Back to Journals » Nature and Science of Sleep » Volume 16
Changes in the Objective Measures of Sleep in Association with Menses Among Female Athletes with Poor Subjective Sleep Quality: Female Athletes with Poor Subjective Sleep Quality Have More Sleep Arousals During Menses
Authors Kawasaki Y , Kasai T , Sakurama Y, Kawana F, Shiroshita N, Koikawa N
Received 10 November 2023
Accepted for publication 11 March 2024
Published 16 April 2024 Volume 2024:16 Pages 381—388
DOI https://doi.org/10.2147/NSS.S449305
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Valentina Alfonsi
Yu Kawasaki,1 Takatoshi Kasai,2– 5 Yuko Sakurama,6 Fusae Kawana,2 Nanako Shiroshita,2 Natsue Koikawa6,7
1Department of Obstetrics and Gynecology, Juntendo University Graduate School of Medicine, Tokyo, Japan; 2Cardiovascular Respiratory Sleep Medicine, Juntendo University Graduate School of Medicine, Tokyo, Japan; 3Department of Cardiovascular Management and Remote Monitoring, Juntendo University Graduate School of Medicine, Tokyo, Japan; 4Department of Cardiovascular Biology and Medicine, Juntendo University Graduate School of Medicine, Tokyo, Japan; 5Sleep and Sleep-Disordered Breathing Center, Juntendo University Hospital, Tokyo, Japan; 6Japanese Center for Research on Women in Sport, Juntendo University, Tokyo, Japan; 7Graduate School of Health and Sports Science, Juntendo University, Chiba, Japan
Correspondence: Takatoshi Kasai, Department of Cardiovascular Biology and Medicine, Juntendo University Graduate School of Medicine, 3-1-3 Hongo, Bunkyo-ku, Tokyo, Japan, Tel +81-33813-3111, Fax +81-5689-0627, Email [email protected]
Purpose: Female athletes with menstrual abnormalities have poor sleep quality. However, whether female athletes with poor sleep quality based on subjective assessment have distinctive changes in objective measures of sleep in association with menses remains unclear. This study aimed to compare changes in objective sleep measurements during and following menses between collegiate female athletes with and without poor subjective sleep quality.
Patients and Methods: Female collegiate athletes (age range/mean ± standard deviation: 18– 22/ 22.2± 1.1) with regular menstrual cycles were recruited. The participants underwent home electroencephalogram monitoring during the first and second nights after the onset of menses and one night between the seventh and 10th nights after menses onset (mid-follicular phase). The Pittsburgh Sleep Quality Index (PSQI) was used to assess the subjective sleep quality. Interactions between the presence of poor subjective sleep quality (ie, PSQI ≥ 6) and changes in objective measures of sleep in association with menses were analyzed.
Results: Data of 45 athletes, including 13 with poor subjective sleep quality, showed that changes in arousal index in athletes with poor subjective sleep quality were distinctive from those in athletes without poor subjective sleep quality (p = 0.036 for interaction). In athletes with poor subjective sleep quality, the arousal index was significantly increased in menses (p for analysis of variance, 0.015), especially on the first night after the onset of menses compared with during the mid-follicular phase (p = 0.016).
Conclusion: Collegiate female athletes with regular menstrual cycles are likely to have poor subjective sleep quality in association with more frequent arousal during the first night after the onset of menses than during the mid-follicular phase.
