster cognitive decline in patients with dementia or MCI in Taiwan. Antipsychotic use did not mediate or contribute to the faster rate of decline.
Regarding the predictors of disease progression, we found that age at dementia diagnosis was associated with a faster rate of cognitive decline. This is consistent with a cohort study of patients with AD receiving anti-dementia medication treatment.32 This might possibly be due to more comorbidities in older populations.
In the era of boxed warnings of antipsychotic prescriptions for patients with dementia, both pharmacological agents and non-pharmacological techniques have shown some efficacy33 in the management of NPSs. Antipsychotic use has been reported to lead to faster cognitive decline in other study context34 but not in this cohort. We infer that this discrepancy may be due to the dementia collaborative team, particularly physicians and case managers paying attention to the safety and effectiveness of antipsychotics and caregiver support with proper education. Choosing to use antipsychotics in a similarly identifiable patient population may be based on clinical decisions and may differ within individualized levels including family factors.
The reason behind NPS being associated with faster cognitive decline can be discussed in several dimensions. Biologically, neuroanatomical correlates of persistent NPS have been proposed.35 In addition, the four major types of dementia have different NPS profiles.36 Psychologically, patients with NPSs have difficulty adhering to cognitive-enhancing activities and anti-dementia drugs. Certain NPSs have been shown to be associated with worse cognitive performance.37 Socially, NPSs are associated with caregiver burden and treatment outcomes.
Neurodegenerative diseases inevitably decline, and strategies to assist people living with dementia and their caregivers are important. Because certain NPSs independently predict faster rates of cognitive decline, a dementia collaborative team should routinely identify and ascertain the NPS for each case. More trials should be conducted on the effect of ameliorating NPS in reducing the functional progression of dementia.38
The present study has some limitations. We derived the medication list at entry into dementia collaborative care to represent antipsychotic use. As the medication review in our study was obtained at entry only, we could not address the issue of duration of antipsychotic use and defined the daily dose in more detail.
Heterogeneity exists in the declining trajectory of serial cognitive testing for each participant. In addition, collaborative dementia care involves an interventional cohort, so the composition and longitudinal effects of the intervention should also be considered. However, these long-term effects warrant further investigation.
Conclusion
This study found that certain NPSs, rather than antipsychotic use, led to faster cognitive decline in a dementia collaborative care model. The present study highlighted that delusions and mood conditions in patients were associated with faster cognitive decline. Therefore, it is essential to check and provide appropriate interventions for NPSs in patients with dementia and their caregivers.
Data Sharing Statement
The raw data supporting the conclusions of this article will be made available by the corresponding author, without undue reservation.
Ethics Approval & Patient Consent Statement
This clinical study was approved by the Institutional Review Board of Changhua Christian Hospital (CCH IRB 220920). The requirement for informed consent from each participant was waived by the institutional review board because of the retrospective chart review design. Attentions were paid on minimal risk of patient data confidentiality and the study was in compliance with the Declaration of Helsinki.
Funding
This study was supported by CCH Grants 112-CCH-IRP-020. The funding source had no role in the design of this study, execution, analyses, or interpretation of the data.
Disclosure
Yen-Jen Chen, Ming-Che Chang, Kai-Ming Jhang and Wen-Fu Wang contributed equally to this work and share first authorship. The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
1. Bhat A, Biswas A, Das G, Lahiri D, Dubey S, Mukherjee A. Behavioral variations among vascular cognitive impairment subtypes - A comparative study. Appl Neuropsychol Adult. 2021;30(4):1–8.
