Back to Journals » Nature and Science of Sleep » Volume 16
Functional Connectivity Changes in Amygdala Subregions of Obstructive Sleep Apnea Patients After Six Months of Continuous Positive Airway Pressure Treatment
Authors Zeng L , Shu Y, Xie W, Zeng Y, Li K, Long T, Huang L, Liu X, Li H , Peng D
Received 1 November 2023
Accepted for publication 16 January 2024
Published 5 February 2024 Volume 2024:16 Pages 99—109
DOI https://doi.org/10.2147/NSS.S442253
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Valentina Alfonsi
Li Zeng,1 Yongqiang Shu,1,2 Wei Xie,3 Yaping Zeng,4 Kunyao Li,5 Ting Long,1 Ling Huang,1 Xiang Liu,1 Haijun Li,1,2 Dechang Peng1,2
1Department of Radiology, the First Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, People’s Republic of China; 2Department of PET, the First Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, People’s Republic of China; 3Department of Radiology, the Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, People’s Republic of China; 4Department of Radiology, Jiangxi Provincial People’s Hospital, Nanchang, People’s Republic of China; 5Department of Radiology, Yongchuan Hospital of Chongqing Medical University, Chongqing, People’s Republic of China
Correspondence: Dechang Peng; Haijun Li, The First Affiliated Hospital, Jiangxi Medical College, Nanchang University, No. 17, Yongwai Zheng Street, Donghu District, Nanchang, Jiangxi Province, 330006, People’s Republic of China, Tel +86 79186427565, Email [email protected]; [email protected]
Purpose: Previous studies demonstrated that there was abnormal functional connectivity (FC) in the amygdala subregions in obstructive sleep apnea (OSA), which was associated with cognitive function. However, it is not clear whether these abnormalities can be improved after continuous positive airway pressure (CPAP) treatment. Therefore, the aim of this research was to investigate the changes in FC of amygdala subregions with other brain regions after 6 months of CPAP treatment (post-CPAP) in patients with OSA.
Patients and Methods: Fifteen OSA patients underwent Magnetic Resonance Imaging prior to CPAP treatment (pre-CPAP) and following CPAP treatment. The amygdala was divided into six subregions, including bilateral dorsal amygdala (DA), medial amygdala (MA) and ventral amygdala (VA). The FC was calculated by using the amygdala subregions as seeds. A paired sample T-test was employed to assess alterations in the amygdala subregions FC of pre-CPAP and post-CPAP OSA patients, and correlation analysis was then conducted to evaluate the association between the changed FC and clinical assessment.
Results: Compared to pre-CPAP OSA patients, post-CPAP OSA patients displayed an enhanced FC between the left DA and the right posterior cingulate cortex (PCC), whereas the FC between the left MA and the right postcentral gyrus, and between the right MA and the left middle frontal gyrus, decreased. Moreover, significant correlation between the FC value of left DA-right PCC and Hamilton Anxiety Inventory scores was found in pre-CPAP OSA patients.
Conclusion: Altered FC between the amygdala subregions and other brain regions in OSA patients induced by CPAP treatment was related to cognitive, emotional, and sensorimotor function. Our study found altered FC between amygdala subregions and cognitive and motor-related brain regions in post-CPAP OSA patients, providing potential neuroimaging indicators for CPAP treatment.
Keywords: obstructive sleep apnea, amygdala, functional connectivity, treatment, emotion
Introduction
Obstructive Sleep Apnea (OSA), the most prevalent form of sleep apnea, is marked by a recurrent partial or total collapse of the upper airway during sleep, leading to intermittent hypoventilation or cessation of airflow despite respiratory effort.1 The overall population’s OSA prevalence ranged from 9% to 38%, with an increase in prevalence with weight and age.2,3 OSA is a public health issue that leads to a range of health problems, including hypertension, atrial fibrillation,4 type 2 diabetes,5 depression, anxiety, and cognitive impairment.6,7 In addition, brain alterations have been observed in patients with OSA, especially in brain regions associated with mood and cognitive function, which are mainly thought to be caused by ischemia-reperfusion injury, oxidative stress, and hypoxemia.8–10 However, the exact neural mechanisms underlying cognitive dysfunction, anxiety and depression caused by OSA remain unclear.
