Back to Journals » Nature and Science of Sleep » Volume 15
Impact of Preoperative Sleep Disturbances on Postoperative Delirium in Patients with Intracranial Tumors: A Prospective, Observational, Cohort Study
Authors Liu Y , Zhang X, Jiang M, Zhang Y, Wang C , Sun Y, Shi Z, Wang B
Received 23 August 2023
Accepted for publication 12 December 2023
Published 21 December 2023 Volume 2023:15 Pages 1093—1105
DOI https://doi.org/10.2147/NSS.S432829
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Valentina Alfonsi
Yang Liu,1 Xiaoyu Zhang,1 Mengyang Jiang,1 Yiqiang Zhang,1 Chenhui Wang,1 Yongxing Sun,1 Zhonghua Shi,2 Baoguo Wang1
1Department of Anesthesiology, Sanbo Brain Hospital, Capital Medical University, Beijing, 100093, People’s Republic of China; 2Department of Intensive Care Medicine, Sanbo Brain Hospital, Capital Medical University, Beijing, 100093, People’s Republic of China
Correspondence: Baoguo Wang, Department of Anesthesiology, Sanbo Brain Hospital, Capital Medical University, No. 50 Xiangshan Yi-Ke-Song Road, Haidian District, Beijing, 100093, People’s Republic of China, Tel +86 13370185075, Email [email protected]
Background: Postoperative delirium (POD) is prevalent in craniotomy patients and is associated with high mortality. Sleep disturbances are receiving increasing attention from clinicians as associated risk factors for postoperative complications. This study aimed to determine the impact of preoperative sleep disturbances on POD in craniotomy patients.
Methods: We recruited 130 patients undergoing elective craniotomy for intracranial tumors between May 1st and December 30th, 2022. Preoperative subjective sleep disturbances were assessed using the Pittsburgh Sleep Quality Index on the day of admission. We also measured objective perioperative sleep patterns using a dedicated sleep monitoring device 3 days before and 3 days after the surgery. POD was assessed twice daily using the Confusion Assessment Model for the Intensive Care Unit within the first week after craniotomy.
Results: Preoperative sleep disturbances were diagnosed in 49% of the study patients, and POD was diagnosed in 22% of all the study patients. Sleep disturbances were an independent risk factor for POD (OR: 2.709, 95% CI: 1.020– 7.192, P = 0.045). Other risk factors for POD were age (OR: 3.038, 95% CI: 1.195– 7.719, P = 0.020) and the duration of urinary catheterization (OR: 1.246, 95% CI: 1.025– 1.513, P = 0.027). Perioperative sleep patterns (including sleep latency, deep sleep duration, frequency of awakenings, apnea-hypopnea index, and sleep efficiency) were significantly associated with POD.
Conclusion: This study demonstrated that preoperative sleep disturbances predispose patients undergoing craniotomy to POD, also inferred a correlation between perioperative sleep patterns and POD. The targeted screening and intervention specifically for sleep disturbances during the perioperative period are immensely required.
Keywords: postoperative delirium, preoperative sleep disturbances, craniotomy, sleep pattern, confusion assessment model for the intensive care unit, Pittsburgh Sleep Quality Index
Introduction
Postoperative delirium (POD) is a self-limiting condition that occurs within hours to days after surgery.1,2 It is marked by manifestations of brain dysfunction including acute disturbances in attention, cognitive function, and thought process.3 POD occurs in 10–50% of patients after general surgery and is associated with a prolonged hospital stay, long-term cognitive impairment, poor quality of life, and increased mortality.4–7 In patients undergoing craniotomy, studies have shown that 14.8% to 33.8% of these patients develop POD.2,8,9 POD can be precipitated by many risk factors including pre-existing cognitive impairment, illnesses, and sleep disturbances.10
Sleep is an essential physiological process of the human body, it is required especially according to the circadian rhythms for optimal functions of the biological processes in the body and to avoid disease onset.11,12 The disruption in the circadian rhythms like a reversed sleeping schedule that leads to a lack of sleep during the night, or sleeping with the lights on can impair the quality of sleep.13,14 Sleep is incredibly vital for the optimal function of the brain, studies show that the lack of sleep causes a systemic low-grade inflammation without the occurrence of infection the brain. Therefore, sustained loss of sleep can cause an increased release of inflammatory cytokines such as TNF-α and IL6. The release of these cytokines activates the microglia which release more inflammatory factors and cytotoxic cytokines in the brain which cause damage to the neurons,15,16 and neural inflammation has been considered a pathophysiological mechanism for POD onset.17 Studies have shown that sleep disturbances are prevalent in patients with intracranial tumors, with the reported incidence ranging from 53% to 61.5%.18,19 However, very little research has been done and only recently has there been an interest in the correlation between POD and sleep disturbances.20
Sleep disturbances are very common during the preoperative period and have been described as vital risk factors for POD.3 Studies have revealed that preoperative sleep disturbances are correlated with a higher likelihood of experiencing POD and consequently lead to delayed recovery after surgery and increased mortality.7,10,21,22 They are a combination of objective evidence and subjective sleep complaints.23 These disturbances are composed of a plethora of sleep facets including sleep duration, sleep latency, and sleep efficiency which can be measured using subjective sleep quality complaints and objective evidence.24 In a study by Ulsa et al, they found that the higher the severity of poor sleep in patients, the higher the likelihood of experiencing hospital-diagnosed delirium. These results did not change when only factoring in postoperative delirium.25 Although numerous other factors can cause delirium, it is important to specifically report on the effect of preoperative sleep disturbance on POD as it is a common occurrence that can affect the prognosis of postoperative patients.