Keywords: electroencephalogram, menstruation, Pittsburgh sleep quality index, sports, women
Introduction
Despite the crucial role of sleep in recovery after training,1,2 few studies have investigated sleep and sleep issues in athletes.3,4 Women are likely to be dissatisfied with their sleep quality,5,6 and this is true in female athletes.7,8 The reasons for such poor “self-reported/subjective” sleep quality in women remain unclear but could be multifactorial. One reason may include menstruation and its related issues.9 Indeed, in our previous study, menstrual abnormality was associated with poor subjective sleep quality in female athletes.8
In terms of objectively evaluating sleep, in women, electroencephalogram (EEG)-based sleep parameters during the mid-follicular (MF) phase and those during the mid- or late-luteal phase were generally compared, and no differences were noted in most sleep parameters except for a minor reduction in rapid eye movement (REM) sleep during the mid- or late-luteal phase.10–12 However, sleep disturbances were reported during the initial few days after menses onset,13,14 and comparisons of objective measures of sleep during and following menses should be considered. Thus, we previously assessed changes in objective sleep measures based on EEG monitoring during and after menses in athletes and found that female collegiate athletes were likely to have poor objective sleep quality during menses.15
In general, despite the likelihood of poor subjective sleep quality in women,5–8 results are not always bad when sleep quality is assessed objectively.16–19 Thus, there are some discrepancies between subjective and objective sleep quality, particularly in women. Whether female athletes with poor sleep quality, based on subjective assessments, have distinctive changes in objective measures of sleep in association with menses remains unclear. Therefore, we conducted subgroup analyses of our previous study, in which changes in objective sleep measures based on EEG monitoring during and following menses in collegiate female athletes were assessed by comparing changes in objective sleep measures in association with menses between those with and without poor subjective sleep quality.
Materials and Methods
Participants
Details were described elsewhere.15 Briefly, healthy female collegiate athletes (age range/mean: 18–22/ 22.2±1.1) with regular menstrual cycles, defined according to the Japanese Society of Obstetrics and Gynecology (ie, menstrual cycles between 25 and 38 days long with a variation of each cycle within 6 days), were recruited from the Juntendo University School of Health and Sports Science. The exclusion criteria were the use of any drugs, including hormonal contraceptives, premenstrual dysphoric disorder, dysmenorrhea, and other gynecological pathologies that may interfere with sleep patterns, shift work, and transmeridian travel within the past 3 months and during the study period. This study adhered to the Declaration of Helsinki and was approved by the Research Ethics Committee of Juntendo University. Informed consent was obtained from all the participants.
Sleep-Related Baseline Assessments
Sleep-related baseline assessments using the following tools were conducted once at the time of enrollment of all participants. The Pittsburgh Sleep Quality Index (PSQI) was used to assess self-reported sleep quality over a 1-month period.20,21 The total score of the seven components ranges from 0 to 21. The Japanese version (PSQI-J)21 was used, with a cutoff point of 5.5, rounded up to >6, to indicate poor subjective sleep quality in this study. The Epworth Sleepiness Scale (ESS)22 is an established tool for evaluating self-reported sleepiness over a few weeks to months.23 The ESS has been translated into Japanese (JESS) and validated.23 In this study, significant self-reported sleepiness was defined as a JESS score of ≥11.24 Restless legs syndrome was assessed based on the international criteria and a positive answer to all five interview questions.24,25 Moreover, the participants were asked the following questions: (1) Have you snored during the past 1 month? “No”, “<1 night per week”, “1–2 nights per week”, or “>2 nights per week”; and (2) Have you displayed apneas during sleep? “No”, “<1 night per week”, “1–2 nights per week”, or “>2 nights per week”.
Sleep Monitoring
Details were shown elsewhere.15 Briefly, all participants underwent overnight home sleep monitoring using a two-channel portable EEG device (ZA-9. Proassist, Ltd., Osaka, Japan), which consists of two pairs of electrodes connected to a transmitter and receiver and provides EEG, electrooculogram, and submental electromyogram. The portable EEG device has only two input channels; however, it can display EEG, electrooculogram (EOG), and electromyogram (EMG). The first channel recorded brain waves from electrodes above the right eye and left mastoid, and the second channel recorded the low-frequency band (EOG) and high-frequency band (EMG) from the left eye and jaw muscle input signals. It is possible to separate and display the data by changing the band filter. After receiving instructions on the portable EEG device, the participants set it up themselves in the four situations described below. All data were manually scored by an experienced sleep technologist based on widely used criteria.26
The participants underwent EEG monitoring on the following four occasions: (1) the first night at any time during the menstrual cycle to adapt to the EEG monitoring system; the obtained data were not scored or used (TEST); (2) the first night after menses onset (M1); (3) the second night after menses onset (M2); and (4) one night between the seventh and tenth nights after menses onset (MF). Daily entries of the menstrual data were collected. The MF phase was chosen because, in previous studies, while objectively evaluating sleep in women, EEG-based sleep parameters during the MF and mid-luteal phases were generally compared, and no differences were noted except for a minor reduction in REM sleep during the mid-luteal phase;10–12 moreover, it is generally easier to capture than the mid-luteal phase. Following the TEST, the order of starting from MF to M1/2 (ie, MF first) or from M1/2 to MF (ie, menses first) depended on the participants. All participants were asked to undergo EEG monitoring during the league’s regular season.
