Back to Journals » Nature and Science of Sleep » Volume 16
Associations Between Sleep Spindle Metrics, Age, Education and Executive Function in Young Adult and Middle-Aged Patients with Obstructive Sleep Apnea
Authors Sui R, Li J, Shi Y, Yuan S, Wang H, Liao J , Gao X, Han D, Li Y, Wang X
Received 19 September 2023
Accepted for publication 18 December 2023
Published 6 January 2024 Volume 2024:16 Pages 1—15
DOI https://doi.org/10.2147/NSS.S436824
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Ahmed BaHammam
Rongcui Sui,1– 3 Jie Li,4 Yunhan Shi,1– 3 Shizhen Yuan,1– 3 Huijun Wang,1– 3 Jianhong Liao,1– 3 Xiang Gao,1– 3 Demin Han,1– 3 Yanru Li,1– 3 Xingjun Wang4
1Department of Otorhinolaryngology Head and Neck Surgery, Beijing Tongren Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Obstructive Sleep Apnea-Hypopnea Syndrome Clinical Diagnosis and Therapy and Research Centre, Capital Medical University, Beijing, People’s Republic of China; 3Key Laboratory of Otolaryngology-Head and Neck Surgery, Ministry of Education, Capital Medical University, Beijing, People’s Republic of China; 4Department of Electronic Engineering, Tsinghua Shenzhen International Graduate School, Tsinghua University, Shenzhen, People’s Republic of China
Correspondence: Demin Han; Yanru Li, Beijing Tongren Hospital, Capital Medical University, 1 Dongjiaominxiang, Dongcheng District, Beijing, People’s Republic of China, Email [email protected]; [email protected]
Purpose: This study aimed to investigate the association between sleep spindle metrics and executive function in individuals with obstructive sleep apnea (OSA). Furthermore, we examined the association of age and education on executive function.
Patients and Methods: A total of 230 (40.90 ± 8.83 years, F/M = 45/185) participants were enrolled. Overnight electroencephalogram (C3-M2) recording detected sleep spindles by a novel U-Net-type neural network that integrates temporal information with time-frequency images. Sleep spindle metrics, including frequency (Hz), overall density (events/min), fast density (events/min), slow density (events/min), duration (sec) and amplitude (μV), were measured. Executive function was assessed using standardized neuropsychological tests. Associations between sleep spindle metrics, executive function, and demographic factors were analyzed using multivariate linear regression.
Results: In fully adjusted linear regression models, higher overall sleep spindle density (TMT-A, B=− 1.279, p=0.009; TMT-B, B=− 1.813, p=0.008), fast sleep spindle density (TMT-A, B=− 1.542, p=0.048; TMT-B, B=− 2.187, p=0.036) and slow sleep spindle density (TMT-A, B=− 1.731, p=0.037; TMT-B, B=− 2.449, p=0.034) were associated with better executive function. And the sleep spindle duration both during N2 sleep time (TMT-A, B=− 13.932, p=0.027; TMT-B, B=− 19.001, p=0.034) and N3 sleep time (TMT-B, B=− 29.916, p=0.009; Stroop-incongruous, B=− 21.303, p=0.035) was independently associated with better executive function in this population. Additionally, age and education were found to be highly associated with executive function.
Conclusion: Specific sleep spindle metrics, higher overall density, fast density and slow density during N2 sleep time, and longer duration during N2 and N3 sleep time, are independent and sensitive indicators of better executive function in young adult and middle-aged patients with OSA. Further research is needed to explore the underlying mechanisms and clinical implications of these findings.