In this present study, we will explore the impact of preoperative sleep disturbances on POD in patients undergoing craniotomy. In addition, we will also investigate the time-dependent characteristics of perioperative sleep patterns in patients with and without POD.
Materials and Methods
Study Design and Participants
This prospective, observational, cohort study was conducted at Sanbo Brain Hospital, Capital Medical University. Ethical approval was obtained from the institutional ethics committee (SBNK-YJ-2022-011-01), and the trial was registered on the Chinese Clinical Trial Registry (ChiCTR2200059425). Patients (≥45 years) undergoing elective craniotomy for intracranial tumors from May 2022 to September 2022 were screened for eligibility. The patient who met one of the following criteria was excluded: (1) history of delirium, cognitive dysfunction, or psychosis; (2) history of stroke; (3) American Society of Anesthesiologists physical status classification > II; (4) having dissemination and distant metastasis; (5) unable to complete assessments due to various reasons. Written informed consent was obtained from all recruited patients or their legal representatives of the study.
Sleep Disturbances Assessment
Sleep quality has previously been reported in POD patients according to studies that used the Pittsburgh Sleep Quality Index.26 Patients were asked to complete the Pittsburgh Sleep Quality Index (PSQI) questionnaires unassisted on the day of admission. The PSQI comprises 7 parts and 18 items in total, which assesses the sleep quality of the subject experienced over the preceding month. A score of 5 was set as an empirically determined cut-off to differentiate individuals with good or poor sleep quality.27 Patients were divided into two groups based on the score of PSQI. The sleep disturbances group was defined as a score of >5, and the non-sleep disturbances group was defined as a score of ≤5.
Objective Perioperative Sleep Patterns Assessment
A dedicated sleep device (SC-500TM; Boshi Linkage Technology, Beijing, China) with a built-in electret condenser microphone (EM246ASSTM; Hakujitsu Technology Co., Tokyo, Japan) was used to monitor the objective perioperative sleep pattern 3 days before and 3 days after craniotomy (Figure 1). The sleep device detected signals in the frequency domain of 0.01 Hz to 10kHz, which could accurately identify signals such as human heart rate, respiration, and body movement (Figure 1). The signals measured by this device were collected and analyzed based on the algorithm of ballistocardiogram, we measured, total sleep duration, deep and light sleep duration, rapid eye movement phase duration, sleep latency, frequency of awakenings, apnea-hypopnea index, and sleep efficiency.28 Studies have demonstrated the reliability of this sleep device by using polysomnography as the golden standard.29,30
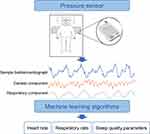 |
Figure 1 Working principle of sleep device. |
Postoperative Delirium Assessment
POD was assessed using the Confusion Assessment Model for the Intensive Care Unit (CAM-ICU). The assessment was conducted twice daily (8:00 am–10:00 am and 6:00 pm–8:00 pm, respectively) within the first week after surgery, following the Richmond Agitation Sedation Scale (RASS) evaluation. The assessment was performed only in patients with RASS higher or equal to −3, and RASS scores of −3 and higher indicate patients who are aroused and can answer questions, and can therefore be assessed by the CAM-ICU. The diagnosis of delirium is based on the presence of the first two criteria in the CAM-ICU flow sheet (ie, acute or fluctuating mental status and inattention) plus at least one of the following criteria (altered level of consciousness and or disorganized thinking). The delirium assessment investigators were unaware of the PSQI score results.
