Back to Journals » Nature and Science of Sleep » Volume 15
Investigating the Role of BDNF in Insomnia: Current Insights
Authors Ditmer M, Gabryelska A, Turkiewicz S, Sochal M
Received 28 July 2023
Accepted for publication 28 November 2023
Published 7 December 2023 Volume 2023:15 Pages 1045—1060
DOI https://doi.org/10.2147/NSS.S401271
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Valentina Alfonsi
Marta Ditmer,* Agata Gabryelska,* Szymon Turkiewicz, Marcin Sochal
Department of Sleep Medicine and Metabolic Disorders, Medical University of Lodz, Lodz, 92-215, Poland
*These authors contributed equally to this work
Correspondence: Marcin Sochal, Department of Sleep Medicine and Metabolic Disorders, Medical University of Lodz, Lodz, 92-215, Poland, Tel +48 661 475 310, Email [email protected]
Abstract: Insomnia is a common disorder defined as frequent and persistent difficulty initiating, maintaining, or going back to sleep. A hallmark symptom of this condition is a sense of nonrestorative sleep. It is frequently associated with other psychiatric disorders, such as depression, as well as somatic ones, including immunomediated diseases. BDNF is a neurotrophin primarily responsible for synaptic plasticity and proper functioning of neurons. Due to its role in the central nervous system, it might be connected to insomnia of multiple levels, from predisposing traits (neuroticism, genetic/epigenetic factors, etc.) through its influence on different modes of neurotransmission (histaminergic and GABAergic in particular), maintenance of circadian rhythm, and sleep architecture, and changes occurring in the course of mood disturbances, substance abuse, or dementia. Extensive and interdisciplinary evaluation of the role of BDNF could aid in charting new areas for research and further elucidate the molecular background of sleep disorder. In this review, we summarize knowledge on the role of BDNF in insomnia with a focus on currently relevant studies and discuss their implications for future projects.
Keywords: sleep, insomnia, brain-derived neurotrophic factor
Introduction
Sleep is vital to cognitive performance and overall health, and sleep disturbances are known to significantly decrease life quality as well as affect the course of other diseases. For example, sleep disorders have been demonstrated to be a risk factor for disease relapse in ulcerative colitis patients.1 In a South Korean study, patients with sleep disorders had a higher risk of developing immunomediated disorders, such as alopecia areata, Graves disease, or Hashimoto thyroiditis.2 According to Young et al, sleeping <7 hours per night puts individuals at a higher risk of developing systemic lupus erythematosus.3 Interestingly, Sang et al found no association between genetically determined insomnia (125 single-nucleotide polymorphisms identified by the authors) and systemic lupus erythematosus, which suggests that the relationship between the two is dynamic and could involve external factors, such as lifestyle and environment.4 Poor sleep quality is also an important risk factor for depression relapse.5
The American Academy of Sleep Medicine defines insomnia disorder as frequent and persistent difficulty initiating, maintaining, or going back to sleep in spite of the right conditions, which results in nonrestorative sleep.6 Insomnia disorder is usually associated with some level of impairment in other domains of life. The cutoff point between chronic and short-term insomnia is 3 months, and symptoms should occur at least 3 times a week.6 Insomnia prevalence varies between studies. Depending on the population and assumed diagnostic criteria, it appears to be 10%–30%.7 It can be a primary disease or a symptom of another condition, such as restless leg syndrome (RLS) or periodic leg movements during sleep. Insomnia also frequently accompanies psychiatric disorders and immunomediated diseases.8–10
The causes of insomnia remain elusive. Insomnia might have genetic predisposing factors, as familial aggregation has been observed.11 Insomnia appears to be tightly related to stress-response and coping mechanisms. The term “sleep reactivity” describes individual propensity to sleep disruptions in response to stressful situations. This trait alongside neuroticism is a risk factor for insomnia development.11,12 Currently, one of the most popular models of insomnia, developed by Spielman et al in 1987, identifies three types of factors at play: predisposing, precipitating, and perpetuating.13
The cornerstone of insomnia treatment is cognitive–behavioral therapy (CBT). CBT focuses on developing certain adaptive strategies, like control of stimuli, relaxation techniques, and coping with stress.14 This approach is effective and results are sustainable over time.14 In their guidelines, the European Sleep Research Society also approves the use of some antidepressants (eg, doxepin, mianserin, mirtazapine, trazodone), benzodiazepine-receptor agonists (Z-drugs), and benzodiazepines.15 Commonly used antihistamines and melatonin are not recommended due to the low quality of available evidence in favor of their use.15
BDNF is a neurotrophin ubiquitous in the central nervous system (CNS), particularly in the prefrontal cortex and the limbic regions, with the highest levels detected in the hippocampus.16,17 Outside the CNS it is also found in the heart, lungs, and liver.17 As a neurotrophic factor, BDNF is responsible for growth and development of neurons, promoting neuro- and gliogenesis.18 BDNF also has antiapoptotic properties; it stimulates the sonic hedgehog and erythropoietin and promotes the expression of Bcl-2 proteins, which together prevent cell death.19 Neurotrophin might also exert antioxidative and antiautophagy effects through ERK1/2 and PI3K/Akt pathways, respectively.19
BDNF is vital for neuroplasticity: it affects the development of new synapses by modulating synaptic arborization and morphogenesis, thus promoting formation of new neuronal circuits and improving cognitive function.20 It also controls glutamatergic transmission by regulating the activity and expression of N-methyl-
Disruptions in BDNF signaling have long been associated with psychiatric disorders, such as dementia or depression, which are frequently accompanied by insomnia.21,22 Patients with Alzheimer’s disease have been shown to have lower levels of this neurotrophin in brain, blood, and cerebrospinal fluid. Moreover, increased serum BDNF was associated with better cognitive faculties in this group.23 Similar observations have been made in patients with major depressive disorders: treatment with antidepressant drugs or electroconvulsive therapy brought about significant improvements in BDNF levels.24 BDNF might also be directly involved in the course of insomnia.18 It shows a strong connection to sleep architecture, the sleep–wake cycle, and comorbidities of this sleep disorder.18
This review aims to investigate the role of BDNF in insomnia and other selected subjects related to this sleep disorder (eg, circadian rhythm, neurodenerative conditions), with a focus on currently relevant studies. We discuss the relationship between BDNF, the sleep–wake cycle, circadian rhythm, the basis of an inherent propensity to insomnia, and the interplay between stress response and the titular neurotrophin.
Neurobiology of BDNF
BDNF was discovered in 1982 by Yves Barde and Hans Thoenen.25 It is a member of the neurotrophin family, and its structure bears a high degree of similarity to other neurotrophins, such as nerve growth factor (NGF), neurotrophin 3, and neurotrophin 4/5. The BDNF gene is located on the 11p chromosome.18 It has five exons, with only the 3’ exon coding for the BDNF protein.18 Transcription of the remaining is tissue-dependent, with exons I–III dominating in different regions of the central nervous system, mainly the cortex and the hippocampus.18
BDNF’s cognate receptor is tropomyosin receptor kinase B (TrkB).18,26 Upon binding of the ligand, TrkB dimerizes and recruits other pathways, such as the MEK–MAPK pathway, ERK pathway, PLCγ pathway, and PI3K–Akt–mTOR pathway.18 The PI3K–Akt–mTOR pathway might be of particular importance to the prosurvival effect BDNF has on neurons, as it is associated with autophagy and has been demonstrated to protect neuronal cells from hypoxic damage.18,27 BDNF might also attenuate activation of microglia, preventing them from releasing proinflammatory cytokines and toxins that could damage neurons.28
BDNF is one of the major factors contributing to synaptic plasticity, which is strengthening or weakening synaptic connections, a process underlying learning or memory formation.29 Inhibition of this pathway has been shown to reduce synaptogenic activity of the synapsin I and NMDAR pathway.29 BDNF-knockout animals display decreased synaptic density to sympathetic neurons.30 In contrast, stimulation of the BDNF pathway promotes dendrite arborization, while dendrite branching is enhanced by TrkB and MAPK pathway–mediated cypin expression.31–35
The BDNF pathway is strongly connected to glutamatergic neurotransmission. It has been demonstrated to regulate the amount of glutamate released to the synapse via MAPK-dependent synapsin I phosphorylation, as well as an increase in intracellular Ca2+ concentration.36–38 It also interacts with the postsynaptic glutamate receptors NMDAR and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, promoting their phosphorylation.39–41 It might exert a protective influence against glutamate excitotoxicity via the Ras–MAPK and PI3K–Akt pathways.42
BDNF is also connected to γ-aminobutyric acid (GABA) signaling. It controls the expression of the GABA synthetic enzyme GAD65.43 BDNF also inhibits endocytosis of GABAAR, a metabotropic GABA receptor, via PI3K, including its downstream target PP2A and PKC pathways.43 ProBDNF has the opposite effect, promoting endocytosis of GABAAR β3 subunits through the RhoA–Rock–PTEN pathway.43 As already mentioned, a majority of neurons in the suprachiasmatic nucleus (SCN) are GABAergic; therefore, it is possible that BDNF fulfills there a major role controlling communication between cells.
It could be suspected that orexin signaling is related to BDNF. This neuropeptide was demonstrated to prompt the expression of the neurotrophin via activation of the OX1R-related pathways: PI3K and PKC.44 Interestingly, mice bereft of another orexin receptor, OX2R, were shown to have more depressive behavior than healthy ones. This could be caused by an in increase in BDNF expression mediated by OX2R-mediated stimulation of the CREB pathway.44,45 Due to the importance of orexin for histaminergic signaling as well as wakefulness, future studies on the role of BDNF in the context of discussed neuropeptides are validated.