Keywords: sleep spindle metrics, executive function, obstructive sleep apnea, young adults, middle-aged adults
Introduction
Obstructive sleep apnea (OSA) has emerged as a global concern due to its high prevalence and the various systemic complications it entails.1,2 OSA is characterized by recurrent episodes of complete or partial collapse of the upper airway during sleep, often accompanied by snoring and frequent arousal. These occurrences lead to hypoxia, sleep fragmentation, and subsequent physiological damage. Epidemiological studies have revealed significant independent associations between OSA and conditions such as hypertension, coronary artery disease, heart failure, and cognitive impairment.3–5
Epidemiology shows that OSA co-morbid with cognitive impairment can be observed in children, adolescents, and adults. In a sleep clinic cohort, the prevalence of cognitive impairment was 47.9% in the entire patient population, rising to more than 55.3% in patients with moderate-to-severe OSA.6 In middle-aged adults, OSA is commonly associated with cognitive impairment in terms of attention, memory, and executive functioning.5 Nemeth et al concluded that patients with OSA are selectively susceptible to higher-order cognitive functions (eg, executive function) compared to general skill learning and sequence-specific learning.7 Furthermore, meta-analyses confirmed that executive function was a vulnerable cognitive domain in adults with OSA.8,9
Executive function is an important domain of cognition in which higher cognitive processes modulate lower cognitive processes, including sustained attention, working memory, and inhibition control. It is supported by a distributed set of brain regions that include the prefrontal cortex (PFC) and the thalamus, particularly the dorsomedial nucleus (MD), which interconnects with the PFC.10 Neuroimaging studies have shown that the MD is a key subcortical node in the prefrontal-parietal “executive control” network that supports working memory, cognitive flexibility, and inhibition control.11 Damage to the thalamic-prefrontal circuit leads to deficits in executive function. Recent studies have reported that executive dysfunction coexists with abnormalities in thalamic-frontal connections in several psychiatric disorders, including schizophrenia.10,12
Sleep spindles, which signal as 11–16 Hz bursts in the electroencephalogram (EEG) of the mammalian brain, represent electrical surface associations of thalamic neuron oscillations. They are a highly inherited feature of the sleep EEG and generated by the thalamic reticular nucleus (TRN) in conjunction with specific thalamic nuclei and are modulated by thalamo-cortical circuit.13 It has been shown that the presence of abnormal sleep spindle activity and deficits in individuals with chronic or early schizophrenia is associated with abnormal thalamo-cortical connectivity compared to healthy individuals.14 Study showed that moderate OSA patients exhibit a lower percentage of slow spindles (11–13 Hz) with deceleration in frontal and parietal regions, in comparison to mild and non-OSA patients.15 Research has demonstrated that older adults with OSA show deficits in fast sleep spindles (14–16 Hz) but exhibit preserved overnight declarative memory consolidation.16 Similarly, in children with obstructive sleep-disordered breathing (SDB), reduced sleep spindle density and intensity has been linked to poorer visual/executive function.17
Abnormalities in thalamo-cortical circuit connectivity are commonly manifested as executive dysfunction and sleep spindle abnormalities. The strength and plasticity of thalamo-cortical circuits, as reflected by the spindle, may underlie an individual’s cognition, especially executive function. The metric of spindle and slow oscillation morphology has been identified as predicted cognitive performance in older adults.18 In healthy middle-aged and older adults (50–91 years old) without sleep disorders, higher spindle density predicted better performance in verbal learning, visual attention, and verbal fluency.19 In children, slow sleep spindle density reflects the developing brain’s ability to acquire and improve executive function learning abilities, such as integrated planning and problem-solving skills.20 Although no direct neuroimaging studies have demonstrated impaired thalamo-cortical connectivity in patients with OSA, thinned prefrontal cortex and reduced thalamic tissue integrity were observed.21,22 This provides a neuroanatomical basis for executive dysfunction and sleep spindle deficits. Recent studies in middle-aged and older adults showed sleep spindle abnormalities were independently associated with OSA severity measures.23 Furthermore, the associations between sleep spindle metrics and functional outcomes was explored in large population-based sample of aging men, and results showed that higher fast spindle density during N3 sleep time was associated with worse executive performance.24 These observations supported the utility of sleep spindle metrics as useful executive function markers in older OSA. Cognitive impairment in OSA patients presents at a younger age; however, our understanding of how this relationship may manifest in the younger OSA patient population is limited.
In this study, we aimed to investigate the association between sleep spindle metrics and executive function in young adult and middle-aged patients with OSA.
Materials and Methods
The study protocol was conducted according to the principles of the Declaration of Helsinki and was approved by the appropriate Institutional Review Board of Beijing Tongren Hospital, Capital Medical University (TREC2022-KY059). The participants signed written informed consent forms before the study for inclusion in the study and for the use of executive function test data and medical records.