The amygdala is a brain region essential for emotion processing and links to emotions such as fear, anxiety, and reward.11 Several previous studies have suggested that atrophy and dysfunction of the amygdala are associated with emotional and cognitive impairment in patients with OSA, which may explain the impairments of emotion and memory in OSA.12–14 Moreover, some studies found that the amygdala consists of different subregions that were involved in various emotional processes through distinct different pathways.15,16 Resting-state functional magnetic resonance imaging (rs-fMRI) was employed by Bickart to divide the amygdala into three subregions: the dorsal amygdala (DA), medial amygdala (MA), and ventral amygdala (VA), which are associated with networks supporting social aversion, social affiliation, and social cognition, respectively.17 Some studies found that amygdala subregions of depression and obsessive-compulsive disorder patients altered.18,19 Previously, we discovered an abnormal functional connectivity (FC) between the amygdala subregions and other brain regions in OSA sufferers, implying that OSA had a selective impact on amygdala subregions which were more sensitive to hypoxia, and that these impairments were connected to both affective and cognitive impairment in those with OSA.20 However, it is not clear whether these abnormalities can be improved after treatment. Therefore, a longitudinal study of changes in the amygdala subregions of patients with OSA is necessary.
Continuous positive airway pressure (CPAP), a primary and successful treatment for OSA patients, can prevent cerebral tissue hypoxia caused by OSA.21 It has been demonstrated in numerous studies to reduce daytime sleepiness symptoms and enhance cardiovascular health and cognitive function.22–24 However, one study proposed that certain brain regions associated with cognitive functioning may be less responsive to CPAP.25 But there are no studies about the underlying neural mechanisms in the amygdala subregions in response to CPAP treatment.
The brain structure and function of individuals with OSA have been profoundly illuminated by neuroimaging studies. According to previous studies, a month of CPAP treatment increased degree centrality (DC) values and changed regional homogeneity (ReHo) in some brain regions, such as the frontal and temporal lobes;26,27 three months of CPAP treatment increased connectivity of the default mode network (DMN);28 but white matter had no reversible changes after short-term CPAP treatment.29 Reports of the influence of CPAP on brain performance in OSA patients using FC, a technique that shows a statistically significant relationship between the time series of anatomically distinct brain regions and displays demonstrable functional connections among widely separated brain regions,30 have been few. In addition, seed-based FC, the most widespread and fundamental FC approach, has been deemed both sensitive and dependable.31 It has been extensively employed in a range of objective assessments of brain functioning, such as in sleep and neuropsychiatric disorders.20,32 However, the FC pattern in the amygdala subregions of patients with OSA after CPAP treatment remains unclear.
Therefore, we hypothesized that the FC of the amygdala subregions in patients with OSA might alter after six months of CPAP treatment. We employ a seed-based resting-state FC approach in this study to investigate any changes in FC pattern between each amygdala subregion and other brain regions of OSA caused by CPAP treatment. Then we investigated the correlation between changed FC and clinical factors to investigate the possible neuroimaging processes that could be responsible for the FC alterations caused by CPAP therapy.
Materials and Methods
Patients
All patients were right-handed, native speakers of Chinese, who had not previously received CPAP treatment, recruited from the Sleep Center of the First Affiliated Hospital of Nanchang University. The diagnostic criteria were in accordance with the clinical practice guidelines for adult OSA proposed by the American Academy of Sleep Medicine (AASM) in 2018.33 Patients with an apnea-hypopnea index (AHI)>15/h, defined as moderate-to-severe OSA, were included in this study. Exclusion criteria were as follows: (1) misuse of illegal drugs or alcohol, and current use of psychotropic drugs; (2) past cardiovascular, neurological, or psychiatric illnesses and diabetes; (3) other sleep disorders; and (4) contraindications to MRI. Finally, 15 patients with OSA who had complied with CPAP therapy for at least 6 months were included in the analysis. We obey the principles of the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University, and all participants gave written consent.
Polysomnography
Patients were asked to abstain from hypnotics, alcohol, and coffee the day prior to undergoing overnight polysomnography (PSG). Items recorded on a PSG monitor (Alice 5 LE, Respironics, Orlando, FL, USA) included body position, thoracic and abdominal breathing movements, snoring, oral and nasal airflow, electroencephalogram, electrooculography, electrocardiography, electrocardiogram, and chin electromyography. The total sleep duration, efficiency, latency, stages, oxygen saturation (SaO2), awakening, and respiratory events were all documented.34 Obstructive apnea was defined as a decrease of more than 90% in airflow or no airflow for at least 10 seconds. Hypopnea was characterized as a ≥ 30% drop in airflow for ≥ 10 seconds associated with a ≥ 3% drop in the oxygen saturation or an arousal. AHI was determined as the total number of apnea and hypopnea events per hour during sleep.