Clinical Data Collection
The patient demographic characteristics (including gender, age, body mass index, years of education, alcohol consumption, and smoking), medical history, cognitive function (Mini-Mental State Examination), and symptoms of depression and anxiety (Hospital Anxiety and Depression Scale) were collected at admission. The laboratory test was collected within 24 hours before surgery.
Anesthesia was performed according to our department routine (Table S1), and data related to surgery and anesthesia were also collected. Pain scores (Numerical Rating Scale) were assessed on the first postoperative day. Postoperative complications and the Barthel index assessed by the Activity of Daily Living scale were collected at hospital discharge (Supplementary File 1). The quality of life was investigated by telephonic interview 3 months after surgery according to the European Organization for Research and Treatment of Cancer, Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30) (Supplementary File 2).
Sample Size Calculation
We calculated the required sample size for the study, assuming the incidence of POD in patients with sleep disturbances is 50% with OR equal to 5.24,31,32 and the incidence of POD in patients without sleep disturbances is 10%.33 Under these assumptions, 102 patients were required when choosing a power of 90% and a two-sided α of 0.05. Considering the dropout rate of 10%, we planned to recruit 114 patients, with 57 patients in each group. PASS 15.0 (NCSS, LLC. Kaysville, Utah, USA) was used for sample size calculation.
Statistical Analysis
The normality of the variable’s distribution was analyzed using the Kolmogorov–Smirnov test. The data were presented as mean ± standard deviation (SD), median (interquartile range, IQR), frequency (percentage), and estimated mean (EM) [95% CI], as appropriate. The differences in categorical variables were assessed using the chi-square and Fisher’s exact tests. The differences in continuous variables were analyzed using the independent sample t-test or Mann–Whitney U-test based on the data distribution. The association between POD and sleep disturbances was analyzed using the multivariate logistic regression analysis with a backward stepwise method. The adjustment was performed based on the risk factors selected by the univariate analysis, including age, neutrophil-to-lymphocyte ratio, postoperative intubation time, length of stay in the intensive care unit (ICU), and duration of urinary catheterization. The time-dependent characteristics of perioperative sleep patterns in patients with and without POD were analyzed using generalized estimating equations with a main effect of time, group, and group-by-time interaction. The missing data on sleep patterns during the study period were considered missing at random and were not imputed. A P-value <0.05 was considered statistically significant in all analyses. All statistical analyses were performed using SPSS Statistics 25.0 (IBM Corp, Armonk, NY, USA).
Results
Characteristics of Study Participants
Of the 213 patients screened in this study, 130 patients (age 57 ± 8 years) were recruited, and 48% (62/130) of the patients were male (Figure 2). Among all the patients studied, 22% of them were diagnosed with POD. About 63 (49%) of the patients recruited were diagnosed with preoperative sleep disturbances according to the PSQI, of which 32% (20/63) of them were diagnosed with POD, this number was significantly higher than that of patients without preoperative sleep disturbances (12%, P=0.006) (Table 1). There was no difference in the time of onset and duration of POD between patients with sleep disturbances and without sleep disturbances (Table 1).
 |
Table 1 Univariate Analysis According to Sleep Disturbances |
 |
Figure 2 Flow chart of included population. |
Preoperative Sleep Disturbances and Postoperative Delirium
The patient characteristics regarding POD status are presented in Table 2 and Table 3. The risk factors analyzed by univariate analysis associated with POD were: age (P = 0.001), sleep disturbances (P = 0.006), PSQI score (P = 0.008), neutrophil-to-lymphocyte ratio (P = 0.025), postoperative intubation time (P = 0.047), length of stay in the ICU (P = 0.047), and duration of urinary catheterization (P = 0.001). After adjusting for potential confounding effects (P<0.05) by multivariate logistic regression analysis, the sleep disturbances still had a significant correlation with POD (OR: 2.709, 95% CI: 1.020–7.192, P = 0.045). Other risk factors for POD were age (OR: 3.038, 95% CI: 1.195–7.719, P = 0.020) and the duration of urinary catheterization (OR: 1.246, 95% CI: 1.025–1.513, P = 0.027) (Table 4).
 |
Table 2 Baseline Characteristics Associated with Postoperative Delirium |
 |
Table 3 Surgical Characteristics Associated with Postoperative Delirium |
 |
Table 4 Variables Associated with Postoperative Delirium on Multivariable Analysis |
Perioperative Sleep Patterns and Postoperative Delirium
We analyzed data on sleep patterns using a dedicated sleep monitoring device before diagnosing POD, excluding data collected after the onset of POD. A total of 480 days of data on sleep patterns were analyzed, 122 days in 22 patients with POD and 358 days in 65 patients without POD (Tables S2 and S3).