2. Manso-Calderón R, Cacabelos-Pérez P, Sevillano-García MD, Herrero-Prieto ME, González-Sarmiento R. The impact of vascular burden on behavioural and psychological symptoms in older adults with dementia: the BEVASDE study. Neurol Sci. 2020;41(1):165–174. doi:10.1007/s10072-019-04071-3
3. Tsai WC, Lin HC, Chang CC, et al. Neuropsychiatric symptoms in parkinson’s disease: association with caregiver distress and disease severity. Int Psychogeriatr. 2020;32(6):733–739. doi:10.1017/S1041610219001510
4. Chen YJ, Wang WF, Jhang KM, Chang MC, Chang CC, Liao YC. Prediction of Institutionalization for patients with dementia in Taiwan according to condition at entry to dementia collaborative care. J Appl Gerontol. 2022;41(5):1357–1364. doi:10.1177/07334648211073129
5. Knopman DS, Berg JD, Thomas R, Grundman M, Thal LJ, Sano M. Nursing home placement is related to dementia progression: experience from a clinical trial. alzheimer’s disease cooperative study. Neurology. 1999;52(4):714–718. doi:10.1212/WNL.52.4.714
6. Nation DA, Ho JK, Dutt S, Han SD, Lai MHC. Neuropsychological decline improves prediction of dementia beyond alzheimer’s disease biomarker and mild cognitive impairment diagnoses. J Alzheimers Dis. 2019;69(4):1171–1182. doi:10.3233/JAD-180525
7. Callahan CM, Boustani MA, Unverzagt FW, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295(18):2148–2157. doi:10.1001/jama.295.18.2148
8. Vickrey BG, Mittman BS, Connor KI, et al. The effect of a disease management intervention on quality and outcomes of dementia care: a randomized, controlled trial. Ann Intern Med. 2006;145(10):713–726. doi:10.7326/0003-4819-145-10-200611210-00004
9. Törmälehto S, Martikainen J, Bell JS, Hallikainen I, Koivisto AM. Use of psychotropic medications in relation to neuropsychiatric symptoms, cognition and functional performance in alzheimer’s disease over a three-year period: kuopio ALSOVA study. Int Psychogeriatr. 2017;29(10):1723–1733. doi:10.1017/S1041610217001090
10. Rouch I, Pongan E, Trombert B, et al. One-year evolution of behavioral and psychological symptoms of dementia in patients initially hospitalized in cognitive behavioral units: the EVITAL prospective cohort. J Alzheimers Dis. 2017;57(1):147–155. doi:10.3233/JAD-161023
11. Pitkänen A, Alanen HM, Kampman O, Leinonen E. Outcome of neuropsychiatric symptoms and daily functioning of patients with dementia treated on an acute psychogeriatric ward. Nord J Psychiatry. 2018;72(7):521–525. doi:10.1080/08039488.2018.1532021
12. Mallo SC, Patten SB, Ismail Z, et al. Does the neuropsychiatric inventory predict progression from mild cognitive impairment to dementia? A systematic review and meta-analysis. Ageing Res Rev. 2020;58:101004. doi:10.1016/j.arr.2019.101004
13. Jiang F, Cheng C, Huang J, Chen Q, Le W. Mild behavioral impairment: an early sign and predictor of alzheimer’s disease dementia. Curr Alzheimer Res. 2022;19(6):407–419. doi:10.2174/1567205019666220805114528
14. Dietlin S, Soto M, Kiyasova V, et al. Neuropsychiatric symptoms and risk of progression to alzheimer’s disease among mild cognitive impairment subjects. J Alzheimers Dis. 2019;70(1):25–34. doi:10.3233/JAD-190025
15. Krell-Roesch J, Syrjanen JA, Machulda MM, et al. Neuropsychiatric symptoms and the outcome of cognitive trajectories in older adults free of dementia: the mayo clinic study of aging. Int J Geriatr Psychiatry. 2021;36(9):1362–1369. doi:10.1002/gps.5528
16. Rosenberg PB, Mielke MM, Han D, et al. The association of psychotropic medication use with the cognitive, functional, and neuropsychiatric trajectory of alzheimer’s disease. Int J Geriatr Psychiatry. 2012;27(12):1248–1257. doi:10.1002/gps.3769
17. Honjo Y, Ide K, Takechi H. Most families tend to realize progress of alzheimer’s disease when behavioural and psychological symptoms are obvious. Psychogeriatrics. 2022;22(3):317–323. doi:10.1111/psyg.12815
18. Chen CT, Chang CC, Chang WN, et al. Neuropsychiatric symptoms in alzheimer’s disease: associations with caregiver burden and treatment outcomes. Qjm. 2017;110(9):565–570. doi:10.1093/qjmed/hcx077