BDNF and Sleep Regulation
Sleep is a complex, involving specific brain activity, which can be shown on electroencephalography (EEG). Sleep architecture is a term describing changes in the EEG patterns seen through the night.46 Two main stages of sleep are non–rapid eye movement (NREM) and REM phases.46 NREM can be further divided into three stages, N1–N3: N2b and N3 are described as slow-wave sleep (SWS).46 Apart from eye movement, those two distinctly differ in terms of muscle tone and heart and respiratory rate.46 Slow-wave activity (SWA) is characterized by EEG power between 0.5 and 4 Hz.46 Sleep progresses in cycles between those phases, usually four to five per night.46
Sleep architecture varies on an individual basis, depending on factors like age, sex or drug use.46 Its alterations have been observed in numerous conditions, including insomnia.46–49 Even though polysomnography is not a diagnostic method for insomnia, changes in sleep architecture might affect one’s perception of sleep duration and its quality, and thus factors shaping sleep structure need to be studied.46 Literature on the subject shows that BDNF might modulate sleep structure. Intraventricular administration of BDNF was demonstrated to increase the duration of both REM and NREM sleep in rabbits, but only NREM in rats.50 What is interesting is that changes in sleep architecture in mice with decreased BDNF production due to heterozygosity negatively affect only REM sleep, while NREM is unaltered.51 This includes decreased overall REM duration and number of phases and increased REM initiation.51
A decrease in BDNF production caused by the Val66Met BDF single-nucleotide polymorphism rs6265, present in 25%–30% of the population, is associated with prolonged initiation and termination of SWA (a type of EEG oscillation present primarily during NREM that is enhanced proportionally by the duration of wake time preceding sleep, a marker of sleep need).52–54 Moreover, those individuals display reduced SWS duration and sleep activity (as measured by delta activity).54 In a study on patients with major depressive disorder, it was revealed that upregulated BDNF production was linked to more intense SWA and higher NREM sleep quality; however, the level of this neurotrophin did not bear any relation to insomnia in this group.55
The mechanism behind BDNF’s action on sleep architecture remains elusive. Some researchers maintain it has little, if any, influence on SWA.56 The mode of BDNF administration seems to be an important modulator of the effects exerted by this protein. Intracortical administration has been shown to enhance SWA, while intraventricular BDNF injections led to SWA reduction.50 It is plausible that the intraventricular mode of injection affect other structures controlling sleep.57 Faraguna et al posited that BDNF is the interface between synaptic plasticity and SWA.57 In a study on rats, animals who displayed more exploratory behavior had higher SWA than their less inquisitive counterparts.57 Moreover, SWA, BDNF production, and exploratory behavior were positively correlated.57
In contrast to the aforementioned studies, Watson et al hypothesized that the BDNF pathway has a greater role in modulation of REM sleep, rather than NREM.50 Mice lacking a form of BDNF receptor TrkB T1 (truncated isoform without the tyrosine kinase domain), did not exhibit any changes in NREM, but REM sleep in this group was profoundly distorted. The researchers noted an increase in REM duration, decrease in REM sleep latency, and sleep fragmentation.50 Those changes resembled the ones seen in patients with depression.50 In vivo inhibition of the BDNF pathway brought contrasting results, decreasing the number of REM duration and episodes.58 BDNF might also be involved in regulation of REM sleep drive: mice with decreased BDNF production after selective REM sleep deprivation do not experience rebound REM sleep.51 This is in line with other studies, which showed that receptors for BDNF can be found in GABAergic and glutamatergic neurons of the midbrain and the brain stem important for sleep regulation. The pedunculopontine tegmentum, located in the upper brain stem, in particular was demonstrated to be vital for REM drive.51,59
BDNF and Circadian Rhythm
Circadian rhythm is a biological clock that follows a 24-hour cycle. It regulates alertness/sleepiness, metabolism, behavior, etc.60,61 The role of the central master clock is fulfilled by the SCN, which coordinates secondary peripheral oscillators in the tissues.60 When endogenous circadian rhythm is altered or there occurs a misalignment between external factors and sleep–wake cycle (eg, shift work), circadian rhythm sleep disorder (CRSDs) might develop.62 These include conditions like advanced/delayed/irregular sleep–wake phase disorder or shift-work sleep disorder. Due to similar symptomatology to insomnia, there are discussions on whether they should be classified together or regarded as a separate group of sleep disturbances. This review assumes the approach of the American Academy of Sleep Medicine, which claims that CRSDs can generate insomnia.63 Nevertheless, thorough differential diagnosis between CRSDs and primary insomnia is necessary in order to apply proper treatment.
The pathophysiology of CRSDs is complex. Research has demonstrated that polymorphisms in clock genes like PER3 or CLOCK might contribute to a susceptibility to sleep disorders.64–66 External factors appear to play a role as well, such as with shift-work sleep disorder.67 Due to its interactions with the circadian rhythm, BDNF might partake in the pathophysiology of CRSDs; however, the literature on this subject remains limited. It has been evidenced, that this neurotrophin and its receptor TrkB are expressed in the SCN.68 As for BDNF, D’Agostino et al showed that it could be indispensable in the maintenance of circadian oscillators: BDNF-knockout zebrafish showed severe impairments in clock gene expression.69 Studies demonstrate that this neurotrophin displays circadian rhythmicity. Piccini et al and Choi et al observed this relationship only in men.70–72 However, in a study by Cain et al, as many as three in four women and one in two men had BDNF circadian rhythmicity.70 The BDNF peal was unrelated to clock time in women, as opposed to men.70 In a study on healthy male volunteers, Begliuomini et al showed that plasma levels of BDNF and cortisol are positively correlated and peak early in the day to later decrease.73
Melatonin is a hormone produced in the pineal gland modulating the adjustment of circadian rhythm to the photoperiod. Jang et al showed that the melatonin precursor N-acetylserotonin might activate TrkB receptors in a circadian-dependent manner, with peaks during the biological night.63 This indolamine was shown to increase BDNF expression in animal models.74 Nevertheless, a recent meta-analysis did not confirm the same effect in humans.75 Future research on the subjects of interplay between the circadian rhythm, melatonin, and BDNF would be desirable. Organic and functional aspects of changes in the circadian rhythm occurring in the course of various CRSDs might be further studied in the context of BDNF and other neurotrophins.
BDNF and Stress
Stress can exert various effects on health, depending on its predictability, intensity, and duration. The importance of stress-induced hyperarousal (in emotional–cognitive and somatic aspects) has long been suggested to play a role in the pathophysiology of insomnia.12 It could be hypothesized that coping strategies (cognitive and behavioral) and sleep reactivity affect the risk of developing insomnia. Studies seem to confirm this. Otsuka et al demonstrated that insomnia is more prevalent in individuals with maladaptive behavioral patterns (ie, substance use, self-blame).76 Other researchers obtained similar results.77,78 Insomnia is also an important diagnostic symptom in anxiety disorders: approximately 70%–90% of these patients suffers from this sleep disorder.79 As Neckelmann et al found, insomnia might also be a risk factor for anxiety disorder (OR 4.9, 95% CI 3.8–6.4).80
This system of sleep regulation might be disrupted by hyperarousal, an element strongly emphasized by insomnia models proposed in recent decades. Acute stress, a usual trigger of insomnia, was related to the increased activation of both sleep- and wake-promoting neurons, which under physiological conditions should be switched off.81 The state of hyperarousal is difficult to exhaustively define: it might involve numerous components, like neurotransmitters, the endocrine system, and genetics.82 Riemann et al in their work adopted a holistic view of this state, describing it as affecting cognitive–emotional, cortical, and physiological domains of functioning.83 Even in the early studies, it was observed that insomnia individuals tend to display signs of enhanced metabolism, like increased body temperature, level of adrenaline, cortisol, and heart rate.82 Hyperarousal in chronic insomnia might also be associated with secondary maladaptive behaviors developed in response to acute insomnia caused by a stressful life event.83 These include rumination about adverse effects of currently experienced sleep disorder, focus on daytime insomnia symptoms, and drug/alcohol abuse, which further perpetuate the sleep disorder, creating a vicious circle.83
Glucocorticoids can cross the blood–brain barrier and fulfill important functions within the central nervous system.84 Under physiological conditions, they participate in the formation and stabilization of new synapses, as well as synaptic pruning.85 Pathological excess of glucocorticoids, like in Cushing’s disease, is well known to have psychiatric consequences, such as mood disorders.86 Individuals afflicted with this disease might also experience regressive changes in the hippocampus and the prefrontal cortex, eg, loss of synaptic density or dendrite arborization.86 According to a recent meta-analysis, patients with insomnia also tend to have an increased level of peripheral cortisol as well as disturbed rhythm of secretion of this protein (ie, normal morning cortisol, increased during daytime and before bedtime), which demonstrated dysregulation of the HPA axis in this group.87 Products of the HPA axis were demonstrated to alter BDNF production and pathway, which might suggest that their negative effects on neurons could be mediated by changes in activity of this neurotrophin.84,88–90