Sleep Stages
The sleep latency decreased immediately after the surgery (please indicate the statistic and p-value for this result), but no change was noted in either group before surgery. Compared with patients without POD, the sleep latency was longer on the first (EM: 36.1, [95% CI: 27.5–44.6] min vs EM: 23.7, [95% CI: 20.5–27.0] min, P = 0.008) and second (EM: 30.7, [95% CI: 23.4–37.9] min vs EM: 22.3, [95% CI: 19.2–25.5] min, P = 0.038) postoperative days in patients with POD (Figure 3A). The overall change in deep sleep duration increased after craniotomy in both groups. There were significant differences between groups one day before surgery (EM: 54.0, [95% CI: 44.7–66.3] min vs EM: 66.6, [95% CI: 58.3–74.8] min, P = 0.047) (Figure 3B). No significant differences were found in the parameters including total sleep duration, light sleep duration, and rapid eye movement phase duration between the two groups (Figure S1).
Events During Sleep
The events of awakening and apnea were also recorded and presented as the frequency of awakenings and the apnea-hypopnea index. The frequency of awakenings significantly increased the day before surgery and gradually decreased in the postoperative period in both groups. Patients with POD had a higher frequency of awakenings on the day before surgery (EM: 6.5, [95% CI: 5.7–7.4] vs EM: 5.1, [95% CI: 4.5–5.7], P = 0.006) and on the third day after surgery (EM: 4.9, [95% CI: 4.0–5.8] vs EM: 3.2, [95% CI: 2.7–3.7], P = 0.001) (Figure 3C). The apnea-hypopnea index was significantly higher on the first postoperative day in both groups and then gradually decreased. Patients with POD had a higher apnea-hypopnea index compared to patients without POD, especially on the day before surgery (EM: 17.1, [95% CI: 14.8–19.4] vs EM: 12.8, [95% CI: 11.0–14.7], P=0.005) and three days after surgery (EM: 19.5, [95% CI: 16.5–22.4] vs EM: 14.8, [95% CI: 13.0–16.7], P = 0.010) (Figure 3D).
Sleep Efficiency
The sleep efficiency remained stable in both groups during the perioperative period. However, the sleep efficiency in patients with POD on the second postoperative day was lower than that in patients without POD (EM: 78.1, [95% CI: 75.2–81.0] % vs EM: 81.8, [95% CI: 80.7–82.8] %, P = 0.021) (Figure 3E).
Clinical Outcomes
Compared with patients without POD, the cost of hospitalization was higher in patients with POD (103,261 [IQR: 97,105, 118,064] RMB vs 96,987 (89,874, 106,597) RMB, P = 0.007). The Barthel index measured at discharge was significantly lower in patients with POD (80.0 [IQR: 70.0, 90.0] vs 90.0 [IQR: 83.8, 100.0], P <0.001). However, no difference was observed in other clinical outcomes (Table 5).
 |
Table 5 Clinical Outcomes Associated with Postoperative Delirium |
Discussion
This study showed that sleep disturbances are associated with POD in patients underwent craniotomy. We found that patients with sleep disturbances (PSQI >5) had 2.7 times the odds of developing POD and these odds were determined to be independent of other risk factors. In addition, our study demonstrated significant differences in perioperative sleep patterns between patients with and without POD.
In our study, the incidence of POD is 32% in patients with sleep disturbances. To the best of our knowledge, our study is the first to investigate the correlation between sleep disturbances and POD in patients receiving craniotomy for intracranial tumors. The rate of POD in our cohort is consistent with findings from previous studies, which range from 21.7% to 50%.21,31 However, these studies were mainly focused on non-neurosurgical patients. Neural inflammation has been demonstrated as a major mechanism for POD development17 and studies showed that sleep disturbances could lead to neural inflammation, characterized by increased levels of pro-inflammatory cytokines and overactivated microglia and astrocytes, as its downstream effect.16,34 The sleep disturbances may further exaggerate the surgery-induced neuroinflammation in post-craniotomy patients. Therefore, it may constitute a significant risk factor for POD. In our study, we found that the neutrophil-to-lymphocyte ratio, a marker for systemic inflammation, was significantly higher in patients with POD, indicating that inflammation plays a role in POD development. However, we did not investigate the inflammation biomarkers of the central nervous system. Therefore, its effect required further study to confirm. Furthermore, cortical and subcortical atrophy has been demonstrated to contribute to the development of POD,35 and sleep disturbances, especially REM-sleep behavior disturbance, can result in cortical and subcortical atrophy.36 In patients with intracranial tumors, cortical and subcortical atrophy frequently occur due to the compression of lesions and surgical procedures. Therefore, sleep disturbances-induced cortical atrophy may aggravate brain atrophy in patients with intracranial tumors, subsequently increasing the risk of POD.