Participants
A total of 180 patients with obstructive sleep apnea (OSA) and 50 primary snorers, who underwent polysomnography (PSG) at Beijing Tongren Hospital, were included in this study (Figure 1). Overnight PSG data were collected from all participants. Inclusion criteria for enrollment were as follows: 1) age between 18 and 59 years; 2) apnea-hypopnea index (AHI) >5 events/h for OSA patients; 3) no reliance on sleep medication for falling asleep; 4) abstention from sleep medication on the night of PSG; 5) absence of active cardiovascular or cerebrovascular disease, uncontrolled diabetes or hypertension, or degenerative diseases (eg, Parkinson’s disease or Alzheimer’s disease); 6) no history of psychiatric disorders; and 7) no history of craniosurgery. All participants underwent comprehensive interviews and evaluations conducted by medical professionals.
 |
Figure 1 A flow diagram for participants. |
Overnight Polysomnography
All participants underwent in-laboratory nocturnal PSG recording. Eight electroencephalogram (EEG) electrodes were positioned according to the standard 10–20 system configuration (F3, F4, C3, C4, O1, O2, M1 and M2). Electrocardiogram (ECG) electrodes were used for heart rate monitoring. Two chin electromyography (EMG) electrodes and a submental EMG were employed to measure chin muscle activity, while two electrooculogram (EOG) electrodes were used to detect eye movements. Respiratory data, including finger pulse oximetry, airflow, chest and abdominal respiratory effort, were collected using a thermistor flow sensor and nasal airflow sensors. Compumedics systems (Compumedics Medics Corporation, Australia) were used for PSG recording and experienced sleep technicians manually scored all PSG measures according to 2017 American Academy of Sleep Medicine (AASM). The AHI, representing the sum of apneic and hypopneic events per hour of sleep, was calculated. OSA was identified by an AHI ≥ 5/h and further categorized as mild (5–15/h), moderate (15–30/h), or severe (≥30/h). Additionally, participants completed the Epworth Sleepiness Scale (ESS) to assess their subjective sleep quality, with global scores >10 indicating excessive daytime sleepiness (EDS).
Executive Function
Trail Making Test A and B (TMT)
The Trail Making Test (TMT) consists of two parts: TMT-A and TMT-B. TMT-A is primarily used to assess psychomotor functions and processing speed requiring participants to connect encircled numbers (1–25) in sequence.25 TMT-B is more complex and measures executive functions such as cognitive flexibility, divided attention, response monitoring, and task switching. In view of the wide variation in the mastery of the letters among Chinese, a revised version of the TMT-B in Chinese was used. It includes circles with numbers from 1 to 13 and boxes with numbers from 1 to 12. Participants are required to connect the numbers in ascending order, starting with the circles and then the boxes. Prior to the test, participants receive practice trials to ensure understanding of the tasks. During the practice test, if participants make errors during the test, the examiner points them out and guides the participants to complete the task correctly. Performance on the test is measured by the time taken to complete TMT-A and TMT-B. To provide a more specific assessment of executive function, the TMT difference (TMT-D) is calculated by subtracting the performance time in TMT Part A from Part B.
Stroop Color-Word Test (SCWT)
The Stroop Color-Word Test (SCWT) is used to evaluate working memory and inhibitory control and is measured by completion times.26 Participants are instructed to respond as quickly and accurately as possible. The test has congruous and incongruous conditions. In the congruous condition, participants read the word in black ink (eg, the word “GREEN” written in black ink) or identify the color of the ink (eg, the word “XX” written in red ink). In the incongruous condition, participants read the color of the ink in which the words are written (eg, the word “GREEN” written in red ink).27 Each test consists of 50 words, and the completion time for identifying all 50 words is measured using a stopwatch.