In a study on rats subjected to chronic stress, an increase in hippocampal, hypothalamic, and pituitary BDNF as well as plasmatic corticosterone in comparison with healthy controls was noted. Other studies with similar stress protocols showed that BDNF mRNA level was decreased in the hippocampal region.91 Those results suggest that alterations in BDNF production are posttranscriptional; however, none of the BDNF precursor forms was measured.91 The authors suggested that such alterations might aid in adaptive processes of the HPA axis due to BDNF’s role in synaptic plasticity.91 This neurotrophin could also participate in restoration of cortisol reserves, depleted during stress.91 What is interesting is that a pattern of time-dependent changes in BDNF levels in the pituitary and hypothalamus in response to an acute stressor were similar in the study group and controls; however, in the hippocampus, an opposite dynamic was observed, with significant decreases in the study group.91 Such an effect could explain atrophy of C3 neurons, observed in chronic stress.91
In a study on suicide attempters, those who had a dysregulated HPA axis (ie, did not experience suppression of cortisol production in the dexamethasone suppression test) showed a negative correlation between posttest cortisol level and plasma BDNF.92 This association was present only in women, which might to some extend elucidate the mechanisms behind differences in suicidal behavior between sexes.92 On the other hand, in a study on subjects with burnout, a psychosomatic disorder thought to stem from working in a high-stress environment, no significant relationship between BDNF and cortisol was noted.93
Another subject worth mentioning in the context of interactions between BDNF and stress is anxiety disorders. There are reports of associations between the Val66Met polymorphism in the BDNF gene and the conditions discussed herein; however, studies on the subject have brought conflicting results.94 According to a meta-analysis by Suliman et al, the level of this neurotrophin on the periphery is reduced in this group of patients, but this decrease is not uniform across the spectrum of anxiety disorders. Obsessive–compulsive disorder appeared to be responsible for BDNF reduction.94
Next to catecholaminergic neurons and glucocorticoid axes, dopaminergic neurons are an important element of stress response.95 The two important neuronal circuits that participate in adaptation to stress are the mesolimbic (reward) and corticolimbic (emotion) system. A10 dopamine-containing neurons are located in the ventral tegmental area (VTA), a part of the mesolimbic system that is responsible for reward, motivation, and executive and affective functions.95 Dopaminergic neurons from the VA project, among others, to the prefrontal cortex (PFC), amygdala, and nucleus accumbens (Nac).88 The mesolimbic system is connected to the corticolimbic system in numerous locations, like the PFC, the hippocampus, the VTA, and the Nac, and is considered to be the major interface between the limbic system and motor regions of the cortex.88 Proper functioning and synchrony between these two systems allows for value-based learning and selection of action based on predicted consequences.88 Impairments of the mesocorticolimbic system have been linked to drug abuse, addiction, and depression.88 The relationship between substance abuse and sleep disorders might be a particularly interesting subject for future research, as it appears to have a bidirectional character: sleep complaints are among the most common in subjects who have a history of addiction, and patients with sleep disorders are at a high risk of developing problems with drugs or alcohol, as they try to self-medicate insomnia.96
Based on studies on human brains and animal models, it appears that BDNF/TrkB expression is decreased in the corticolimbic system, inhibiting BDNF-mediated neuroplasticity in this region; the opposite occurs in the mesolimbic system.88 Those alterations might increase the neuronal plasticity in the mesolimbic region, rendering individuals more prone to social stress or substance addiction.88 It could be suspected that alterations in the BDNF signaling seen in insomnia could impair proper adaptive response to stress and instead facilitate the development of maladaptive habits, which would further exacerbate already existing problems with sleep, creating a vicious circle. The relationship between stress, BDNF signaling, and insomnia needs to be perceived as mutual. Disruption in one of those elements, caused by eg, trauma, could precipitate impairment of the others.
BDNF and Sleep Deficiency
Sleep deficiency might have either a chronic or acute character, which seem to have opposed effects on the organism. It might be conceptualized as a disorder in its own right, as it accompanies a vast number of conditions. Studies conducted up to this point on the subject of changes in peripheral BDNF level in insomnia consistently show BDNF reduction in individuals afflicted with this sleep disorder, regardless of sex or accompanying psychiatric conditions; findings are summarized in Table 1.16 Among all characteristics of insomnia, ie, troubles falling asleep or maintaining this state, early-morning awakenings, etc., BDNF might be the most related to short sleep duration. According to Furihata, there were no differences between individuals with normal sleep duration (above 6 hours), regardless of the presence of insomnia.97 However, in a group with short (<6 hours) sleep duration, BDNF levels were significantly lower only in subjects with insomnia.97 In a study by Giese et al, BDNF concentration was negatively correlated with the severity of insomnia symptoms, as measured with the insomnia severity index (a scale based on DSM-IV criteria) and fatigue.98 Moreover, in their study there were no differences in BDNF concentration between individuals diagnosed with RLS/periodic leg movements during sleep and those without.98 RLS medication (ropinirole, antidepressants, etc.) did not affect the level of neurotrophin, which suggests that sleep disorders other than insomnia might not impact BDNF production.98 There could be some variability depending on the biological material used in the study, ie, serum or plasma. About 99% of circulating BDNF is accumulated in platelets, which release it during coagulation.99 Thus, in terms of serum, clotting time is crucial to results.99 Trajkovska et al observed a decrease in serum BDNF in probes stored >6 months.100 BDNF concentration might also differ on an individual basis, depending on sex, age, or physical activity.99 In subjects with insomnia and short sleep duration (<6 hours), serum level of BDNF was positively correlated with neuropsychological faculties measured with tests on spatial span and brief visuospatial memory and the identical pair test.101
 |
Table 1 Summary of findings of studies on the subject of BDNF in insomnia |
Acute sleep deficiency, ie, sleep deprivation, might increase BDNF both systemically in plasma/serum and locally in certain brain regions, eg, in the hippocampus.20,107 It appears to be more related to lack of NREM sleep, as isolated REM sleep deprivation in general does not seem to strongly affect production of this neurotrophin.107 However, there are studies showing elevated BDNF in the brain stem following REM sleep deprivation.20,108
A causal relationship between different types of sleep deficiency and BDNF used to be explained in the context of the biphasic model of stress, which is tightly connected to glutamate signaling in the prefrontal cortex.109 Namely, acute stress (ie, sleep deprivation) enhances glutamatergic signaling in the PFC.109 This process takes place thanks to genomic and nongenomic mechanisms, triggered by activation of the MR–GR pathway by corticosterone.109 Intensification of glutamatergic transmission might promote further adaptive changes in function, morphology, and number of synapses, promoting working memory and adaptation.109 Long-term stress (ie, insomnia) has opposite effects, producing reduction of dendritic complexity and atrophy, which might ultimately severely impair behavior.109
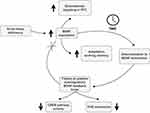
Nevertheless, this model does not fully answer the question of why chronic sleep deficiency actually leads to BDNF depletion. As shown in Figure 1, BDNF, due to a positive autoregulatory feedback loop is able to stimulate its own transcription in a CREB-mediated mechanism, as well as TrkB surface expression. Additionally, TrkB might be transactivated from adrenergic, dopaminergic or glucocorticoid activity in a manner similar to that caused by neurotrophins.16 Interestingly, it appears that prolonged BDNF exposure might actually lead to a decrease in TrkB expression on the cell surface, leading to desensitization, which could ultimately reduce BDNF production.110,111 Further studies on the BDNF pathway in insomnia would be desirable to elucidate the time point as well as exact mechanism of BDNF depletion in this sleep disorder.
BDNF is primarily produced by neurons; however, studies have shown that it can also be released by astrocytes and microglia. Zhang et al found that insomnia was associated with increased serum level of S100B, GFAP, BDNF, and GDNF, which could suggest astrocyte dysfunction.104 In another study, both short sleep deprivation and chronic sleep restriction were shown to increase the rate of astrocytic phagocytosis involving presynaptic parts of larger synapses.112 The pattern of changes in lipid peroxidation in synaptoneurosomes and the expression of MERTK, the gene responsible for astrocytic phagocytosis, was also similar, and thus authors concluded this was a cleaning of overused synaptic components during wake time.112 However, in contrast to SD, chronic sleep restriction was associated with microglial activation and phagocytosis without other overt signs of neuroinflammation.112 Such low-level, long-term microglial activation (microglial priming) could be associated with pathologically enhanced response to other insults, thus being detrimental to the brain.112 Those results show that alterations in morphology and functioning of glial cells might be relevant to the pathophysiology of insomnia, and further studies on this subject would be highly desirable.