Neuropsychological Assessment
At baseline (pre-CPAP) and after six months of CPAP follow-up (post-CPAP), all patients with OSA were given the Epworth Sleepiness Scale (ESS), Pittsburgh Sleep Quality Index (PSQI), Hamilton Depression Inventory (HAMD), Hamilton Anxiety Inventory (HAMA), and Montreal Cognitive Assessment (MoCA). The ESS is used to gauge daytime sleepiness, with a range of 0 to 24, with higher scores indicating more intense sleepiness. The PSQI, a measure of subjective sleep quality, is used to evaluate scores ranging from 0 to 21, with higher scores indicating poorer quality. The HAMD and HAMA were then utilized to evaluate the patients’ levels of depression and anxiety. Generally, a score of less than 7 is considered normal, 7–17 implies potential depression, 17–24 implies definite depression, and >24 implies extreme depression. HAMA scores greater than 14 indicate significant anxiety symptoms. MoCA’s assessment of cognitive functioning yields a total score and seven syndrome scores, including visual space and execution, naming, delayed memory, attentional function, language, abstracting, and orientating. A total MoCA score below 26 is considered to indicate cognitive impairment. All surveys and MRI acquisitions were done on the same day.
CPAP Treatment
All patients were deemed eligible for CPAP treatment with the CPAP standardized auto-adjustment model (YH-480, Yuwell, Jiangsu, China) after the clinician health education intervention. The therapeutic pressure of the ventilator was set to 4–20 cmH2O, with automatic pressure titration according to the patient’s condition. The treatment duration was six months, with a frequency of at least 4 h per night and at least 5 days per week. The integrated circuit card of the ventilator, which logged machine usage time automatically, thereby affirming compliance.
MRI Data Acquisition
Using a 3.0 Tesla magnetic resonance scanner (Siemens, Munich, Germany) with an 8-channel phased-array head coil, two acquisitions of MRI data were conducted the day after the PSG test and within 1 week of the completion of six months of CPAP treatment. Subjects were asked to lie on the scan bed with their eyes closed, and to remain awake, tranquil, and relaxed while not thinking about anything during the scans. To reduce the MRI scanner’s noise, soft earplugs were employed, while a pad was utilized to stabilize the head and reduce movement. Routine MRI data were collected from the brain: axis T2WI [pulse repetition time (TR) = 3000 ms, echo time (TE) = 122 ms, field of view (FOV) = 240 mm × 240 mm, matrix = 256 × 256, layer thickness = 5 mm] and axial T1WI (TR = 600 ms, TE = 10 ms, FOV = 240 mm ×240 mm, matrix = 256×256, layer thickness = 5 mm). An echo-planar imaging (EPI) sequence was utilized to acquire resting-state blood oxygen level-dependent data of the entire brain(TR = 2000 ms, TE = 30 ms, FOV = 230 mm × 230 mm, matrix = 64 × 64, layer thickness = 4.0 mm), for the purpose of obtaining rs-fMRI data.
Image Processing and Analysis
Image preprocessing and data analysis performed by DPABI (Chinese Academy of Sciences, Beijing, China, http://rfmri.org/dpabi), which was based on SPM 12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) and running on MATLAB 2018b (MathWorks, Natick, MA, USA). The following steps were taken: (1) transforming the image data from DICOM to NIFTI format; (2) eliminating the initial 10 time points; (3) slice timing correction; (4) the data of the remaining 230 time points were corrected for 3D head movements, and the maximum displacement (x, y, z) > 2.0 mm, maximum rotation > 2.0° or frame displacement of any of the 230 volumes exceeding 2.0 standard deviations were excluded; (5) aligning the functional images of each subject with the EPI template.(6) normalizing the image space to the Montreal Neuroscience Institute (MNI) template, resampling it to 3×3×3 mm3 voxel size, and then smoothing it with a 6-mm full width at half-maxima (FWHM) Gaussian smoothing kernel, the data were filtered using a bandwidth of 0.01~0.08 Hz; (7) linear regression was then employed to regress 24 head movement parameters and white matter and cerebrospinal fluid signals.
Bickart’s research determined six subregions of the bilateral amygdala to be regions of interest (ROI), each with a radius of 3 mm: DA (MNI coordinates ±22,-4,-12), MA (±14,-4,-20), and VA (±28,-4,-22)17 (Figure 1). The Pearson correlation coefficients between each ROI and the other voxels in the entire brain were determined by extracting their mean time series, thus forming a brain-wide Pearson correlation coefficient pattern map with each amygdala subregion as the seed, otherwise known as a FC pattern map. To make the results more consistent with a standard normal distribution, Fisher’s r-to-z transformation was then applied.