Spindle Detection
All PSG data were exported to standardized European Data Format from which overnight C3-M2 EEG recordings underwent automated artifact detection using a previously validated algorithm.28 In this study, a new U-Net type neural network29 integrating temporal information with time-frequency images was constructed to enhance spindle detection in N2/N3 sleep. To obtain time-frequency images, we employ the technique of Continuous Wavelet Transform (CWT), and the Morlet wavelet was chosen as the basis wavelet function. With the information from both the time domain and the time-frequency domain, a multi-modal fusion approach using a U-Net architecture was trained for sleep spindle detection (11–16 Hz, ≥0.5 and ≤3 s) (Figure S1.). Fast sleep spindles (13–16 Hz) and slow sleep spindles (11–13 Hz) were detected. The training dataset used in this study was collected from the Beijing Tongren Hospital, and the dataset were partitioned into three splits ie the training set, validation and test set. The model hyperparameters and evaluation metrics were set to be consistent with the previous work SUMO.30 The validated model achieved an F1 score of 83% on the test set, surpassing the performance of existing state-of-the-art methods and reaching the level of expert recognition (Table S1).
As the model provides the start and end time points of each spindle, we can calculate the duration of a spindle by subtracting the start time from the end time. The amplitude is obtained by subtracting the minimum value from the maximum value of the signal within that time period, and the frequency is calculated as the average frequency of the signal during that time period.
Statistical Analysis
Data analysis was conducted using IBM SPSS statistical software (version 24.0). Descriptive statistics are presented as mean ± standard deviation (SD) for normal distribution data, and median (IQR) was presented for non-normal distribution data. Differences in population characteristics and sleep spindle metrics were determined by one-way analysis of variance (ANOVA) for normal distribution variables and the Mann–Whitney U-test for non-normal distribution variables.
Univariate and multivariate linear regression models including Bonferroni correction were used to examine the relationships between sleep spindle metrics during N2/N3 sleep, age/education, and executive function. The models were adjusted for covariates such as age, AHI, total sleep time (TST), body mass index (BMI), minimum oxygen saturation (min SpO2), education, gender, and arousal index (AI). Unstandardized beta (B) coefficients with 95% confidence intervals (CI) are reported. Variance inflation factor values for all covariates were close to 1, indicating the absence of multicollinearity. As the oxygen desaturation index (ODI) was highly correlated with AHI, min SpO2 was used to assess sleep hypoxemia. The p < 0.05 was statistically different, p < 0.01 was statistically significant, and p < 0.001 was extremely statistically significant.
Results
Characteristics of Participants
The study included a total of 230 participants. OSA prevalence (AHI ≥ 5/h) was 78.3% (n=180, F/M = 20/160), and 34.8% have severe OSA (AHI ≥ 30/h) (n=80, F/M = 7/73). Table 1 displays the participants characteristics, revealing no significant differences in age, education, body mass index (BMI), neck circumference (NC) in patients with OSA. Participants with severe OSA showed more pronounced hypoxia, as reflected by higher AHI, longer apnea/hypopnea duration, lower minimum oxygen saturation (min-SpO2), and increased sleep fragmentation, as indicated by the arousal index.
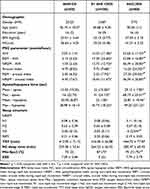 |
Table 1 Characteristics of Participants |
Spindle Metrics
The study found significant differences in various metrics related to sleep spindles during N2 sleep between mild/moderate OSA (n=100, 40.68 ± 9.30 years), severe OSA (n=80, 40.5 (11) years) and primary snorers (n=50, 36.19 ± 10.07 years) (Table 2). Specifically, decreased overall spindle density, fast spindle density, and slow spindle density were observed in the mild/moderate OSA patients compared to simple snorers. A similar difference in spindle density was observed in patients with severe OSA compared to mild/moderate OSA. Additionally, the severe OSA patients exhibited a significant decrease in these sleep spindle metrics compared to the mild/moderate OSA patients (0.67 (2.16) vs 1.49 (2.51); 0.30 (1.22) vs 0.78 (1.10); 0.26 (0.88) vs 0.54 (1.07), respectively, p<0.05). Moreover, the duration of sleep spindles showed a decreasing trend with the severity of OSA in comparison to primary snorers, although this difference was not statistically different. During N3 sleep time, decreased overall spindle density, fast spindle density, and slow spindle density were also observed in the mild/moderate OSA and severe OSA patients compared to simple snorers. The severe OSA patients also exhibited a significant decreased sleep spindle metrics compared to the mild/moderate OSA patients (0.29 (0.81) vs 0.77 (0.99); 0.14 (0.41) vs 0.43 (0.59); 0.10 (0.36) vs 0.32 (0.34), respectively, p<0.05).