Obstructive sleep apnea (OSA) is a common disorder characterized by recurrent collapse of the upper airways during sleep, causing intermittent hypoxia.99 This condition is associated with sleep fragmentation caused by frequent subconscious awakenings, which could be considered a form of sleep deficiency. BDNF has been quite extensively studied in the context of OSA, primarily due to its connections with cognitive decline.99 Studies conducted to date are heterogeneous.99 A recent meta-analysis demonstrated that peripheral levels of BDNF are unaltered by OSA or its standard treatment—continuous positive airway pressure therapy.113
Molecular Basis of Insomnia
Insomnia is a disorder with a complex background, involving interactions between psychological and somatic aspects of health. It frequently overlaps with disrupted BDNF production, as is the case in depression or obsessive–compulsive disorder.94,114 Several systems have been implicated in the pathophysiology of insomnia, one of them being GABA signaling. Besides having a major role in sleep initiation and maintenance, it is also a target for insomnia medications (ie, benzodiazepines), which increase the affinity of GABA receptors.115 According to a study by Morgan et al, individuals with primary insomnia tend to have increased levels of this neurotransmitter in the occipital region, as measured by proton magnetic resonance spectroscopy (1H-MRS); moreover, GABA was negatively correlated with a polysomnographic parameter—wake after sleep onset.116 The authors explained this as an allogenic response to hyperarousal.116 However, Plante et al obtained opposite results, showing stark reductions of GABA in the brain.115 Discrepancies between studies could be attributed to, among others, timing of data acquisition, differences in sleep quality between respective control groups, as well as method of objective sleep assessment (polysomnography or actigraphy).115 Oral administration of GABA might show benefits related to early stages of sleep, ie, increased duration of the first NREM, and decreased sleep latency, but they might be related to its stress-relieving effects.117 Glutamatergic neurotransmission is tightly connected to GABA. Glutamate is an excitatory neurotransmitter, which might be converted into GABA by glutamate decarboxylase 1, thus supplying inhibitory synapses.118 L-glutamate in atoxic concentrations might be also found extrasynaptically in the area surrounding neurons.119 The relationship between insomnia and glutamatergic transmission is still under investigation. It appears to be of special importance in neurodegenerative disorders, which are linked to glutamate excitotoxicity.120 Glutamate signaling has a major role in regulation of circadian rhythm by regulating the functioning of the SCN, the central master clock.121 A majority of neurons in this structure are GABAergic; GABA release might be promoted by astrocytic glutamate via activation of the NR2C subunit of the glutamate NMDAR, which inhibits activation of postsynaptic neurons.121 Selective NR2C blockade impairs the circadian variability of neuronal membrane potential as well as expression of clock genes.121 Glutamate has two main transporters that regulate its working concentration in the brain: vesicular glutamate transporters (VGLUTs) and excitatory amino acid transporters (EAATs).121 In animal models, alteration of EAAT1 expression changed the sleep phenotype in Drosophila, whereas injection of glutamate into SCN induced phase shifts.121 Such disruptions might negatively affect the functioning of the Zeitgeber and contribute to the development of sleep disruptions associated with impaired circadian rhythm.121 Models involving sleep deprivation give further insight into the link between sleep and mode of neurotransmission. Sleep deprivation in mice was demonstrated to increase the expression of GLT1, which removes excessive glutamate.121 Rats subjected to this form of sleep deficiency had a higher concentration of extrasynaptic glutamate.121 Increase in BDNF following acute sleep deprivation might be of importance here, as it was shown to protect neurons against excitotoxicity and oxidative damage.17 Interestingly, in a study on mice subjected to chronic sleep restriction, the study group sustained less damage following the injection of NMDA (NMDAR agonist used to mimic the actions of glutamate) to the nucleus basalis magnocellularis.122 Thus, it appears that there are mechanisms protecting the central nervous system from the damage inflicted by deregulation of the glutamatergic system associated with different forms of sleep deficiency, but this subject remains underinvestigated. More studies are validated to broaden the knowledge on the topic of interactions between glutamatergic signaling and insomnia.
Histamine is also considered to be an important neurotransmitter in the pathophysiology of insomnia. In the brain, it is produced only by the tuberomamillary nucleus (TMN); it acts as an REM suppressant and promotes wakefulness.118 It has three main receptors: stimulatory H1, H2, and inhibitory H3. H1 and H3 appear to be of the most relevance for sleep. H1 antagonists (or rather inverse agonists, reducing the activity of constitutively active receptors) are known to cause sleepiness, such as diphenhydramine.118 Pharmacological inhibition of H1 in rats increased NREM; animals bereft of this receptor acted in a soporific way when compared to the control group.118 Substances that disturb H3 signaling (ie, ciprofloxacin) were shown to suppress sleep in animal studies.118 Interestingly, other studies on animals with impaired histaminergic signaling have demonstrated that such damage might be to some extent compensated by other arousal systems, but those mechanisms fail when a higher level of arousal is required, such as in high-stress environments.118 Studies regarding regulation feeding behavior also bring interesting results. Gotoh et al have demonstrated that food intake might be modulated by interactions between BDNF, corticotropin-releasing factor and histamine.123 Namely, BDNF in the ventromedial nucleus (VMH) regulates histamine production in the TMN via activation of corticotropin-releasing factor (CRF) neurons in the paraventricular nucleus (PVN).123 The released histamine in turn inhibits different groups of feeding neurons and BDNF neurons located in the VMH and PVN via the H1 receptor.123 Weight gain caused by the use of second-generation antipsychotic drugs was also associated with BDNF concentration in the hypothalamus and on the periphery.124 Similar studies appear to be needed on the subject of disruptions of sleep and circadian rhythm.
Orexins are produced by neurons in the hypothalamic region.125 They project to various locations in the brain that contain structures important for sleep regulation, such as the ventrolateral preoptic nucleus (VLPO), locus coeruleus (LC), and the TMN.125 Orexin neurons control the release of histamine form the TMN neurons; notably, there is no feedback loop, as histamine does not influence orexin neurons.125 Current medications targeting this mode of neurotransmission might be classified into two types: dual orexin receptor antagonist (DORA), antagonists of both orexin receptor type 1 (OX1R) and OX2R, which include relatively popular lemborexant and suvorexant, and selective orexin receptor antagonist (SORA), targeting only OX2R, such as seltorexant.125 The latter group is still under development.125 To date, studies report that they decrease sleep latency as well as wake after sleep onset, while increasing total sleep time.125 Orexin might also promote BDNF production, thus exerting neuroprotective effects on neurons.44
Saper et al have proposed a flip-flop switch model of sleep regulation, presenting a neuronal pathway consisting of sleep-promoting GABAergic neurons in the VLPO that cooperate with neuronal pathways promoting the wakeful state, namely the orexinergic neurons in the hypothalamus, noradrenergic LC, histaminergic TMN, serotonergic dorsal raphe nuclei (DRN) as well as cholinergic neurons in the pontine laterodorsal and pedunculopontine tegmental (LDT/PPT) nuclei.126,127 The ascending reticular system in the brain is also vital for cortical arousal.127 Alternations between phases are controlled by the switch located in the thalamus.127
Insomnia is currently regarded as a polygenic disorder.128 Genome-wide analyses as well as twin studies show that insomnia shows significant heritability and an overlap with major depression, bipolar disorder as well as schizophrenia.128–130 The risk of insomnia increases proportionally to the number of variants, which individually have little contribution to the final outcome.131 Van Someren has proposed that genetic alterations conditioning enhanced activity of the salience network, a circuit consisting primarily of the dorsal anterior cingulate cortex and the anterior insula, might be associated with lower amounts of SWS due to increased propensity to arousal.131 In his review, he also stressed the importance of disturbances in REM sleep, caused by noradrenaline input from the LC, to impaired stress adaptation, restoration and synaptic plasticity in the course of insomnia.131
Studies have revealed that sleep–wake rhythm, sleep timing, etc. are regulated by genes responsible for stress response and circadian rhythm; specific genotypes described to date include variances of genes responsible for GABAergic signaling and clock genes, as well as polymorphism of a serotonin transporter promoter variant (5-HTTLPR), a potential mediator between stress and depression.132,133 Individuals homozygous for the Clock gene variant 3111C/C Clock tend to suffer from insomnia more frequently than healthy controls.130 A missense mutation GABA-A β3 subunit might be associated with chronic insomnia.134
Stress or trauma early in development has long been connected to sleep disorder; a possible mechanism behind this effect involves epigenetic regulations. In contrast to rather static genetic factors, they have a dynamic character and might alter in response to environmental cues, thus providing more insight into changes occurring in this sleep disorder.83 Studies have demonstrated that stress in the prenatal period and early life development might have long-lasting consequences, increasing the reactivity of the HPA axis and impairing its negative feedback control.130 Other studies on animals connect early-life stress to alterations in sleep architecture (eg, increased sleep fragmentation, decreasing deep SWS in relation to total sleep time).130,135 Epigenetic regulation involves mechanisms like histone modification, regulatory noncoding RNA, as well as DNA methylation, which appears to be especially important to sleep disorders. Sammallahti et al have found that decreased methylation at cg24815001 in newborns was liked with longer sleep duration.136 Similar change at cg02753354 resulted in longer sleep onset latency.136 According to other studies, PPARA methylation in adolescent girls was inversely associated with sleep fragmentation.128 Koopman-Verhoeff et al have identified that DNA methylation in the 17q21.31 region (which includes, among others, MAPT, a regulator of Tau proteins, involved in neuronal functioning) was associated with sleep duration.137 Other authors also found associations between DNA methylation, sleep duration, and chronotype in children and adolescents.128 Ancelin et al have revealed that four single-nucleotide polymorphisms in the BDNF gene as well as higher methylation of BDNF promoter I were connected to increased wake after sleep onset (WASO), a polysomnographic parameter frequently disrupted in insomnia as well as other sleep disorders.138 Interestingly, such a relationship was not present in psychotropic drug users, suggesting their modifying effect on BDNF production.138 Relationships between sleep and epigenetics might have a mutual character. Lahtinen et al in their study on adult individuals with subjective sleep deficiency revealed that they tend to have altered methylation of genes associated with nervous system development, mainly hypomethylation.139
Sleep disturbances share a connection with neurodegenerative disorders. In a study by Elwood et al, severe excessive daytime sleepiness was a strong predictor of vascular dementia OR of 4.44 (95% CI 2.05–9.61).140 In cases of Lewy body dementia, sleep disturbances, such as REM behavior disorder (RBD), are estimated to occur in ca. 90% of patients.141 Prevalence of sleep disorders in patients with AD, the most common type of dementia, varies from 14% to 69%.142 Moreover, neurodegenerative processes are associated with disruptions in circadian rhythm: in AD subjects, autopsies revealed loss of neurons expressing vasopressin, vasoactive intestinal peptide (VIP), and melatonin receptor type 1 in the SCN.143 Melatonin level might also be increasingly reduced in the course of AD.143 Conversely, sleep loss can further exacerbate the course of the disease, increasing Aβ content and markers of neuroinflammation in the cerebrospinal fluid143 BDNF might also act as a link between neurodegeneration and chronic sleep deficiency. Studies have consistently shown decrease of this neurotrophin in both AD and insomnia patients. In this case, reduction of BDNF is induced by accumulation of Aβ and Tau. It is associated with apoptosis of neurons, due to lack of antiapoptotic stimulation, which in AD usually begins in the entorhinal cortex, spreading to hippocampus, amygdala and other structures (limbic system, neocortex) in a symmetrical fashion.144 Similarly, in individuals with insomnia, hippocampal volume on magnetic resonance imaging (MRI) was reduced on both sides compared to their counterparts with no sleep problems.130 Memory impairments, traditionally associated with insomnia, might be related to such findings; nevertheless, such studies were impossible to replicate.130,145 People with insomnia disorder also tend to display reduced gray matter in areas like the parietal, orbitofrontal and middle cingulate cortex.145
The molecular background of insomnia is an interdisciplinary and active area of research. Studies on the subject of genetic overlap between this sleep disorder and other psychiatric or somatic diseases would be desirable. Projects comparing different insomnia phenotypes as well as their interactions with comorbidities would provide more insight into the pathophysiology of this sleep disorder.