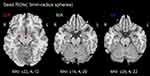 |
Figure 1 The amygdala subregions. Abbreviations: ROI, region of interest; DA, dorsal amygdala; MA, medial amygdala; VA, ventral amygdala; MNI, Montreal Neuroscience Institute; R, right, L; left. |
Statistical Analysis
The Kolmogorov–Smirnov test was applied to demographic and clinical data to ascertain if the data followed a normal distribution. Paired t-test and paired-on-sample rank-sum test were applied for data with normal and abnormal distribution, respectively. The p <0.05 were considered statistically significant. A single-sample t-test was initially employed to evaluate the spatial distribution of FC in each amygdala subregion and the entire brain prior to and following CPAP. Subsequently, a paired t-test was conducted with head movement as the covariate, based on the amygdala subregions as the seed point, using DPABI software to analyze FC disparities between the pre-CPAP and post-CPAP. A Gaussian random field theory (GRF) two-tailed correction was used to consider statistical differences at the voxel level with p < 0.01 and at the cluster level with p < 0.05. Pearson’s and Spearman correlation analyses were then conducted to explore the correlation between FC discrepancy and clinical and neuropsychological variables at pre-CPAP and post-CPAm,P, with data having a normal and non-normal distribution, respectively.
Results
Demographic and Clinical Assessment Results
Table 1 displays the demographic and clinical characteristics of patients with OSA before and after CPAP. Compared to pre-CPAP OSA, we observed a statistically significant difference in ESS, HAMA, HAMD, MoCA, and delayed recall of MoCA scale scores post-CPAP (p <0.05). However, no significant differences were found in body mass index (BMI) or PSQI scores.
 |
Table 1 Characteristics of Participant Demographics and Clinical Assessment Information |
Functional Connectivity Differences
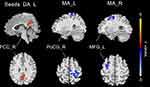
Before and after CPAP treatment, the FC patterns of the various functional amygdala subregions in OSA patients were similar (Figure 2). In comparison to pre-CPAP, the FC between the left DA and the right posterior cingulate cortex (PCC) was notably augmented, whereas the FC between the left MA and the right postcentral gyrus (PoCG) and the FC between the right MA and the left middle frontal gyrus (MFG) were diminished in post-CPAP OSA patients (Table 2 and Figure 3). No statistically noteworthy disparity in bilateral VA was observed between pre- and post-CPAP patients with OSA.
 |
Table 2 Brain Areas Showing Functional Connectivity Disparities with Amygdala Subregions Between Pre-CPAP OSA and Post-CPAP OSA |
Correlation Analysis
A positive correlation (r = 0.558, p = 0.031) between FC values of the left DA-right PCC in pre-CPAP OSA and HAMA was observed (Figure 4). However, no significant relationship between altered FC and ESS, HAMA, HAMD, MoCA, or delayed recall was found in patients with OSA after CPAP.
Discussion
To the best of our knowledge, this study is the first to investigate the impact of CPAP treatment on the FC between amygdala subregions and the entire brain in OSA patients. After six months of CPAP, the FC of the left DA with the right PCC was increased, which was correlated with the HAMA in pre-CPAP OSA, while the FC of the left MA with the right PoCG and the right MA with the left MFG were both decreased compared to the baseline. In addition, anxiety, depressive mood, daytime sleepiness, cognitive function, and delayed recall improved after CPAP. These results suggest that CPAP treatment can partially reverse brain damage in patients with OSA and provide important clues to the debate regarding the pathogenesis of emotional and cognitive impairment and its reversibility in OSA.
After six months of CPAP treatment, a marked improvement in FC between the left DA and the right PCC was observed in this study. The DA plays functionally specific role in the control of behavior and processes olfactory, emotional, and socially relevant information.35,36 Moreover, the PCC, a central node of the DMN, has been found to have a significant impact on spatial orientation and memory function,37,38 and is strongly connected to other cognitively relevant structures, such as parahippocampus.39 Previous studies have also revealed that OSA patients have an altered volume and metabolism of the PCC40,41 and that FC between it and various brain regions related to cognitive function has been altered, suggesting that PCC dysfunction is an important neuropathophysiological mechanism of neurocognitive impairment.42,43 In addition, a previous study found that the functional connectivity in the DMN is associated with hypoxemia.44 Our results, in agreement with prior studies that demonstrate CPAP treatment boosts DMN connectivity28 and further demonstrate that CPAP treatment is seen to contribute to the functional integration of the amygdala and DMN. This enhanced FC could be attributed to CPAP to enhance glucose uptake in the DMN,45 a key factor in emotional processing and cognitive function. We also found that the FC values between the left DA and right PCC in patients with OSA at baseline were positively correlated with HAMA, which could explain in part the emotional dysfunction caused by impaired PCC.