Statistical Analysis
Continuous variables are summarized as mean and standard deviation or median (interquartile range), as appropriate. Categorical variables are presented as numbers (percentages). Changes in sleep parameters across occasions were assessed using repeated-measures one-way analysis of variance (ANOVA) and Tukey’s test for multiple comparisons. Sleep-onset latency (SOL) and wake after sleep onset were naturally log-transformed because they were not normally distributed. To assess the effect of poor subjective sleep quality on changes in sleep parameters, first-order interactions in two-way repeated-measures ANOVA models were examined by entering interaction terms between the instances of EEG monitoring and PSQI subgroups (ie, time-by-subgroup interaction). Repeated-measures ANOVA was performed for each subgroup if significant time-by-subgroup interactions were found. If the within-subgroup ANOVA was significant, post hoc Bonferroni pairwise comparisons were performed. P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 23 (IBM Corp., Armonk, NY, USA).
Results
Participant Characteristics
Overall, 52 healthy female collegiate athletes with regular menstrual cycles were enrolled. All participants were instructed by their team to record their menstrual cycles regularly, allowing us to confirm regular menstrual cycles and non-use of oral contraceptives. The data of seven female athletes could not be used because they could not complete all the EEG monitoring occasions for personal reasons. No differences in age, type of competition, or PSQI score were found between the dropouts and completers (data not shown). Thus, data from 45 female athletes were analyzed. Among them, 32 (71.1%) had PSQI <6 (ie, without poor subjective sleep quality), and 13 (28.9%) had PSQI ≥6 (ie, with poor subjective sleep quality). Regarding the PSQI subscale, significant differences were observed in subjective sleep quality, sleep latency, sleep duration, and daytime dysfunction between female athletes with and without poor subjective sleep quality (p=0.001, p<0.001, p=0.004, and p<0.001, respectively). Their characteristics are summarized in Table 1. None of the patients had any symptoms of restless legs syndrome. Two in each group (6.3% in PSQI <6 and 15.4% in PSQI ≥6) reported snoring <1 night per week, one in each group (3.1% in PSQI <6 and 7.7% in PSQI ≥6) reported snoring 1–2 nights per week (p = 0.472) for the previous 1 month, and none reported experiencing apnea during sleep.
 |
Table 1 Subject Characteristics |
Interactions Between Poor Subjective Sleep Quality and Changes in Objective Measures of Sleep in M1, M2, and MF
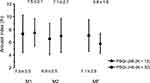
No time-by-subgroup interactions were found between changes in the objective measures of sleep other than the arousal index and the presence or absence of poor subjective sleep quality (Table 2). However, a significant interaction was found between changes in the arousal index and the presence or absence of poor subjective sleep quality (Figure 1), indicating that changes in the arousal index across the menstrual cycle in athletes with poor subjective sleep quality were different from those in athletes without poor subjective sleep quality.
 |
Table 2 Changes in Objective Measures of Sleep Other Than Arousal Index in Participants with and without Poor Subjective Sleep Quality |
Changes in Arousal Index in M1, M2, and MF Within Athletes with and without Poor Subjective Sleep Quality
In athletes without poor subjective sleep quality, the arousal index was similar across the menstrual cycle (p for ANOVA, 0.131). In athletes with poor subjective sleep quality, the arousal index varied significantly across the menstrual cycle (p for ANOVA, 0.015; Figure 1) and was significantly increased in M1 compared with MF (p = 0.016), while no difference was observed between M2 and MF (p = 0.111) and between M1 and M2 (p > 0.999) (Figure 1).