 |
Table 2 Sleep Spindle Metrics in OSA Patients |
Executive Function Performance
The performance of executive function was assessed by Trail Making Test A and B (TMT) and Stroop Color-Word Test (SCWT) (Table 3). Specially, patients with OSA took longer to complete both in the TMT and SCWT tests compared to primary snorers. The completion time of TMT showed a significant increase in mild/moderate OSA (TMT-A, 30.80 ± 9.45 vs 26.49± 8.73, p<0.05; TMT-B, 47.39 ± 17.64 vs 39.13 ± 9.20, p<0.05; TMT-D, 16.34 (8.48) vs 11.80 ± 9.17, p<0.05) and severe OSA patients (TMT-A, 28.48 (12.12) vs 26.4 9± 8.73, p<0.05; TMT-B, 46.05 (17.35) vs 39.13 ± 9.20, p<0.05; TMT-D, 17.64 (12.33) vs 11.80 ± 9.17, p<0.05) compared to primary snorers. The completion time of the Stroop-word test (23.09 ± 5.15 vs 20.21 (5.22), p<0.05) and Stroop-colour test (27.25 (7.67) vs 24.83 (5.21), p<0.05) showed a significant increase in severe OSA patients compared to mild/moderate OSA.
 |
Table 3 Executive Function Assessment |
Associations of Age and Executive Function
The associations between age and performance on the TMT and SCWT tests are presented in Table 4. In the adjusted model, a decline in executive function was observed with increasing age in young adult and middle-aged patients with OSA. In young adult and middle-aged OSA patients, there was a significant increase in completion times for TMT-A (B=0.359, p<0.001), TMT-B (B=0.661, p<0.001), and TMT-D (B=0.283, p=0.002) as age increased. Similarly, the completion times for Stroop-word (B=0.197, p<0.001), Stroop-colour (B=0.233, p<0.001), and Stroop-incongruous tasks (B=0.494, p<0.001) also increased with age. In primary snorers, completion time of TMT-B (B=0.316, p=0.048) and Stroop-colour test (B=0.296, p=0.021) also increased with age.
 |
Table 4 The Association Between Age and Executive Function |
Associations of Education and Executive Function
The associations between education and performance on the TMT and SCWT in OSA patients and primary snorers were presented in Table 5. In patients with OSA, higher levels of education were associated with better executive function performance. Specifically, individuals with higher education levels demonstrated shorter completion times on both TMT tests (TMT-A, B=−1.125, p<0.001; TMT-B, B=−1.932, p<0.001; TMT-D, B=−0.777, p=0.025) and SCWT tests (Stroop-word, B=−0.518, p=0.002; Stroop-color, B=−0.714 p=0.001; Stroop-incongruous, B=−1.601, p<0.001). However, in primary snorers, completion time on the TMT tests and SCWT tests appeared to be independent of education.
 |
Table 5 The Association Between Education and Executive Function. |
Associations of Sleep Spindle Metrics and Executive Function
The associations between sleep spindle metrics and executive function in both young adult and middle-aged patients with OSA and primary snorers are presented in Tables 6–8. In the adjusted model, higher overall, fast and slow sleep spindle density and longer sleep spindle duration during N2 sleep time were associated with reduced completion time on the TMT-A test (B=−1.279, p=0.009; B=−1.542, p=0.048; B=−1.731, p=0.037; B=−13.932, p=0.027, respectively) and TMT-B test (B=−1.813, p=0.008; B=−2.187, p=0.036; B=−2.449, p=0.034; B=−19.001, p=0.034, respectively) in young adult and middle-aged patients with OSA. Similarly, in the adjusted model, longer duration of sleep spindle during N3 sleep time was associated with reduced completion time on the TMT-B test and Stroop-incongruous test (B=−29.916, p=0.009; B=−21.303, p=0.035, respectively) in young adult and middle-aged patients with OSA. In the unadjusted model, higher overall, fast and slow sleep spindle density and longer sleep spindle duration during N2 sleep time were associated with better performance in Stroop-incongruous test (B=−2.183, p<0.001; B=−3.165, p<0.001; B=−2.382, p=0.039; B=−17.664, p=0.002; respectively). However, in the adjusted model, the association was diminished. Moreover, executive function was found to be independent of sleep spindle metrics in primary snorers. No other adjusted associations of N2/N3 sleep spindle metrics with completion times of the TMT-D, Stroop-word, and Stroop-colour tests were found in young adult and middle-aged patients with OSA and primary snorers.