A diminished FC between the left MA and right PoCG was discovered. MA plays a crucial role in social behavioral responses, contains both sensory and behavioral properties.46 PoCG, as a somatosensory center, belongs to the sensorimotor network (SMN). SMN’s dysfunction is associated with sleep disorders47 and OSA severity, which may lead to reduced information-processing speed and executive dysfunction.45 However, patients with OSA exhibit inconsistent alterations in the SMN. Studies have revealed that ReHo,48 FC,13 and regional cerebral blood flow49 are diminished in the precentral and postcentral gyrus, which may be due to a change in sensory input and motor output in the upper airway, resulting in a decrease of lingual muscle tissue tone and airway collapse.13,50 A previous study showed regional ReHo activities increased in the bilateral PoCG in OSA patients before treatment and short-term CPAP treatment reduced ReHo signals in the PoCG, which was further correlated with improved sleep quality.51 Our earlier research revealed an augmented FC in the precentral gyrus and PoCG,31,52 which could be a compensatory mechanism for the previously mentioned decreased function. Thus, our findings suggest that CPAP treatment can effectively reverse the compensatory responses induced by OSA.
In addition, Post-CPAP patients with OSA showed a decrease in FC between the right MA and the left MFG, which was part of the Frontoparietal Control Network (FPN) and was linked to executive function and cognitive control, as well as working memory.53,54 Studies have indicated a decrease in ReHo and fractional amplitude of low-frequency fluctuations in the left MFG, implying that nocturnal intermittent hypoxemia may impair FPN function, resulting in cognitive dysfunction.48,55 Moreover, some studies have revealed an increase in DC values of the MFG in patients with OSA treated with CPAP for one month26 and an augmentation in connectivity of the MFG after three months of CPAP treatment,28 suggesting that CPAP treatment can restore brain damage caused by OSA. Our previous studies have found enhanced FC between the amygdala, hippocampus, insula, and FPN, reflecting a potential compensatory mechanism of FPN function.20,31,52 Consequently, the FC between the right MA and left MFG may be indicative of a withdrawal of adaptive compensatory mechanisms. CPAP has been shown to enhance brain plasticity and promote metabolic alterations in the frontal and connecting brain regions,56 suggesting that frontal plasticity may explain the FC alterations in this study.
Our prior research demonstrated a noteworthy decrease in FC between the right DA and the right prefrontal cortex,20 yet this investigation revealed no noteworthy enhancement in the prefrontal cortex - in agreement with some prior studies.57,58 Two explanations may be given for this: the inability of six months CPAP treatment to completely restore brain function and clinical changes in those with OSA, and the potential for hypoxic damage in OSA to cause neuronal loss, which, unlike neuronal dysfunction, cannot be reversed by CPAP.59 Further studies are required to assess the impact of complete adherence to long-term CPAP treatment.
Limitations
This study has several limitations. The sample size being limited to 15 subjects, mainly men, necessitates further validation of the results. Furthermore, due to the absence of a placebo-treated group, we were unable to compare and observe if those with OSA treated with placebo would display alterations in brain function akin to those observed in our study. Finally, the PSG’s lack in patients’ post-treatment and the inaccuracy of AHI data from the ventilator’s integrated circuit card necessitate a deeper investigation into the impact of treatment and its connection to the restoration of sleep architecture.
Conclusion
In conclusion, we found that abnormal FC between amygdala subregions and other brain regions after CPAP treatment can be partly reversed in patients with OSA, which are important for emotional function. These findings provide a new imaging perspective to further understand the underlying neural mechanisms of CPAP treatment response, thus suggesting a new direction for exploring CPAP treatment assessment in patients with OSA.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant No. 81860307), Clinical Research Center For Medical Imaging In Jiangxi Province (No.20223BCG74001), the Natural Science Foundation Project of Jiangxi, China (Grant Nos. 20202BABL216036, 20181ACB20023), Science and technology plan project of Jiangxi Administration of Traditional Chinese Medicine (Grant No 2023A0278), and Department of Health Project and Jiangxi Province, China (Grant No. 202210211).
Disclosure
The authors declare that the study was conducted without any commercial or financial ties that could be seen as a possible conflict of interest.