Discussion
These findings provide novel insights into the relationship between menstruation and sleep quality in female athletes. First, 28.9% of the female athletes with regular menstrual cycles had poor subjective sleep quality, as assessed using the PSQI. Second, changes in one objective measure of sleep (arousal index) differed between participants with and without poor subjective sleep quality. Finally, the arousal index increased during menses, particularly at M1, which increased significantly compared to MF in female athletes with poor subjective sleep quality, whereas no such variation was observed in female athletes without poor subjective sleep quality. These findings suggest that a substantial number of female athletes have poor subjective sleep quality even when only female athletes with regular menstrual cycles were enrolled, and that poor subjective sleep quality may be partially explained by the contrast of the arousal index between menses and the MF phase (ie, increase in menses, but decrease in MF). Therefore, greater cortical arousal was observed during menses in female athletes with poor sleep than in those with good sleep quality.
The percentage of female athletes with poor subjective sleep quality (PSQI ≥6) in previous studies7,8 ranged from 48.8% to 50.0%. The 28.9% of female athletes who reported poor subjective sleep quality in this study was less than that in previous studies. This may be because only female athletes without menstrual irregularities were included in this study, confirming the association between subjective sleep quality and menstrual abnormalities, as shown in a previous study.8 It is difficult to compare female athletes with other populations regarding the percentage of athletes with poor subjective sleep quality. The applicability of this method to other populations and variations in the cutoff values for PSQI27 in various populations remain to be elucidated. However, approximately one in three healthy female athletes who do not have menstrual cycle problems remain dissatisfied with their sleep; thus, sleep interventions in female athletes are an urgent issue.
In the present study, a significant increase in the arousal index was observed during menstruation compared with the follicular phase in female athletes who showed poor subjective sleep quality. The accentuated arousal response during menstruation may be due to discomfort during menstrual sleep. Sleep-disordered breathing, represented by obstructive sleep apnea syndrome, is the leading disorder that causes sleep fragmentation,28 but it is also known to cause sleep fragmentation in discomfort due to restless leg syndrome29 and discomfort due to the external environment, such as the intensive care unit,30 which can also cause sleep fragmentation. Furthermore, an interventional study that used gonadotropin-releasing hormone agonists to mimic menopause in healthy women and evaluated the association between nighttime discomfort and objective sleep measures reported that nighttime discomfort was associated with sleep fragmentation.31 Koikawa et al15 showed a significant association between sanitary products and the percentage of N3 sleep during menstruation. This finding suggests that discomfort during menstruation may affect sleep quality.
Furthermore, changes in the core body temperature (CBT) due to fluctuations in sex hormones may explain this increased arousal response. Increased CBT owing to fluctuations in sex hormones during the menstrual cycle increases arousal during sleep.32–34 An increase in progesterone levels contributes to an increase in CBT, and a decrease in estradiol levels leads to a decrease in CBT; the progesterone/estradiol ratio influences CBT variability.34 Therefore, CBT tends to drop during the menstrual and follicular phases of the menstrual cycle and rises during the luteal phase. Particularly, women in the follicular phase performed less during the day than those in the luteal phase when exposed to transient sleep deprivation.32,34 Increased deep body temperature is potentially protective against disturbances in arousal levels.32 Consequently, female athletes who perceived poor subjective sleep quality may have had CBT-related sleep vulnerabilities.