19. Chen YJ, Jhang KM, Wang WF, Lin GC, Yen SW, Wu HH. Applying apriori algorithm to explore long-term care services usage status-variables based on the combination of patients with dementia and their caregivers. Front Psychol. 2022;13:1022860. doi:10.3389/fpsyg.2022.1022860
20. Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9(Suppl 1):173–6; discussion 7–8. doi:10.1017/S1041610297004870
21. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. doi:10.1212/WNL.44.12.2308
22. Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233–239. doi:10.1176/jnp.12.2.233
23. Jhang KM, Chang MC, Lo TY, Lin CW, Wang WF, Wu HH. Using The Apriori Algorithm To Classify The Care Needs Of Patients With Different Types Of Dementia. Patient Prefer Adherence. 2019;13:1899–1912. doi:10.2147/PPA.S223816
24. Jhang KM, Wang WF, Chang HF, Liu YH, Chang MC, Wu HH. Care needs of community-residing male patients with vascular cognitive impairment. Neuropsychiatr Dis Treat. 2020;16:2613–2621. doi:10.2147/NDT.S277303
25. Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6(1):45–58; discussion 62. doi:10.1017/S1041610294001602
26. Defrancesco M, Marksteiner J, Kemmler G, et al. Specific neuropsychiatric symptoms are associated with faster progression in alzheimer’s disease: results of the prospective dementia registry (PRODEM-Austria). J Alzheimers Dis. 2020;73(1):125–133. doi:10.3233/JAD-190662
27. Edwin TH, Strand BH, Persson K, Engedal K, Selbæk G, Knapskog AB. Trajectories and risk factors of dementia progression: a memory clinic cohort followed up to 3 years from diagnosis. Int Psychogeriatr. 2021;33(8):779–789. doi:10.1017/S1041610220003270
28. Eldholm RS, Barca ML, Persson K, et al. Progression of alzheimer’s disease: a longitudinal study in Norwegian memory clinics. J Alzheimers Dis. 2018;61(3):1221–1232. doi:10.3233/JAD-170436
29. Haaksma ML, Rizzuto D, Leoutsakos JS, et al. Predicting cognitive and functional trajectories in people with late-onset dementia: 2 population-based studies. J Am Med Dir Assoc. 2019;20(11):1444–1450. doi:10.1016/j.jamda.2019.03.025
30. Sung PS, Lee KP, Lin PY, et al. Factors associated with cognitive outcomes after first-ever ischemic stroke: the impact of small vessel disease burden and neurodegeneration. J Alzheimers Dis. 2021;83(2):569–579. doi:10.3233/JAD-210587
31. Kueper JK, Speechley M, Lingum NR, Montero-Odasso M. Motor function and incident dementia: a systematic review and meta-analysis. Age Ageing. 2017;46(5):729–738. doi:10.1093/ageing/afx084
32. Yang YH, Wu MN, Chou PS, Su HC, Lin SH, Sung PS. Longitudinal neuropsychological outcome in Taiwanese alzheimer’s disease patients treated with medication. Curr Alzheimer Res. 2018;15(5):474–481. doi:10.2174/1567205014666171010112518
33. Tampi RR, Bhattacharya G, Marpuri P. Managing Behavioral and Psychological Symptoms of Dementia (BPSD) in the era of boxed warnings. Curr Psychiatry Rep. 2022;24(9):431–440. doi:10.1007/s11920-022-01347-y
34. Oh ES, Rosenberg PB, Rattinger GB, Stuart EA, Lyketsos CG, Leoutsakos JS. Psychotropic medication and cognitive, functional, and neuropsychiatric outcomes in Alzheimer’s Disease (AD). J Am Geriatr Soc. 2021;69(4):955–963. doi:10.1111/jgs.16970
35. Poulin SP, Bergeron D, Dickerson BC. Risk factors, neuroanatomical correlates, and outcome of neuropsychiatric symptoms in alzheimer’s disease. J Alzheimers Dis. 2017;60(2):483–493. doi:10.3233/JAD-160767
36. Kazui H, Yoshiyama K, Kanemoto H, et al. Differences of behavioral and psychological symptoms of dementia in disease severity in four major dementias. PLoS One. 2016;11(8):e0161092. doi:10.1371/journal.pone.0161092
37. Lü W, Duan J, Zhang W, Yang W, Yu W. Relationship between neuropsychiatric symptoms and cognitive functions in patients with cognitive impairment. Psychogeriatrics. 2021;21(5):773–782. doi:10.1111/psyg.12738
38. Cheng ST, Li KK, Or PPL, Losada A. Do caregiver interventions improve outcomes in relatives with dementia and mild cognitive impairment? A comprehensive systematic review and meta-analysis. Psychol Aging. 2022;37(8):929–953. doi:10.1037/pag0000696
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