In addition, a meta-analysis was conducted for 18 studies, resulting in a pooled incidence of POD after craniotomy of 19%.37 In our cohort, the incidence of POD was 21.9% which was relatively high. The difference in incidence might be attributed to the location of neurosurgery, different delirium assessment tools, and the time of assessments. In this study, 31.5% of patients had a surgical location in the prefrontal or parietal lobes closely associated with cognitive function, which may explain the higher incidence of POD. Additionally, the POD was defined as within 7 days after the operation in our study, this was longer than previous primary studies which were conducted within ≤3 days.37 Our data showed that 32% (9/28) of the patients presented with POD after the third postoperative day; this may further explain the relatively higher incidence of POD.
Perioperative sleep disturbances have also been shown to increase the risk of postoperative cognitive dysfunction.38 However, no studies have been conducted on neurosurgical patients. In the present study, we investigated the association between perioperative sleep patterns and POD in patients undergoing craniotomy in depth. We derived parameters related to sleep stages, events during sleep, and sleep efficiency using a dedicated sleep monitoring device.
Firstly, for the parameters of sleep stages, both sleep latency and deep sleep duration showed significant differences between patients with and without POD (please show statistics when stating these results and p-value). However, no differences were found in total sleep duration, light sleep duration, and rapid eye movement phase duration. Consistent with our findings, previous studies found that reduced sleep time and prolonged sleep latency recorded by an electroencephalogram on the first postoperative day were associated with increased incidence and severity of POD in orthopedic patients.38 Furthermore, deep sleep has been demonstrated to be associated with an increase in the convective exchange of cerebrospinal fluid with interstitial fluid to reduce the concentration of β-amyloid and cerebral metabolic wastes via the glymphatic pathway.39 Meanwhile, deep sleep could facilitate memory consolidation, especially for hippocampus-dependent memories.40 Hence, the loss of deep sleep may be causally associated with delirium.
Secondly, we also observed a higher frequency of awakenings and apnea during sleep in patients with POD (Figure 3). A higher frequency of awakenings indicates a more pronounced degree of sleep fragmentation. In a multicenter study, the researchers recruited 150 ICU patients and recorded their sleep via actigraphy. The study found that sleep fragmentation was related to worse cognitive performance assessed by the Repeatable Battery for the Assessment of Neuropsychological Status after seven days of ICU discharge.41 Evidence suggests that sleep fragmentation is linked to impaired spatial memory, verbal fluency, attention, and executive function, contributing to cognitive dysfunction in older adults.42 Additionally, intermittent hypoxemia caused by sleep apnea can mediate cognitive dysfunction via decreasing brain-derived neurotrophic factor levels, damaging the blood-brain barrier, and increasing the activation of pro-inflammatory factors and microglia.43 The Toll-like receptor 4-myeloid differentiation primary response 88 (TLR2-MyD88) signaling pathway is one of the mechanistic pathways by which hypoxia triggers neuroinflammatory responses in hippocampal CA1 and dentate gyrus (DG) areas.44 The CA1 and DG areas are essential in consolidating hippocampus-dependent learning and memory and maintaining normal cognitive function.45
Thirdly, our study found a significant difference in sleep efficiency between patients with and without POD (Figure 3). Meanwhile, there was no difference in total sleep duration, further demonstrating that patients with POD have a longer sleep latency and have more difficulty falling asleep.
All in all, pre-existing sleep disturbances combined with poor perioperative sleep quality could induce overlapping pathophysiological pathways of delirium and lead to the occurrence of POD. However, the present study did not calculate the sample size based on perioperative sleep patterns. Therefore, the impact of perioperative sleep patterns on POD is explorative. Given the results of the current study, we plan to design a new protocol to investigate this relationship.
The follow-up results of this study found that the patients in the POD group had a higher hospitalization cost during their hospital stay and poor mobility at the time of discharge. These results were in line with previous findings.46 Although the course of POD is usually transient and characterized by fluctuations, POD is also associated with an increased risk of long-term cognitive impairment.6 The EORTC QLQ-C30 showed that patients with POD had lower cognitive function scores 3 months after surgery than those without POD, but the result did not differ significantly. The outcome may be attributed to our inadequate sample size and the scale’s relatively simple and rough evaluation of cognitive function, which only assesses two dimensions: attention and memory.