In the present study, poor objective sleep quality, as indicated by an increase in the arousal index, was associated with poor subjective sleep quality. Objective and subjective sleep outcomes may reflect the different aspects of sleep.35 Some reports have shown that there is usually a discrepancy between the subjective and objective measures of sleep.36,37 Despite this, in female athletes with regular menstrual cycles, poor subjective sleep quality is associated with an increased arousal index during menstruation. Sleep fragmentation is reported to be more strongly perceived as poor subjective sleep quality than as poor objective sleep quality.38 It is likely that sleep fragmentation, expressed as an increase in the arousal index during menstruation, causes subjective sleep quality deterioration. In addition, a recent scoping review investigating how various physiological signals recorded during sleep relate to subjectively perceived sleep quality reported that increased sleep fragmentation was associated with poor subjective sleep quality.39 In sleep interventions for female athletes, particular attention should be paid to the increased arousal index during menstruation. Further studies are warranted to determine how sleep fragmentation during menses can be improved in athletes.
Our study had some limitations. First, the lack of overnight polysomnography is a major limitation. Although nobody in each group reported experiencing apnea during sleep, only one participant in each group reported snoring for 1–2 nights per week. However, no significant difference in terms of snoring between the two groups was found, which might be due to snoring or other forms of sleep-disordered breathing on objective sleep measures. In addition, the presence or absence of other sleep disorders such as periodic leg movements was not formally confirmed. Second, we lacked data on productive hormone levels and body temperatures. Because all our participants had regular menstrual cycles based on regularly recorded cycles and because we focused on the first and second days after menses onset and the seventh and tenth nights after menses onset, their phases were adequately captured. Third, the participants were not controlled for physical activity, diet, caffeine intake, or daytime naps on the day of measurement. All participants were recruited from the Juntendo University School of Health and Sports Science, and their competition levels and lifestyle rhythms were considered consistent. This study aimed to assess the natural sleep patterns of female collegiate athletes.
Conclusion
An increased arousal response during menstrual sleep in female athletes was shown to be an important factor causing both objective and subjective sleep quality impairments. Although further research is needed to determine the causes of increased arousal response during menstruation, efforts to reduce nighttime discomfort during menstruation (eg, proper use and selection of sanitary products) may improve objective and subjective sleep quality.
Funding
This study was supported by the MEXT*-Supported Program for the Strategic Research Foundation at Private Universities, 2014–2018 (*Ministry of Education, Culture, Sports, Science and Technology); Japanese Center for Research on Women in Sport, Juntendo University; Project for Fostering, Survey research for the strategic strengthening of female athletes 2017–2018 by the Japan Sports Agency; the Juntendo University Young Investigator Joint Project Award 2015 (K1517), a Grant-in-Aid for Scientific Research (Grant Number, 26507010); JSPS KAKENHI (Grant Number, JP17K09527; JP18K15904; JP21K08116; JP21K16034; JP21K17604); and a grant to The Intractable Respiratory Diseases and Pulmonary Hypertension Research Group from the Ministry of Health, Labor and Welfare (20FC1027). These funding sources do not have any other roles in this study.
Disclosure
Fusae Kawana and Takatoshi Kasai are affiliated with a department endowed by Philips Respironics, ResMed, Teijin Home Healthcare, and Fukuda Denshi. Nanako Shiroshita and Takatoshi Kasai are affiliated with a department endowed by Paramount Bed. The authors report no other conflicts of interest in this work.
References
1. Myllymaki T, Rusko H, Syvaoja H, Juuti T, Kinnunen ML, Kyrolainen H. Effects of exercise intensity and duration on nocturnal heart rate variability and sleep quality. Eur J Appl Physiol. 2012;112(3):801–809. doi:10.1007/s00421-011-2034-9
2. Samuels C. Sleep, recovery, and performance: the new frontier in high-performance athletics. Neurol Clin. 2008;26(1):169–80; ix–x. doi:10.1016/j.ncl.2007.11.012
3. Simpson NS, Gibbs EL, Matheson GO. Optimizing sleep to maximize performance: implications and recommendations for elite athletes. Scand J Med Sci Sports. 2017;27(3):266–274. doi:10.1111/sms.12703
4. Leeder J, Glaister M, Pizzoferro K, Dawson J, Pedlar C. Sleep duration and quality in elite athletes measured using wristwatch actigraphy. J Sports Sci. 2012;30(6):541–545. doi:10.1080/02640414.2012.660188
5. Akerstedt T, Knutsson A, Westerholm P, Theorell T, Alfredsson L, Kecklund G. Sleep disturbances, work stress and work hours: a cross-sectional study. J Psychosom Res. 2002;53(3):741–748.