References
1. Gottlieb DJ, Punjabi NM. Diagnosis and Management of Obstructive Sleep Apnea: a Review. JAMA. 2020;323(14):1389. doi:10.1001/jama.2020.3514
2. Wang SH, Keenan BT, Wiemken A, et al. Effect of Weight Loss on Upper Airway Anatomy and the Apnea–Hypopnea Index. The Importance of Tongue Fat. Am J Respir Crit Care Med. 2020;201(6):718–727. doi:10.1164/rccm.201903-0692OC
3. Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. doi:10.1016/j.smrv.2016.07.002
4. Yeghiazarians Y, Jneid H, Tietjens JR, et al. Obstructive Sleep Apnea and Cardiovascular Disease: a Scientific Statement From the American Heart Association. Circulation. 2021;144(3):e56–e67. doi:10.1161/CIR.0000000000000988
5. Lin J, Song H, Liang M, et al. Advances in the study of OSA and diabetic foot. Diabetol Metab Syndr. 2022;14(1):70. doi:10.1186/s13098-022-00842-9
6. Liguori C, Maestri M, Spanetta M, et al. Sleep-disordered breathing and the risk of Alzheimer’s disease. Sleep Med Rev. 2021;55:101375. doi:10.1016/j.smrv.2020.101375
7. Vanek J, Prasko J, Genzor S, et al. Obstructive sleep apnea, depression and cognitive impairment. Sleep Med. 2020;72:50–58. doi:10.1016/j.sleep.2020.03.017
8. Rosenzweig I, Glasser M, Polsek D, Leschziner GD, Williams SCR, Morrell MJ. Sleep apnoea and the brain: a complex relationship. Lancet Respir Med. 2015;3(5):404–414. doi:10.1016/S2213-2600(15)00090-9
9. Goldstein AN, Walker MP. The Role of Sleep in Emotional Brain Function. Annu Rev Clin Psychol. 2014;10(1):679–708. doi:10.1146/annurev-clinpsy-032813-153716
10. Grandner MA. Sleep, Health, and Society. Sleep Med Clin. 2017;12(1):1–22. doi:10.1016/j.jsmc.2016.10.012
11. Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517(7534):284–292. doi:10.1038/nature14188
12. Tahmasian M, Rosenzweig I, Eickhoff SB, et al. Structural and functional neural adaptations in obstructive sleep apnea: an activation likelihood estimation meta-analysis. Neurosci Biobehav Rev. 2016;65:142–156. doi:10.1016/j.neubiorev.2016.03.026
13. Park B, Palomares JA, Woo MA, et al. Disrupted functional brain network organization in patients with obstructive sleep apnea. Brain Behav. 2016;6(3):e00441. doi:10.1002/brb3.441
14. Park KM, Kim J. Alterations of Limbic Structure Volumes in Patients with Obstructive Sleep Apnea. Can J Neurol Sci. 2022;1–8. doi:10.1017/cjn.2022.303
15. Han HJ, Lee K, Kim HT, Kim H. Distinctive amygdala subregions involved in emotion-modulated Stroop interference. Soc Cogn Affect Neurosci. 2014;9(5):689–698. doi:10.1093/scan/nst021
16. Amunts K, Kedo O, Kindler M, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol. 2005;210(5–6):343–352. doi:10.1007/s00429-005-0025-5
17. Bickart KC, Hollenbeck MC, Barrett LF, Dickerson BC. Intrinsic Amygdala-Cortical Functional Connectivity Predicts Social Network Size in Humans. J Neurosci. 2012;32(42):14729–14741. doi:10.1523/JNEUROSCI.1599-12.2012
18. Jacob Y, Morris LS, Verma G, Rutter SB, Balchandani P, Murrough JW. Altered hippocampus and amygdala subregion connectome hierarchy in major depressive disorder. Transl Psychiatry. 2022;12(1):209. doi:10.1038/s41398-022-01976-0
19. Cao L, Li H, Liu J, et al. Disorganized functional architecture of amygdala subregional networks in obsessive-compulsive disorder. Commun Biol. 2022;5(1):1184. doi:10.1038/s42003-022-04115-z
20. Yu H, Chen L, Li H, et al. Abnormal resting-state functional connectivity of amygdala subregions in patients with obstructive sleep apnea. NDT. 2019;15:977–987. doi:10.2147/NDT.S191441
21. Schwarz EI, Furian M, Schlatzer C, Stradling JR, Kohler M, Bloch KE. Nocturnal cerebral hypoxia in obstructive sleep apnoea: a randomised controlled trial. Eur Respir J. 2018;51(5). doi:10.1183/13993003.00032-2018
22. Wang G, Goebel JR, Li C, Hallman HG, Gilford TM, Li W. Therapeutic effects of CPAP on cognitive impairments associated with OSA. J Neurol. 2020;267(10):2823–2828. doi:10.1007/s00415-019-09381-2