The present study systematically investigated the correlation between sleep disturbances and POD, including the sleep disturbances presented at the admission and an in-depth analysis of perioperative sleep patterns. The results from this study elucidate a correlation between sleep disturbances and POD, and thus may help in reducing the occurrence of POD. Notably, 30% to 40% of cases of delirium are preventable.47 The clinical care strategies for elderly postoperative patients and the perioperative use of melatonin have been demonstrated to reduce the incidence of POD.48,49 Thus, targeted strategies to identify patients at risk of POD in advance and intervene aggressively might lead to significantly improved clinical outcomes.
However, there are also some limitations. First, polysomnography is the golden standard for monitoring sleep patterns, but due to the cumbersome procedures, we chose to use a dedicated sleep device. Although we did not test its reliability in patients undergoing craniotomy, previous studies have demonstrated its significant correlation with polysomnography. For the sleep stages, the mean agreement ratio was 77.5% compared with polysomnography.29 The accuracy, sensitivity, and specificity of the estimated apnea hypopnea index were 87.3%, 85.7%, and 88.1%, respectively, compared with polysomnography.30 Second, this was a single-center cohort study, which may limit the generalizability of our result to other institutions. However, this study was conducted in one of the top neurosurgical specialty hospitals in China. Patients admitted to the hospital include various neurosurgical conditions from most parts of China; the annual surgery volume is about 4000 cases. Therefore, our research unit ensured the diversity of neurosurgical patients.
Conclusions
In conclusion, we found that sleep disturbances were independent risk factors for POD. In addition, perioperative sleep patterns, including sleep latency, deep sleep duration, frequency of awakenings, apnea-hypopnea index, and sleep efficiency, were found to be associated with POD. From our results, we can infer the correlation between sleep disturbances and POD. Therefore, ameliorating sleep disturbances may help in lowering the likelihood of POD onset in patients undergoing craniotomy.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author (Baoguo Wang) upon request.
Ethics Statements
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Sanbo Brain Hospital of Capital Medical University (SBNK-YJ-2022-011-01). All patients had signed an informed consent form for inclusion.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by the Application and Evaluation of Active Health Cloud Platform in China, National Key Research and Development Program of China (2018YFC2000704).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Han Y, Miao M, Li P, et al. EEG-parameter-guided anesthesia for prevention of emergence delirium in children. Brain Sci. 2022;12(9):1195. doi:10.3390/brainsci12091195
2. Li S, Li R, Li M, et al. Dexmedetomidine administration during brain tumour resection for prevention of postoperative delirium: a randomised trial. Br J Anaesth. 2023;130(2):e307–e316. doi:10.1016/j.bja.2022.10.041
3. Leung JM, Sands LP, Newman S, et al. Preoperative sleep disruption and postoperative delirium. J Clin Sleep Med. 2015;11(8):907–913. doi:10.5664/jcsm.4944
4. Hughes CG, Boncyk CS, Culley DJ, et al. American society for enhanced recovery and perioperative quality initiative joint consensus statement on postoperative delirium prevention. Anesth Analg. 2020;130(6):1572–1590. doi:10.1213/ANE.0000000000004641
5. Li T, Li J, Yuan L, et al. Effect of regional vs general anesthesia on incidence of postoperative delirium in older patients undergoing hip fracture surgery: the RAGA randomized trial. JAMA. 2022;327(1):50–58. doi:10.1001/jama.2021.22647
6. Goldberg TE, Chen C, Wang Y, et al. Association of delirium with long-term cognitive decline: a meta-analysis. JAMA Neurol. 2020;77(11):1373–1381. doi:10.1001/jamaneurol.2020.2273
7. Bramley P, McArthur K, Blayney A, McCullagh I. Risk factors for postoperative delirium: an umbrella review of systematic reviews. Int J Surg. 2021;93:106063. doi:10.1016/j.ijsu.2021.106063