6. Lindberg E, Janson C, Gislason T, Bjornsson E, Hetta J, Boman G. Sleep disturbances in a young adult population: can gender differences be explained by differences in psychological status? Sleep. 1997;20(6):381–387.
7. Koikawa N, Shimada S, Suda S, Murata A, Kasai T. Sex differences in subjective sleep quality, sleepiness, and health-related quality of life among collegiate soccer players. Sleep Biol Rhythms. 2016;14(4):377–386. doi:10.1007/s41105-016-0068-4
8. Kawasaki Y, Kasai T, Koikawa N, et al. Sex differences in factors associated with poor subjective sleep quality in athletes. J Sports Med Phys Fitness. 2020;60(1):140–151. doi:10.23736/s0022-4707.19.09875-x
9. Baker FC, Lee KA. Menstrual cycle effects on sleep. Sleep Med Clin. 2018;13(3):283–294. doi:10.1016/j.jsmc.2018.04.002
10. Baker FC, Sassoon SA, Kahan T, et al. Perceived poor sleep quality in the absence of polysomnographic sleep disturbance in women with severe premenstrual syndrome. J Sleep Res. 2012;21(5):535–545. doi:10.1111/j.1365-2869.2012.01007.x
11. Shechter A, Boivin DB. Sleep, hormones, and circadian rhythms throughout the menstrual cycle in healthy women and women with premenstrual dysphoric disorder. Int J Endocrinol. 2010;2010:259345. doi:10.1155/2010/259345
12. Driver HS, Werth E, Dijk DJ, Borbély AA. The menstrual cycle effects on sleep. Sleep Med Clin. 2008;3(1):1–11. doi:10.1016/j.jsmc.2007.10.003
13. Baker FC, Driver HS. Self-reported sleep across the menstrual cycle in young, healthy women. J Psychosom Res. 2004;56(2):239–243. doi:10.1016/S0022-3999(03)00067-9
14. Kravitz HM, Janssen I, Santoro N, et al. Relationship of day-to-day reproductive hormone levels to sleep in midlife women. Arch Intern Med. 2005;165(20):2370–2376. doi:10.1001/archinte.165.20.2370
15. Koikawa N, Takami Y, Kawasaki Y, et al. Changes in the objective measures of sleep between the initial nights of menses and the nights during the midfollicular phase of the menstrual cycle in collegiate female athletes. J Clin Sleep Med. 2020;16(10):1745–1751. doi:10.5664/jcsm.8692
16. Krishnan V, Collop NA. Gender differences in sleep disorders. Curr Opin Pulm Med. 2006;12(6):383–389. doi:10.1097/01.mcp.0000245705.69440.6a
17. van den Berg JF, Miedema HM, Tulen JH, Hofman A, Neven AK, Tiemeier H. Sex differences in subjective and actigraphic sleep measures: a population-based study of elderly persons. Sleep. 2009;32(10):1367–1375. doi:10.1093/sleep/32.10.1367
18. Ehlers CL, Kupfer DJ. Slow-wave sleep: do young adult men and women age differently? J Sleep Res. 1997;6(3):211–215. doi:10.1046/j.1365-2869.1997.00041.x
19. Walsleben JA, Kapur VK, Newman AB, et al. Sleep and reported daytime sleepiness in normal subjects: the sleep heart health study. Sleep. 2004;27(2):293–298. doi:10.1093/sleep/27.2.293
20. Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213.
21. Doi Y, Minowa M, Uchiyama M, et al. Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res. 2000;97(2–3):165–172.
22. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545.