23. Antic NA, Catcheside P, Buchan C, et al. The Effect of CPAP in Normalizing Daytime Sleepiness, Quality of Life, and Neurocognitive Function in Patients with Moderate to Severe OSA. Sleep. 2011;34(1):111–119. doi:10.1093/sleep/34.1.111
24. Labarca G, Saavedra D, Dreyse J, Jorquera J, Barbe F. Efficacy of CPAP for Improvements in Sleepiness, Cognition, Mood, and Quality of Life in Elderly Patients With OSA: systematic Review and Meta-analysis of Randomized Controlled Trials. Chest. 2020;158(2):751–764. doi:10.1016/j.chest.2020.03.049
25. Munoz A, r ML, Barbe F, Pericas J, Agusti AG. Long-term effects of CPAP on daytime functioning in patients with sleep apnoea syndrome. Eur Respir J. 2000;15(4):676–681. doi:10.1034/j.1399-3003.2000.15d09.x
26. Li P, Shu Y, Liu X, et al. The Effects of CPAP Treatment on Resting-State Network Centrality in Obstructive Sleep Apnea Patients. Front Neurol. 2022;13:801121. doi:10.3389/fneur.2022.801121
27. Li H, Li L, Kong L, et al. Frequency‑Specific Regional Homogeneity Alterations and Cognitive Function in Obstructive Sleep Apnea Before and After Short-Term Continuous Positive Airway Pressure Treatment. NSS. 2021;13:2221–2238. doi:10.2147/NSS.S344842
28. Dalmases M, Solé-Padullés C, Torres M, et al. Effect of CPAP on Cognition, Brain Function, and Structure Among Elderly Patients With OSA. Chest. 2015;148(5):1214–1223. doi:10.1378/chest.15-0171
29. Liu X, Wei Z, Chen L, et al. Effects of 3-month CPAP therapy on brain structure in obstructive sleep apnea: a diffusion tensor imaging study. Front Neurol. 2022;13:913193. doi:10.3389/fneur.2022.913193
30. Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201
31. Kong L, Li H, Shu Y, et al. Aberrant Resting-State Functional Brain Connectivity of Insular Subregions in Obstructive Sleep Apnea. Front Neurosci. 2022;15:765775. doi:10.3389/fnins.2021.765775
32. Kim WS, Shen G, Liu C, et al. Altered amygdala-based functional connectivity in individuals with attenuated psychosis syndrome and first-episode schizophrenia. Sci Rep. 2020;10(1):17711. doi:10.1038/s41598-020-74771-w
33. Malhotra RK, Kirsch DB, Kristo DA, et al. Polysomnography for Obstructive Sleep Apnea Should Include Arousal-Based Scoring: an American Academy of Sleep Medicine Position Statement. J Clin Sleep Med. 2018;14(7):1245–1247. doi:10.5664/jcsm.7234
34. Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13(03):479–504. doi:10.5664/jcsm.6506
35. Heimer L, Van Hoesen GW. The limbic lobe and its output channels: implications for emotional functions and adaptive behavior. Neurosci Biobehav Rev. 2006;30(2):126–147. doi:10.1016/j.neubiorev.2005.06.006
36. Gopal A, Clark E, Allgair A, et al. Dorsal/ventral parcellation of the amygdala: relevance to impulsivity and aggression. Psychiatry Res. 2013;211(1):24–30. doi:10.1016/j.pscychresns.2012.10.010
37. Leech R, Braga R, Sharp DJ. Echoes of the brain within the posterior cingulate cortex. J Neurosci. 2012;32(1):215–222. doi:10.1523/JNEUROSCI.3689-11.2012
38. Foster BL, Koslov SR, Aponik-Gremillion L, Monko ME, Hayden BY, Heilbronner SR. A tripartite view of the posterior cingulate cortex. Nat Rev Neurosci. 2023;24(3):173–189. doi:10.1038/s41583-022-00661-x
39. Vanneste S, Luckey A, McLeod SL, Robertson IH, To WT. Impaired posterior cingulate cortex-parahippocampus connectivity is associated with episodic memory retrieval problems in amnestic mild cognitive impairment. Eur J Neurosci. 2021;53(9):3125–3141. doi:10.1111/ejn.15189
40. Filipovic B, Đuric V, Filipovic N, et al. Anatomical Brain Changes and Cognitive Abilities in Patients with Obstructive Sleep Apnea Syndrome and Nonalcoholic Fatty Liver Disease. Can J Gastroenterol Hepatol. 2021;2021:8873652. doi:10.1155/2021/8873652
41. Yaouhi K, Bertran F, Clochon P, et al. A combined neuropsychological and brain imaging study of obstructive sleep apnea. J Sleep Res. 2009;18(1):36–48. doi:10.1111/j.1365-2869.2008.00705.x
42. Li HJ, Nie X, Gong H, Zhang W, Nie S, Peng DC. Abnormal resting-state functional connectivity within the default mode network subregions in male patients with obstructive sleep apnea. NDT. 2016;203. doi:10.2147/NDT.S97449