8. Wang CM, Huang HW, Wang YM, et al. Incidence and risk factors of postoperative delirium in patients admitted to the ICU after elective intracranial surgery: a prospective cohort study. Eur J Anaesthesiol. 2020;37(1):14–24. doi:10.1097/eja.0000000000001074
9. Chen H, Jiang H, Chen B, et al. The incidence and predictors of postoperative delirium after brain tumor resection in adults: a cross-sectional survey. World Neurosurg. 2020;140:e129–e139. doi:10.1016/j.wneu.2020.04.195
10. Li X, Wang Y, Liu J, et al. Effects of perioperative interventions for preventing postoperative delirium: a protocol for systematic review and meta-analysis of randomized controlled trials. Medicine. 2021;100(29):e26662. doi:10.1097/md.0000000000026662
11. Huang Y, Ainsley JA, Reijmers LG, Jackson FR. Translational profiling of clock cells reveals circadianly synchronized protein synthesis. PLoS biol. 2013;11(11):e1001703. doi:10.1371/journal.pbio.1001703
12. Karki S, Castillo K, Ding Z, et al. Circadian clock control of eIF2α phosphorylation is necessary for rhythmic translation initiation. Proc Natl Acad Sci USA. 2020;117(20):10935–10945. doi:10.1073/pnas.1918459117
13. Gao L, Li P, Gaykova N, et al. Circadian rest-activity rhythms, delirium risk, and progression to dementia. Ann Neurol. 2023;93(6):1145–1157. doi:10.1002/ana.26617
14. Skeldon AC, Phillips AJ, Dijk DJ. The effects of self-selected light-dark cycles and social constraints on human sleep and circadian timing: a modeling approach. Sci Rep. 2017;7:45158. doi:10.1038/srep45158
15. Zhu B, Dong Y, Xu Z, et al. Sleep disturbance induces neuroinflammation and impairment of learning and memory. Neurobiol Dis. 2012;48(3):348–355. doi:10.1016/j.nbd.2012.06.022
16. Ni P, Dong H, Zhou Q, Wang Y, Sun M, Qian Y. Preoperative sleep disturbance exaggerates surgery-induced neuroinflammation and neuronal damage in aged mice. Mediators Inflammation. 2019;2019:8301725. doi:10.1155/2019/8301725
17. Taylor J, Parker M, Casey CP, et al. Postoperative delirium and changes in the blood-brain barrier, neuroinflammation, and cerebrospinal fluid lactate: a prospective cohort study. Br J Anaesth. 2022;129(2):219–230. doi:10.1016/j.bja.2022.01.005
18. Jeon MS, Dhillon HM, Koh ES, et al. Exploring sleep disturbance among adults with primary or secondary malignant brain tumors and their caregivers. Neurooncol Pract. 2021;8(1):48–59. doi:10.1093/nop/npaa057
19. Willis KD, Ravyts SG, Lanoye A, Loughan AR. Sleep disturbance in primary brain tumor: prevalence, risk factors, and patient preferences. Support Care Cancer. 2022;30(1):741–748. doi:10.1007/s00520-021-06476-3
20. O’Gara BP, Gao L, Marcantonio ER, Subramaniam B. Sleep, pain, and cognition: modifiable targets for optimal perioperative brain health. Anesthesiology. 2021;135(6):1132–1152. doi:10.1097/aln.0000000000004046
21. Wang H, Zhang L, Luo Q, Li Y, Yan F. Effect of sleep disorder on delirium in post-cardiac surgery patients. Can J Neurol Sci. 2020;47(5):627–633. doi:10.1017/cjn.2020.62
22. Ibala R, Mekonnen J, Gitlin J, et al. A polysomnography study examining the association between sleep and postoperative delirium in older hospitalized cardiac surgical patients. J Sleep Res. 2021;30(5):e13322. doi:10.1111/jsr.13322
23. Cok OY, Seet E, Kumar CM, Joshi GP. Perioperative considerations and anesthesia management in patients with obstructive sleep apnea undergoing ophthalmic surgery. J Cataract Refract Surg. 2019;45(7):1026–1031. doi:10.1016/j.jcrs.2019.02.044
24. Lu L, Wang SB, Rao W, et al. The prevalence of sleep disturbances and sleep quality in older Chinese adults: a comprehensive meta-analysis. Behav Sleep Med. 2019;17(6):683–697. doi:10.1080/15402002.2018.1469492
25. Ulsa MC, Xi Z, Li P. Association of poor sleep burden in middle age and older adults with risk for delirium during hospitalization. J Gerontol. 2022;77(3):507–516. doi:10.1093/gerona/glab272
26. Zheng J, Wang L, Wang W, et al. Association and prediction of subjective sleep quality and postoperative delirium during major non-cardiac surgery: a prospective observational study. BMC Anesthesiol. 2023;23(1):306. doi:10.1186/s12871-023-02267-x
27. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4
28. Mitsukura Y, Sumali B, Nagura M, Fukunaga K, Yasui M. Sleep stage estimation from bed leg ballistocardiogram sensors. Sensors. 2020;20(19):5688. doi:10.3390/s20195688