23. Takegami M, Suzukamo Y, Wakita T, et al. Development of a Japanese version of the Epworth Sleepiness Scale (JESS) based on item response theory. Sleep Med. 2009;10(5):556–565. doi:10.1016/j.sleep.2008.04.015
24. Fukuhara S, Takegami M, Suzukamo Y, et al. [The Japanese version of the Epworth Sleepiness Scale (JESS): major differences and revisions from previously used ‘Japanese’ versions] Nihonngoban the Epworth Sleepiness Scale (JESS): koremade shiyousareteita ookuno nihonngoban tono omona sai to kaitei (in Japanese). Nihon Kokyuki Gakkai Zasshi. 2006;44(11):896–898. Japanese.
25. Allen RP, Picchietti DL, Garcia-Borreguero D, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria--history, rationale, description, and significance. Sleep Med. 2014;15(8):860–873. doi:10.1016/j.sleep.2014.03.025
26. Berry RB, Brooks R, Gamaldo C, et al. AASM scoring manual updates for 2017 (Version 2.4). J Clin Sleep Med. 2017;13(5):665–666. doi:10.5664/jcsm.6576
27. Fabbri M, Beracci A, Martoni M, Meneo D, Tonetti L, Natale V. Measuring subjective sleep quality: a review. Int J Environ Res Public Health. 2021;18(3). doi:10.3390/ijerph18031082
28. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. doi:10.1513/pats.200709-155MG
29. Trenkwalder C, Paulus W. Restless legs syndrome: pathophysiology, clinical presentation and management. Nat Rev Neurol. 2010;6(6):337–346. doi:10.1038/nrneurol.2010.55
30. Pisani MA, D’Ambrosio C. Sleep and delirium in adults who are critically Ill: a contemporary review. Chest. 2020;157(4):977–984. doi:10.1016/j.chest.2019.12.003
31. Joffe H, Crawford S, Economou N, et al. A gonadotropin-releasing hormone agonist model demonstrates that nocturnal hot flashes interrupt objective sleep. Sleep. 2013;36(12):1977–1985. doi:10.5665/sleep.3244
32. Vidafar P, Gooley JJ, Burns AC, et al. Increased vulnerability to attentional failure during acute sleep deprivation in women depends on menstrual phase. Sleep. 2018;41(8). doi:10.1093/sleep/zsy098
33. Wright KP, Badia P. Effects of menstrual cycle phase and oral contraceptives on alertness, cognitive performance, and circadian rhythms during sleep deprivation. Behav Brain Res. 1999;103(2):185–194. doi:10.1016/s0166-4328(99)00042-x
34. Grant LK, Gooley JJ, St Hilaire MA, et al. Menstrual phase-dependent differences in neurobehavioral performance: the role of temperature and the progesterone/estradiol ratio. Sleep. 2019;43(2). doi:10.1093/sleep/zsz227
35. Shuster AE, Simon KC, Zhang J, et al. Good sleep is a mood buffer for young women during menses. Sleep. 2023. doi:10.1093/sleep/zsad072
36. Kaplan KA, Hirshman J, Hernandez B, et al. When a gold standard isn’t so golden: lack of prediction of subjective sleep quality from sleep polysomnography. Biol Psychol. 2017;123:37–46. doi:10.1016/j.biopsycho.2016.11.010
37. Edinger JD, Fins AI, Glenn DM, et al. Insomnia and the eye of the beholder: are there clinical markers of objective sleep disturbances among adults with and without insomnia complaints? J Consult Clin Psychol. 2000;68(4):586–593.
38. Herbert V, Pratt D, Emsley R, Kyle SD. Predictors of nightly subjective-objective sleep discrepancy in poor sleepers over a seven-day period. Brain Sci. 2017;7(3). doi:10.3390/brainsci7030029
39. McCarter SJ, Hagen PT, St Louis EK, et al. Physiological markers of sleep quality: a scoping review. Sleep Med Rev. 2022;64:101657. doi:10.1016/j.smrv.2022.101657
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.