43. Li H, Li L, Shao Y, et al. Abnormal Intrinsic Functional Hubs in Severe Male Obstructive Sleep Apnea: evidence from a Voxel-Wise Degree Centrality Analysis. PLoS One. 2016;11(10):e0164031. doi:10.1371/journal.pone.0164031
44. Chang YT, Chen YC, Chen YL, et al. Functional connectivity in default mode network correlates with severity of hypoxemia in obstructive sleep apnea. Brain Behav. 2020;10(12):e01889. doi:10.1002/brb3.1889
45. Fernandes M, Mari L, Chiaravalloti A, et al. 18F-FDG PET, cognitive functioning, and CSF biomarkers in patients with obstructive sleep apnoea before and after continuous positive airway pressure treatment. J Neurol. 2022;269(10):5356–5367. doi:10.1007/s00415-022-11182-z
46. Raam T, Hong W. Organization of neural circuits underlying social behavior: a consideration of the medial amygdala. Curr Opin Neurobiol. 2021;68:124–136. doi:10.1016/j.conb.2021.02.008
47. Cross NE, Memarian N, Duffy SL, et al. Structural brain correlates of obstructive sleep apnoea in older adults at risk for dementia. Eur Respir J. 2018;52(1):1800740. doi:10.1183/13993003.00740-2018
48. Zhou L, Shan X, Peng Y, et al. Reduced regional homogeneity and neurocognitive impairment in patients with moderate-to-severe obstructive sleep apnea. Sleep Medicine. 2020;75:418–427. doi:10.1016/j.sleep.2020.09.009
49. Baril AA, Gagnon K, Arbour C, et al. Regional Cerebral Blood Flow during Wakeful Rest in Older Subjects with Mild to Severe Obstructive Sleep Apnea. Sleep. 2015;38(9):1439–1449. doi:10.5665/sleep.4986
50. Ruehland WR, Rochford PD, Pierce RJ, et al. Genioglossus muscle responses to resistive loads in severe OSA patients and healthy control subjects. J Appl Physiol. 2019;127(6):1586–1598. doi:10.1152/japplphysiol.00186.2019
51. Song X, Roy B, Vacas S, et al. Brain Regional Homogeneity Changes After Short-term Positive Airway Pressure Treatment in Patients with Obstructive Sleep Apnea. Sleep Med. 2022;91:12–20. doi:10.1016/j.sleep.2022.02.005
52. Liu X, Chen L, Duan W, et al. Abnormal Functional Connectivity of Hippocampal Subdivisions in Obstructive Sleep Apnea: a Resting-State Functional Magnetic Resonance Imaging Study. Front Neurosci. 2022;16:850940. doi:10.3389/fnins.2022.850940
53. Briggs RG, Lin YH, Dadario NB, et al. Anatomy and White Matter Connections of the Middle Frontal Gyrus. World Neurosurg. 2021;150:e520–e529. doi:10.1016/j.wneu.2021.03.045
54. Xu P, Wang M, Zhang T, Zhang J, Jin Z, Li L. The role of middle frontal gyrus in working memory retrieval by the effect of target detection tasks: a simultaneous EEG-fMRI study. Brain Struct Funct. 2023. doi:10.1007/s00429-023-02687-y
55. Bai J, Wen H, Tai J, et al. Altered Spontaneous Brain Activity Related to Neurologic and Sleep Dysfunction in Children With Obstructive Sleep Apnea Syndrome. Front Neurosci. 2021;15:595412. doi:10.3389/fnins.2021.595412
56. Ju G, Yoon IY, Lee SD, Kim YK, Yoon E, Kim JW. Modest Changes in Cerebral Glucose Metabolism in Patients with Sleep Apnea Syndrome after Continuous Positive Airway Pressure Treatment. Respiration. 2012;84(3):212–218. doi:10.1159/000338117
57. Toth M, Faludi B, Kondakor I. Effects of CPAP-Therapy on Brain Electrical Activity in Obstructive Sleep Apneic Patients: a Combined EEG Study Using LORETA and Omega Complexity: reversible Alterations of Brain Activity in OSAS. Brain Topogr. 2012;25(4):450–460. doi:10.1007/s10548-012-0243-0
58. Marillier M, Gruet M, Baillieul S, et al. Impaired cerebral oxygenation and exercise tolerance in patients with severe obstructive sleep apnea syndrome. Sleep Med. 2018;51:37–46. doi:10.1016/j.sleep.2018.06.013
59. Tonon C, Vetrugno R, Lodi R, et al. Proton magnetic resonance spectroscopy study of brain metabolism in obstructive sleep apnoea syndrome before and after continuous positive airway pressure treatment. Sleep. 2007;30(3):305–311. doi:10.1093/sleep/30.3.305
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.