Conclusion and Future Prospects
Even after years of research, numerous aspects of relationships between BDNF and insomnia remain elusive. Current studies chart a few new fields for research. First, it would be interesting to further explore the relationship between sleep regulation, architecture, and BDNF, which could in the future allow for a more personalized management of sleep disorders, especially in patients administered medications that modify the production of this neurotrophin, such as antidepressants. This topic is related to CRSD. Sudying the role of BDNF in those conditions would facilitate better understanding of differences between sleep disorders related to circadian rhythm and primary insomnia. Advancements in this field would allow for development of targeted treatment protocols for those conditions. Interactions between BDNF and stress might be an interesting field for future research in the context of both epigenetic changes regarding DNA methylation in different sleep disturbances as well as individual traits predisposing to insomnia, such as neuroticism. Another interdisciplinary field is the subject of BDNF in substance abuse, as it might involve both neuropsychological predisposing factors, neurodegeneration, as well as imbalance in certain neuronal circuits, mesolimbic and mesocortical in particular.
Expanding the knowledge about BDNF’s connections to other signaling pathways appears to be particularly important due to the ongoing search for better strategies in treatment of sleep disorders. Orexin is a particularly salient subject in this regard due to its potential antidepressant effect. It would also be of interest to gain insight into the interactions between different SORAs/DORAs and BDNF. Projects aiming to investigate the exact processes behind BDNF reduction in neurodegenerative diseases as well as potential compensational mechanisms observed in certain studies (ie, increase in peripheral BDNF) might be desirable. It would also be interesting in the context of searching early biomarkers for dementia. In a 2020 study by Cechova et al, proBDNF:BDNF ratio was a more reliable marker for cognitive performance than any of those proteins alone.146 Another question is whether early diagnosis and proper treatment of sleep disturbances might delay the onset of dementia, decreasing the disease burden in society.
The relationship between BDNF and insomnia is multifaceted. The titular neurotrophin might have a major influence on neurotransmitters like histamine, orexin, and GABA which regulate sleep and wakefulness. Due to its neuroprotective properties, it could be involved in the pathophysiology of neurodegenerative conditions, which are frequently accompanied by various sleep disorders. BDNF might also be implicated in formation of traits predisposing to insomnia, like neuroticism, as well as other epigenetic/genetic factors. Studies have also demonstrated its importance for circadian rhythm and sleep architecture. Broadening the knowledge on insomnia will certainly improve lives of patients afflicted with insomnia as well as aid in development of new molecular targets for treatment. The present review might aid in selecting research subjects as well as offer a new perspective on BDNF’s role in sleep medicine and adjacent fields.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ananthakrishnan AN, Long MD, Martin CF, Sandler RS, Kappelman MD. Sleep disturbance and risk of active disease in patients with Crohn’s disease and ulcerative colitis. Clin Gastroenterol Hepatol. 2013;11(8):965–971. doi:10.1016/j.cgh.2013.01.021
2. Seo HM, Kim TL, Kim JS. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: a Korean population-based retrospective cohort study. Sleep. 2018;41(9). doi:10.1093/sleep/zsy111
3. Young KA, Munroe ME, Harley JB, et al. Less than 7 hours of sleep per night is associated with transitioning to systemic lupus erythematosus. Lupus. 2018;27(9):1524–1531. doi:10.1177/0961203318778368
4. Sang N, Gao RC, Zhang MY, Wu ZZ, Wu ZG, Wu GC. Causal relationship between sleep traits and risk of systemic lupus erythematosus: a two-sample Mendelian randomization study. Front Immunol. 2022;13:918749. doi:10.3389/fimmu.2022.918749
5. Serchov T, Clement HW, Schwarz MK, et al. Increased signaling via adenosine A1 receptors, sleep deprivation, imipramine, and ketamine inhibit depressive-like behavior via induction of Homer1a. Neuron. 2015;87(3):549–562. doi:10.1016/j.neuron.2015.07.010
6. Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487–504. doi:10.5664/jcsm.27286
7. Bhaskar S, Hemavathy D, Prasad S. Prevalence of chronic insomnia in adult patients and its correlation with medical comorbidities. J Family Med Prim Care. 2016;5(4):780–784. doi:10.4103/2249-4863.201153
8. Ditmer M, Gabryelska A, Turkiewicz S, Bialasiewicz P, Malecka-Wojciesko E, Sochal M. Sleep problems in chronic inflammatory diseases: prevalence, treatment, and new perspectives: a narrative review. J Clin Med. 2021;11(1):67. doi:10.3390/jcm11010067
9. Sochal M, Binienda A, Ditmer M, et al. Relation between symptoms of insomnia, depression, sleep quality, anti-tumor necrosis factor therapy and disrupted circadian clock genes’ expression in inflammatory bowel disease. Pol Arch Intern Med. 2023. doi:10.20452/pamw.16487
10. Sochal M, Ditmer M, Binienda A, et al. Relation between selected sleep parameters, depression, anti-tumor necrosis factor therapy, and the brain-derived neurotrophic factor pathway in inflammatory bowel disease. Metabolites. 2023;13(3):450. doi:10.3390/metabo13030450
11. Harvey C-J, Gehrman P, Espie CA. Who is predisposed to insomnia: a review of familial aggregation, stress-reactivity, personality and coping style. Sleep Med Rev. 2014;18(3):237–247. doi:10.1016/j.smrv.2013.11.004
12. Kalmbach DA, Cuamatzi-Castelan AS, Tonnu CV, et al. Hyperarousal and sleep reactivity in insomnia: current insights. Nat Sci Sleep. 2018;10:193–201. doi:10.2147/nss.S138823
13. Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10(4):541–553. doi:10.1016/S0193-953X(18)30532-X
14. Haynes J, Talbert M, Fox S, Close E. Cognitive behavioral therapy in the treatment of insomnia. South Med J. 2018;111(2):75–80. doi:10.14423/smj.0000000000000769
15. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700. doi:10.1111/jsr.12594
16. Ballesio A, Zagaria A, Curti DG, et al. Peripheral brain-derived neurotrophic factor (BDNF) in insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2023;67:101738. doi:10.1016/j.smrv.2022.101738
17. Miranda M, Morici JF, Zanoni MB, Bekinschtein P. Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Review. Front Cell Neurosci. 2019;13. doi:10.3389/fncel.2019.00363
18. Sochal M, Ditmer M, Gabryelska A, Białasiewicz P. The role of brain-derived neurotrophic factor in immune-related diseases: a narrative review. J Clin Med. 2022;11(20):6023. doi:10.3390/jcm11206023
19. Chen SD, Wu CL, Hwang WC, Yang DI. More insight into BDNF against neurodegeneration: anti-apoptosis, anti-oxidation, and suppression of autophagy. Int J Mol Sci. 2017;18(3). doi:10.3390/ijms18030545
20. Rahmani M, Rahmani F, Rezaei N. The brain-derived neurotrophic factor: missing link between sleep deprivation, insomnia, and depression. Neurochem Res. 2020;45(2):221–231. doi:10.1007/s11064-019-02914-1
21. Molano J, Vaughn BV. Approach to insomnia in patients with dementia. Neurol Clin Pract. 2014;4(1):7–15. doi:10.1212/CPJ.0b013e3182a78edf
22. Nutt D, Wilson S, Paterson L. Sleep disorders as core symptoms of depression. Dialogues Clin Neurosci. 2008;10(3):329–336. doi:10.31887/DCNS.2008.10.3/dnutt
23. Gao L, Zhang Y, Sterling K, Song W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl Neurodegener. 2022;11(1):4. doi:10.1186/s40035-022-00279-0