29. Kurihara Y, Watanabe K. Sleep-stage decision algorithm by using heartbeat and body-movement signals. IEEE Trans Syst Man Cybern. 2012;42(6):1450–1459. doi:10.1109/tsmca.2012.2192264
30. Ding F, Cotton-Clay A, Fava L, et al. Polysomnographic validation of an under-mattress monitoring device in estimating sleep architecture and obstructive sleep apnea in adults. Sleep Med. 2022;96:20–27. doi:10.1016/j.sleep.2022.04.010
31. Flink BJ, Rivelli SK, Cox EA, et al. Obstructive sleep apnea and incidence of postoperative delirium after elective knee replacement in the nondemented elderly. Anesthesiology. 2012;116(4):788–796. doi:10.1097/ALN.0b013e31824b94fc
32. Fadayomi AB, Ibala R, Bilotta F, Westover MB, Akeju O. A systematic review and meta-analysis examining the impact of sleep disturbance on postoperative delirium. Crit Care Med. 2018;46(12):e1204–e1212. doi:10.1097/CCM.0000000000003400
33. Patel J, Baldwin J, Bunting P, Laha S. The effect of a multicomponent multidisciplinary bundle of interventions on sleep and delirium in medical and surgical intensive care patients. Anaesthesia. 2014;69(6):540–549. doi:10.1111/anae.12638
34. Wadhwa M, Prabhakar A, Ray K, et al. Inhibiting the microglia activation improves the spatial memory and adult neurogenesis in rat hippocampus during 48 h of sleep deprivation. J Neuroinflammation. 2017;14(1):222. doi:10.1186/s12974-017-0998-z
35. Racine AM, Touroutoglou A, Abrantes T, et al. Older patients with alzheimer’s disease-related cortical atrophy who develop post-operative delirium may be at increased risk of long-term cognitive decline after surgery. J Alzheimers Dis. 2020;75(1):187–199. doi:10.3233/JAD-190380
36. Rahayel S, Gaubert M, Postuma RB, et al. Brain atrophy in Parkinson’s disease with polysomnography-confirmed REM sleep behavior disorder. Sleep. 2019;42(6). doi:10.1093/sleep/zsz062
37. Kappen PR, Kakar E, Dirven CMF, et al. Delirium in neurosurgery: a systematic review and meta-analysis. Neurosurg Rev. 2022;45(1):329–341. doi:10.1007/s10143-021-01619-w
38. Evans JL, Nadler JW, Preud’homme XA, et al. Pilot prospective study of post-surgery sleep and EEG predictors of post-operative delirium. Clin Neurophysiol. 2017;128(8):1421–1425. doi:10.1016/j.clinph.2017.05.004
39. Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018;17(11):1016–1024. doi:10.1016/s1474-4422(18)30318-1
40. Girardeau G, Lopes-Dos-Santos V. Brain neural patterns and the memory function of sleep. Science. 2021;374:
41. Wilcox ME, McAndrews MP, Van J, et al. Sleep fragmentation and cognitive trajectories after critical illness. Chest. 2021;159(1):366–381. doi:10.1016/j.chest.2020.07.036
42. Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident alzheimer’s disease and cognitive decline in older persons. Sleep. 2013;36(7):1027–1032. doi:10.5665/sleep.2802
43. Liu X, Ma Y, Ouyang R, et al. The relationship between inflammation and neurocognitive dysfunction in obstructive sleep apnea syndrome. J Neuroinflammation. 2020;17(1):229. doi:10.1186/s12974-020-01905-2
44. Li W, Yu Y, Li D, et al. TLR2 deficiency attenuated chronic intermittent hypoxia-induced neurocognitive deficits. Int Immunopharmacol Apr. 2020;81:106284. doi:10.1016/j.intimp.2020.106284
45. Pilly PK, Howard MD, Bhattacharyya R. Modeling contextual modulation of memory associations in the hippocampus. Front Hum Neurosci. 2018;12:442. doi:10.3389/fnhum.2018.00442
46. de la Varga-Martinez O, Gutierrez-Bustillo R, Munoz-Moreno MF, Lopez-Herrero R, Gomez-Sanchez E, Tamayo E. Postoperative delirium: an independent risk factor for poorer quality of life with long-term cognitive and functional decline after cardiac surgery. J Clin Anesth. 2023;85:111030. doi:10.1016/j.jclinane.2022.111030
47. Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35(4):350–364. doi:10.1093/ageing/afl005
48. Chen CC, Li HC, Liang JT, et al. Effect of a modified hospital elder life program on delirium and length of hospital stay in patients undergoing abdominal surgery: a cluster randomized clinical trial. JAMA Surgery. 2017;152(9):827–834. doi:10.1001/jamasurg.2017.1083
49. Han Y, Wu J, Qin Z, et al. Melatonin and its analogues for the prevention of postoperative delirium: a systematic review and meta-analysis. J Pineal Res. 2020;68(4):e12644. doi:10.1111/jpi.12644
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