24. Nazareth AM. BDNF, A focus to major depression. Open J Psychol. 2021;1(1):10–21.
25. Rosenfeld RD, Zeni L, Haniu N, et al. Purification and identification of brain-derived neurotrophic factor from human serum. Protein Expr Purif. 1995;6(4):465–471. doi:10.1006/prep.1995.1062
26. Gabryelska A, Turkiewicz S, Ditmer M, et al. BDNF and proBDNF serum protein levels in obstructive sleep apnea patients and their involvement in insomnia and depression symptoms. J Clin Med. 2022;11(23):7135. doi:10.3390/jcm11237135
27. Gabryelska A, Sochal M. Evaluation of HIF-1 involvement in the BDNF and ProBDNF signaling pathways among obstructive sleep apnea patients. Int J Mol Sci. 2022;23(23):14876. doi:10.3390/ijms232314876
28. S-Y W, Pan B-S, Tsai S-F, et al. BDNF reverses aging-related microglial activation. J Neuroinflam. 2020;17(1):210. doi:10.1186/s12974-020-01887-1
29. Walsh EI, Smith L, Northey J, Rattray B, Cherbuin N. Towards an understanding of the physical activity-BDNF-cognition triumvirate: a review of associations and dosage. Ageing Res Rev. 2020;60:101044. doi:10.1016/j.arr.2020.101044
30. Causing CG, Gloster A, Aloyz R, et al. Synaptic innervation density is regulated by neuron-derived BDNF. Neuron. 1997;18(2):257–267. doi:10.1016/S0896-6273(00)80266-4
31. Alonso M, Medina JH, Pozzo-Miller L. ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learn Mem. 2004;11(2):172–178. doi:10.1101/lm.67804
32. Horch HW, Krüttgen A, Portbury SD, Katz LC. Destabilization of cortical dendrites and spines by BDNF. Neuron. 1999;23(2):353–364. doi:10.1016/s0896-6273(00)80785-0
33. Lom B, Cohen-Cory S. Brain-derived neurotrophic factor differentially regulates retinal ganglion cell dendritic and axonal arborization in vivo. J Neurosci. 1999;19(22):9928–9938. doi:10.1523/jneurosci.19-22-09928.1999
34. Niblock MM, Brunso-Bechtold JK, Riddle DR. Insulin-like growth factor I stimulates dendritic growth in primary somatosensory cortex. J Neurosci. 2000;20(11):4165. doi:10.1523/JNEUROSCI.20-11-04165.2000
35. Kwon M, Fernández JR, Zegarek GF, Lo SB, Firestein BL. BDNF-promoted increases in proximal dendrites occur via CREB-dependent transcriptional regulation of cypin. J Neurosci. 2011;31(26):9735–9745. doi:10.1523/jneurosci.6785-10.2011
36. Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci. 2000;3(4):323–329. doi:10.1038/73888
37. Pascual M, Climent E, Guerri C. BDNF induces glutamate release in cerebrocortical nerve terminals and in cortical astrocytes. NeuroReport. 2001;12(12):2673–2677. doi:10.1097/00001756-200108280-00017
38. Chen A, Xiong LJ, Tong Y, Mao M. Neuroprotective effect of brain-derived neurotrophic factor mediated by autophagy through the PI3K/Akt/mTOR pathway. Mol Med Rep. 2013;8(4):1011–1016. doi:10.3892/mmr.2013.1628
39. Caldeira MV, Melo CV, Pereira DB, et al. Brain-derived neurotrophic factor regulates the expression and synaptic delivery of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits in hippocampal neurons. J Biol Chem. 2007;282(17):12619–12628. doi:10.1074/jbc.M700607200
40. Lin SY, Wu K, Levine ES, Mount HT, Suen PC, Black IB. BDNF acutely increases tyrosine phosphorylation of the NMDA receptor subunit 2B in cortical and hippocampal postsynaptic densities. Brain Res Mol Brain Res. 1998;55(1):20–27. doi:10.1016/s0169-328x(97)00349-5
41. Suen P-C, Wu K, Levine ES, et al. Brain-derived neurotrophic factor rapidly enhances phosphorylation of the postsynaptic N-methyl-d-aspartate receptor subunit 1. Proc Natl Acad Sci. 1997;94(15):8191–8195. doi:10.1073/pnas.94.15.8191
42. Almeida RD, Manadas BJ, Melo CV, et al. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005;12(10):1329–1343. doi:10.1038/sj.cdd.4401662
43. Porcher C, Medina I, Gaiarsa JL. Mechanism of BDNF modulation in GABAergic synaptic transmission in healthy and disease brains. Front Cell Neurosci. 2018;12:273. doi:10.3389/fncel.2018.00273
44. Song J, Kim E, Kim C-H, Song H-T, Lee JE. The role of orexin in post-stroke inflammation, cognitive decline, and depression. Molecular Brain. 2015;8(1):16. doi:10.1186/s13041-015-0106-1
45. Guo Y, Feng P. OX2R activation induces PKC-mediated ERK and CREB phosphorylation. Exp Cell Res. 2012;318(16):2004–2013. doi:10.1016/j.yexcr.2012.04.015
46. Erman MK. Sleep architecture and its relationship to insomnia. J Clin Psychiatry. 2001;62(Suppl 10):9–17.
47. Yetton BD, McDevitt EA, Cellini N, Shelton C, Mednick SC. Quantifying sleep architecture dynamics and individual differences using big data and Bayesian networks. PLoS One. 2018;13(4):e0194604. doi:10.1371/journal.pone.0194604
48. Drake CL, Roehrs T, Roth T. Insomnia causes, consequences, and therapeutics: an overview. Depress Anxiety. 2003;18(4):163–176. doi:10.1002/da.10151
49. Moline M, Murphy P, Pinner K, et al. 0369 effect of lemborexant on sleep architecture in older adults with insomnia disorder. Sleep. 2019;42(Supplement_1):A150–A150. doi:10.1093/sleep/zsz067.368
50. Watson AJ, Henson K, Dorsey SG, Frank MG. The truncated TrkB receptor influences mammalian sleep. Am J Physiol Regul Integr Comp Physiol. 2015;308(3):R199–R207. doi:10.1152/ajpregu.00422.2014
51. Garner JM, Chambers J, Barnes AK, Datta S. Changes in brain-derived neurotrophic factor expression influence sleep-wake activity and homeostatic regulation of rapid eye movement sleep. Sleep. 2018;41(2). doi:10.1093/sleep/zsx194
52. Henechowicz TL, Chen JL, Cohen LG, Thaut MH, Li Z. The prevalence of the Val66Met polymorphism in musicians: possible evidence for compensatory neuroplasticity from a pilot study. PLoS One. 2021;16(6):e0245107. doi:10.1371/journal.pone.0245107
53. Greene RW, Frank MG. Slow wave activity during sleep: functional and therapeutic implications. Neuroscientist. 2010;16(6):618–633. doi:10.1177/1073858410377064
54. Mongrain V, Warby SC. Determinants of cortical synchrony. Sleep. 2012;35(3):309–310. doi:10.5665/sleep.1680
55. Monteiro BC, Monteiro S, Candida M, et al. Relationship between brain-derived neurotrofic factor (Bdnf) and sleep on depression: a critical review. Clin Pract Epidemiol Ment Health. 2017;13(1):213–219. doi:10.2174/1745017901713010213
56. Kushikata T, Kubota T, Fang J, Krueger JM. Neurotrophins 3 and 4 enhance non-rapid eye movement sleep in rabbits. Neurosci Lett. 2003;346(3):161–164. doi:10.1016/s0304-3940(03)00564-0
57. Faraguna U, Vyazovskiy VV, Nelson AB, Tononi G, Cirelli C. A causal role for brain-derived neurotrophic factor in the homeostatic regulation of sleep. J Neurosci. 2008;28(15):4088–4095. doi:10.1523/jneurosci.5510-07.2008
58. Muheim CM, Singletary KG, Frank MG. A chemical-genetic investigation of BDNF-NtrkB signaling in mammalian sleep. Sleep. 2022;45(2). doi:10.1093/sleep/zsab237
59. Kaczmarski P, Sochal M, Strzelecki D, Białasiewicz P, Gabryelska A. Influence of glutamatergic and GABAergic neurotransmission on obstructive sleep apnea. Review. Front Neurosci. 2023;17. doi:10.3389/fnins.2023.1213971
60. Reddy S, Reddy V, Sharma S. Physiology, circadian rhythm. In: StatPearls. StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC.; 2023.
61. Gabryelska A, Turkiewicz S, Karuga FF, Sochal M, Strzelecki D, Białasiewicz P. Disruption of circadian rhythm genes in obstructive sleep apnea patients—possible mechanisms involved and clinical implication. Int J Mol Sci. 2022;23(2):709. doi:10.3390/ijms23020709
62. Reid KJ, Zee PC. Chapter 41 - circadian disorders of the sleep–wake cycle. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine.
63. Sack RL, Auckley D, Auger RR, et al. Circadian rhythm sleep disorders: part I, basic principles, shift work and jet lag disorders. An American Academy of Sleep Medicine review. Sleep. 2007;30(11):1460–1483. doi:10.1093/sleep/30.11.1460
64. Archer SN, Carpen JD, Gibson M, et al. Polymorphism in the PER3 promoter associates with diurnal preference and delayed sleep phase disorder. Sleep. 2010;33(5):695–701. doi:10.1093/sleep/33.5.695
65. Semenova NV, Madaeva IM, Bairova TI, et al. 3111T/C clock gene polymorphism in women with insomnia. Bull Exp Biol Med. 2017;163(4):461–464. doi:10.1007/s10517-017-3828-5
66. Charrier A, Olliac B, Roubertoux P, Tordjman S. Clock genes and altered sleep-wake rhythms: their role in the development of psychiatric disorders. Int J Mol Sci. 2017;18(5):938. doi:10.3390/ijms18050938
67. Wickwire EM, Geiger-Brown J, Scharf SM, Drake CL. Shift work and shift work sleep disorder: clinical and organizational perspectives. Chest. 2017;151(5):1156–1172. doi:10.1016/j.chest.2016.12.007
68. Liang FQ, Sohrabji F, Miranda R, Earnest B, Earnest D. Expression of brain-derived neurotrophic factor and its cognate receptor, TrkB, in the rat suprachiasmatic nucleus. Exp Neurol. 1998;151(2):184–193. doi:10.1006/exnr.1998.6804
69. D’Agostino Y, Frigato E, Noviello TMR, et al. Loss of circadian rhythmicity in bdnf knockout zebrafish larvae. iScience. 2022;25(4):104054. doi:10.1016/j.isci.2022.104054
70. Cain SW, Chang AM, Vlasac I, et al. Circadian rhythms in plasma brain-derived neurotrophic factor differ in men and women. J Biol Rhythms. 2017;32(1):75–82. doi:10.1177/0748730417693124
71. Piccinni A, Marazziti D, Del Debbio A, et al. Diurnal variation of plasma brain-derived neurotrophic factor (BDNF) in humans: an analysis of sex differences. Chronobiol Int. 2008;25(5):819–826. doi:10.1080/07420520802387773
72. Choi SW, Bhang S, Ahn JH. Diurnal variation and gender differences of plasma brain-derived neurotrophic factor in healthy human subjects. Psychiatry Res. 2011;186(2–3):427–430. doi:10.1016/j.psychres.2010.07.028
73. Begliuomini S, Lenzi E, Ninni F, et al. Plasma brain-derived neurotrophic factor daily variations in men: correlation with cortisol circadian rhythm. J Endocrinol. 2008;197(2):429–435. doi:10.1677/joe-07-0376
74. Miranda-Riestra A, Estrada-Reyes R, Torres-Sanchez ED, Carreño-García S, Ortiz GG, Benítez-King GM. A neurotrophic factor? Molecules. 2022;27(22):7742. doi:10.3390/molecules27227742
75. Shokri-Mashhadi N, Darand M, Rouhani MH, Yahay M, Feltham BA, Saraf-Bank S. Effects of melatonin supplementation on BDNF concentrations and depression: a systematic review and meta-analysis of randomized controlled trials. Behav Brain Res. 2023;436:114083. doi:10.1016/j.bbr.2022.114083
76. Otsuka Y, Itani O, Matsumoto Y, Kaneita Y. Associations between coping strategies and insomnia: a longitudinal study of Japanese workers. Sleep. 2022;45(2). doi:10.1093/sleep/zsab244
77. Kowalczuk K, Krajewska-Kułak E, Sobolewski M. Relationships between sleep problems and stress coping strategies adopted by nurses including socio-occupational factors. Front Psychiatry. 2021;12:660776. doi:10.3389/fpsyt.2021.660776
78. Serafin LI, Fukowska M, Zyskowska D, Olechowska J, Czarkowska-Pączek B. Impact of stress and coping strategies on insomnia among Polish novice nurses who are employed in their field while continuing their education: a cross-sectional study. BMJ Open. 2021;11(12):e049787. doi:10.1136/bmjopen-2021-049787
79. Soehner AM, Harvey AG. Prevalence and functional consequences of severe insomnia symptoms in mood and anxiety disorders: results from a nationally representative sample. Sleep. 2012;35(10):1367–1375. doi:10.5665/sleep.2116
80. Neckelmann D, Mykletun A, Dahl AA. Chronic insomnia as a risk factor for developing anxiety and depression. Sleep. 2007;30(7):873–880. doi:10.1093/sleep/30.7.873
81. Cano G, Mochizuki T, Saper CB. Neural circuitry of stress-induced insomnia in rats. J Neurosci. 2008;28(40):10167–10184. doi:10.1523/jneurosci.1809-08.2008
82. Dressle RJ, Riemann D. Hyperarousal in insomnia disorder: current evidence and potential mechanisms. J Sleep Res. 2023;e13928. doi:10.1111/jsr.13928
83. Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14(1):19–31. doi:10.1016/j.smrv.2009.04.002
84. Sudheimer KD, O’Hara R, Spiegel D, et al. Cortisol, cytokines, and hippocampal volume interactions in the elderly. Original Research. Front Aging Neurosci. 2014;6. doi:10.3389/fnagi.2014.00153
85. Hall BS, Moda RN, Liston C. Glucocorticoid mechanisms of functional connectivity changes in stress-related neuropsychiatric disorders. Neurobiol Stress. 2015;1:174–183. doi:10.1016/j.ynstr.2014.10.008
86. Jeanneteau F, Chao MV. Are BDNF and glucocorticoid activities calibrated? Neuroscience. 2013;239:173–195. doi:10.1016/j.neuroscience.2012.09.017
87. Dressle RJ, Feige B, Spiegelhalder K, et al. HPA axis activity in patients with chronic insomnia: a systematic review and meta-analysis of case–control studies. Sleep Med Rev. 2022;62:101588. doi:10.1016/j.smrv.2022.101588
88. Jeanneteau F, Borie A, Chao MV, Garabedian MJ. Bridging the gap between brain-derived neurotrophic factor and glucocorticoid effects on brain networks. Neuroendocrinology. 2019;109(3):277–284. doi:10.1159/000496392
89. Suri D, Vaidya VA. Glucocorticoid regulation of brain-derived neurotrophic factor: relevance to hippocampal structural and functional plasticity. Neuroscience. 2013;239:196–213. doi:10.1016/j.neuroscience.2012.08.065
90. Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuro Psychopharmacol Biol Psychiatry. 2012;39(1):112–119. doi:10.1016/j.pnpbp.2012.05.018
91. Naert G, Ixart G, Maurice T, Tapia-Arancibia L, Givalois L. Brain-derived neurotrophic factor and hypothalamic-pituitary-adrenal axis adaptation processes in a depressive-like state induced by chronic restraint stress. Mol Cell Neurosci. 2011;46(1):55–66. doi:10.1016/j.mcn.2010.08.006
92. Ambrus L, Lindqvist D, Träskman-Bendz L, Å W. Hypothalamic–pituitary–adrenal axis hyperactivity is associated with decreased brain-derived neurotrophic factor in female suicide attempters. Nordic J Psychiatry. 2016;70(8):575–581. doi:10.1080/08039488.2016.1184310
93. Onen Sertoz O, Tolga Binbay I, Koylu E, Noyan A, Yildirim E, Elbi Mete H. The role of BDNF and HPA axis in the neurobiology of burnout syndrome. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1459–1465. doi:10.1016/j.pnpbp.2008.05.001
94. Suliman S, Hemmings S, Seedat S. Brain-Derived Neurotrophic Factor (BDNF) protein levels in anxiety disorders: systematic review and meta-regression analysis. Original Research. Front Integrat Neurosci. 2013;7. doi:10.3389/fnint.2013.00055
95. Baik J-H. Stress and the dopaminergic reward system. Exp Mol Med. 2020;52(12):1879–1890. doi:10.1038/s12276-020-00532-4
96. Rosenblum M. Substance abuse and insomnia. Minn Med. 2017;100(3):38–39.
97. Furihata R, Saitoh K, Otsuki R, et al. Association between reduced serum BDNF levels and insomnia with short sleep duration among female hospital nurses. Sleep Med. 2020;68:167–172. doi:10.1016/j.sleep.2019.12.011
98. Giese M, Unternährer E, Hüttig H, et al. BDNF: an indicator of insomnia? Mol Psychiatry. 2014;19(2):151–152. doi:10.1038/mp.2013.10
99. Gabryelska A, Turkiewicz S, Ditmer M, Sochal M. Neurotrophins in the neuropathophysiology, course, and complications of obstructive sleep apnea—a narrative review. Int J Mol Sci. 2023;24(3):1808. doi:10.3390/ijms24031808
100. Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol. 2008;11(8):1169–1180. doi:10.1017/S1461145708009309
101. Fan TT, Chen WH, Shi L, et al. Objective sleep duration is associated with cognitive deficits in primary insomnia: BDNF may play a role. Sleep. 2019;42(1). doi:10.1093/sleep/zsy192
102. Sanchez-Garcia S, Moreno-Tamayo K, Ramirez-Aldana R, et al. Insomnia impairs both the Pro-BDNF and the BDNF levels similarly to older adults with cognitive decline: an exploratory study. Int J Mol Sci. 2023;24(8):7387. doi:10.3390/ijms24087387
103. Satyanarayanan SK, Chien YC, Chang JP, et al. Melatonergic agonist regulates circadian clock genes and peripheral inflammatory and neuroplasticity markers in patients with depression and anxiety. Brain Behav Immun. 2020;85:142–151. doi:10.1016/j.bbi.2019.03.003
104. Zhang P, Li YX, Zhang ZZ, et al. Astroglial mechanisms underlying chronic insomnia disorder: a clinical study. Nat Sci Sleep. 2020;12:693–704. doi:10.2147/nss.S263528
105. Mikoteit T, Brand S, Eckert A, Holsboer-Trachsler E, Beck J. Brain-derived neurotrophic factor is a biomarker for subjective insomnia but not objectively assessable poor sleep continuity. J Psychiatr Res. 2019;110:103–109. doi:10.1016/j.jpsychires.2018.12.020
106. Deuschle M, Schredl M, Wisch C, et al. Serum brain-derived neurotrophic factor (BDNF) in sleep-disordered patients: relation to sleep stage N3 and rapid eye movement (REM) sleep across diagnostic entities. J Sleep Res. 2018;27(1):73–77. doi:10.1111/jsr.12577
107. Schmitt K, Holsboer-Trachsler E, Eckert A. BDNF in sleep, insomnia, and sleep deprivation. Ann Med. 2016;48(1–2):42–51. doi:10.3109/07853890.2015.1131327
108. Xue J, Li H, Xu Z, et al. Paradoxical sleep deprivation aggravates and prolongs incision-induced pain hypersensitivity via BDNF signaling-mediated descending facilitation in rats. Neurochem Res. 2018;43(12):2353–2361. doi:10.1007/s11064-018-2660-2
109. Musazzi L, Treccani G, Popoli M. Functional and structural remodeling of glutamate synapses in prefrontal and frontal cortex induced by behavioral stress. Front Psychiatry. 2015;6:60. doi:10.3389/fpsyt.2015.00060
110. Cunha C, Brambilla R, Thomas KL. A simple role for BDNF in learning and memory? Front Mol Neurosci. 2010;3:1. doi:10.3389/neuro.02.001.2010
111. Frank L, Ventimiglia R, Anderson K, Lindsay RM, Rudge JS. BDN F down-regulates neurotrophin responsiveness, TrkB protein and TrkB mRNA levels in cultured rat hippocampal neurons. Eur J Neurosci. 1996;8(6):1220–1230. doi:10.1111/j.1460-9568.1996.tb01290.x
112. Bellesi M, de Vivo L, Chini M, Gilli F, Tononi G, Cirelli C. Sleep loss promotes astrocytic phagocytosis and microglial activation in mouse cerebral cortex. J Neurosci. 2017;37(21):5263–5273. doi:10.1523/jneurosci.3981-16.2017
113. Khalaji A, Behnoush AH, Shobeiri P, Saeedian B, Teixeira AL, Rezaei N. Association between brain-derived neurotrophic factor levels and obstructive sleep apnea: a systematic review and meta-analysis. Sleep Breath. 2023;27(3):829–841. doi:10.1007/s11325-022-02707-x
114. Nordahl H, Havnen A, Hansen B, Ost LG, Kvale G. Sleep disturbances in treatment-seeking OCD-patients: changes after concentrated exposure treatment. Scand J Psychol. 2018;59(2):186–191. doi:10.1111/sjop.12417
115. Plante DT, Jensen JE, Winkelman JW. The role of GABA in primary insomnia. Sleep. 2012;35(6):741–742. doi:10.5665/sleep.1854
116. Morgan PT, Pace-Schott EF, Mason GF, et al. Cortical GABA levels in primary insomnia. Sleep. 2012;35(6):807–814. doi:10.5665/sleep.1880
117. Hepsomali P, Groeger JA, Nishihira J, Scholey A. Effects of Oral Gamma-Aminobutyric Acid (GABA) administration on stress and sleep in humans: a systematic review. Front Neurosci. 2020;14:923. doi:10.3389/fnins.2020.00923
118. Scammell TE, Jackson AC, Franks NP, Wisden W, Dauvilliers Y. Histamine: neural circuits and new medications. Sleep. 2019;42(1):zsy183. doi:10.1093/sleep/zsy183
119. Mormile R, Mazzei G, Vittori G, de Michele M, Squarcia U. Insomnia and shift-work sleep disorder: a crosstalk between glutamate excitotoxicity and decreased GABAergic neurotransmission? Sleep Biol Rhythms. 2012;10(4):340–341. doi:10.1111/j.1479-8425.2012.00574.x
120. Wang R, Reddy PH. Role of glutamate and NMDA receptors in Alzheimer’s disease. J Alzheimers Dis. 2017;57(4):1041–1048. doi:10.3233/jad-160763
121. He S, Zhang X, Glutamate QS. Glutamate transporters, and circadian rhythm sleep disorders in neurodegenerative diseases. ACS Chem Neurosci. 2019;10(1):175–181. doi:10.1021/acschemneuro.8b00419
122. Novati A, Hulshof HJ, Granic I, Meerlo P. Chronic partial sleep deprivation reduces brain sensitivity to glutamate N-methyl-D-aspartate receptor-mediated neurotoxicity. J Sleep Res. 2012;21(1):3–9. doi:10.1111/j.1365-2869.2011.00932.x
123. Gotoh K, Masaki T, Chiba S, et al. Brain-derived neurotrophic factor, corticotropin-releasing factor, and hypothalamic neuronal histamine interact to regulate feeding behavior. J Neurochem. 2013;125(4):588–598. doi:10.1111/jnc.12213
124. Ren J, Chen Y, Fang X, et al. Correlation of Orexin-A and brain-derived neurotrophic factor levels in metabolic syndrome and cognitive impairment in schizophrenia treated with clozapine. Neurosci Letters. 2022;782:136695. doi:10.1016/j.neulet.2022.136695
125. Mogavero MP, Silvani A, Lanza G, DelRosso LM, Ferini-Strambi L, Ferri R. Targeting orexin receptors for the treatment of insomnia: from physiological mechanisms to current clinical evidence and recommendations. Nat Sci Sleep. 2023;15:17–38. doi:10.2147/nss.S201994
126. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726–731. doi:10.1016/s0166-2236(00)02002-6
127. Wang Z-J, Liu J-F. The molecular basis of insomnia: implication for therapeutic approaches. Drug Dev Res. 2016;77(8):427–436. doi:10.1002/ddr.21338
128. Palagini L, Geoffroy PA, Gehrman PR, Miniati M, Gemignani A, Riemann D. Potential genetic and epigenetic mechanisms in insomnia: a systematic review. J Sleep Res. 2023;e13868. doi:10.1111/jsr.13868
129. Stein MB, McCarthy MJ, Chen CY, et al. Genome-wide analysis of insomnia disorder. Mol Psychiatry. 2018;23(11):2238–2250. doi:10.1038/s41380-018-0033-5
130. Palagini L, Drake CL, Gehrman P, Meerlo P, Riemann D. Early-life origin of adult insomnia: does prenatal-early-life stress play a role? Sleep Med. 2015;16(4):446–456. doi:10.1016/j.sleep.2014.10.013
131. Van Someren EJW. Brain mechanisms of insomnia: new perspectives on causes and consequences. Physiol Rev. 2021;101(3):995–1046. doi:10.1152/physrev.00046.2019
132. Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68(5):444–454. doi:10.1001/archgenpsychiatry.2010.189
133. Palagini L, Biber K, Riemann D. The genetics of insomnia--evidence for epigenetic mechanisms? Sleep Med Rev. 2014;18(3):225–235. doi:10.1016/j.smrv.2013.05.002
134. Hamet P, Tremblay J. Genetics of the sleep-wake cycle and its disorders. Metabolism. 2006;55:S7–S12. doi:10.1016/j.metabol.2006.07.006
135. Wang ZJ, Liu JF. The molecular basis of insomnia: implication for therapeutic approaches. Drug Dev Res. 2016;77(8):427–436. doi:10.1002/ddr.21338
136. Sammallahti S, Koopman-Verhoeff ME, Binter A-C, et al. Longitudinal associations of DNA methylation and sleep in children: a meta-analysis. Clin Epigenet. 2022;14(1):83. doi:10.1186/s13148-022-01298-4
137. Koopman-Verhoeff ME, Mulder RH, Saletin JM, et al. Genome-wide DNA methylation patterns associated with sleep and mental health in children: a population-based study. J Child Psychol Psychiatry. 2020;61(10):1061–1069. doi:10.1111/jcpp.13252
138. Ancelin M-L, Jaussent I, Ritchie K, Besset A, Ryan J, Dauvilliers Y. Brain-derived neurotrophic factor (BDNF) variants and promoter I methylation are associated with prolonged nocturnal awakenings in older adults. J Sleep Res. 2023;32(4):e13838. doi:10.1111/jsr.13838
139. Lahtinen A, Puttonen S, Vanttola P, et al. A distinctive DNA methylation pattern in insufficient sleep. Sci Rep. 2019;9(1):1193. doi:10.1038/s41598-018-38009-0
140. Peter CE, Antony JB, Mark F, Janet P, Clive M, John EJG. Sleep disturbance and daytime sleepiness predict vascular dementia. J Epidemiol Community Health. 2011;65(9):820. doi:10.1136/jech.2009.100503
141. Elder GJ, Lazar AS, Alfonso-Miller P, Taylor JP. Sleep disturbances in Lewy body dementia: a systematic review. Int J Geriatr Psychiatry. 2022;37(10). doi:10.1002/gps.5814
142. Lloret MA, Cervera-Ferri A, Nepomuceno M, Monllor P, Esteve D, Lloret A. Is sleep disruption a cause or consequence of Alzheimer’s disease? Reviewing its possible role as a biomarker. Int J Mol Sci. 2020;21(3):1168. doi:10.3390/ijms21031168
143. Shen Y, Lv QK, Xie WY, et al. Circadian disruption and sleep disorders in neurodegeneration. Transl Neurodegener. 2023;12(1):8. doi:10.1186/s40035-023-00340-6
144. Johnson KA, Fox NC, Sperling RA, Klunk WE. Brain imaging in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(4):a006213. doi:10.1101/cshperspect.a006213
145. Leerssen J, Blanken TF, Pozzi E, et al. Brain structural correlates of insomnia severity in 1053 individuals with major depressive disorder: results from the ENIGMA MDD Working Group. Transl Psychiatry. 2020;10(1):425. doi:10.1038/s41398-020-01109-5
146. Cechova K, Angelucci F, Markova H, et al. Ratio of serum proBDNF to BDNF and its association with cognitive performance and brain morphometry in mild cognitive impairment. Alzheimer’s Dementia. 2020;16(S6):e046340. doi:10.1002/alz.046340
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.