 |
Table 6 The Association Between Sleep Spindle Metrics and TMT-A Test |
 |
Table 7 The Association Between Sleep Spindle Metrics and TMT-B Test |
 |
Table 8 The Association Between Sleep Spindle Metrics and Stroop-Incongruous Test |
Discussion
This study represents a pioneering investigation into the connections between sleep spindle metrics and executive function in young adult and middle-aged patients with OSA and primary snorers while diligently considering potential confounding factors. Remarkably, young adult and middle-aged patients with OSA exhibited a significant correlation between impaired executive function, as assessed through the TMT and SCWT tests, and deficits of specific sleep spindle metrics. Moreover, advanced age and lower educational attainment demonstrated associations with impaired executive function in young adult and middle-aged patients with OSA. Intriguingly, no apparent association was observed between sleep spindle metrics and executive function in primary snorers.
It is widely acknowledged that OSA presents a significant risk for cognitive impairment.31,32 Extensive research has demonstrated that individuals with OSA often exhibit cognitive impairments, including deficits in attention, memory, executive function, psychomotor function, and language abilities.5,33 Our findings reveal that individuals with OSA experience difficulties in executive function when compared to primary snorers. This aligns with previous research, demonstrating that individuals with OSA who experience pronounced hypoxia (as indicated by AHI and min-SpO2) and frequent sleep fragmentation (as measured by the arousal index) are particularly susceptible to executive dysfunction.33,34
In our study, patients with severe OSA had significantly decreased total sleep spindle density, fast sleep spindle density, and slow sleep spindle density. This result was partially consistent with a large community-based cohort study on OSA and sleep spindles, in which slow sleep spindle density decreased with increasing OSA severity.23 Furthermore, our findings suggested that impaired executive performance in young and middle-aged OSA patients is associated with reduced overall, fast and slow sleep spindle density during N2 sleep time. Moreover, it has been shown that CPAP treatment for six months could reverse cognitive function and quantitative sleep EEG changes in OSA patients.35 These results strongly support previous findings in the field that altered sleep spindle morphology (lower spindle frequency, higher amplitude and density) could predict cognitive performance.15,18,24,36,37 In addition, our study makes a novel contribution by identifying the duration of the sleep spindle both during N2 and N3 sleep times as a significant marker of executive function.
In our study, we found a negative association between age and executive performance in both young adult and middle-aged patients with OSA and primary snorers. Imbalances in thalamo-cortical circuits are part of the natural aging process. In particular, executive functions that depend on cognitive functions in the medial temporal and prefrontal cortex show significant age-related declines.38 Consistent with previous research, we identified a decline in psychomotor functions, processing speed, and higher-order executive functions such as cognitive flexibility, divided attention, response monitoring, and task switching with increasing age.39 Supporting previous findings, our study also revealed a decline in inhibitory control, specifically, the ability to refrain from incorrect responses, with aging in both young adult and middle-aged patients with OSA and primary snorers.40
We found that education plays an important role in preserving executive functions in young adult and middle-aged patients with OSA. Illiteracy and low level of educational attainment increase the incidence of cognitive impairment,41 on the contrary, high level of education as one of the indicators of cognitive reserve (CR) delays cognitive decline.42,43 Recent studies have shown that CR contributes to the enhancement of thalamo-cortical functional connectivity in young adults and stabilizes cognitive variability in middle-aged adults.44,45 In young adult and middle-aged patients with OSA, we discovered that greater CR (as measured by years of education) was associated with improved executive function. This suggests that CR acts protectively against executive dysfunction in young adult and middle-aged patients with OSA. A recent regression study conducted with elderly individuals also highlighted the association between CR and attention, executive function, verbal memory, and working memory, partially supporting our findings in young adult and middle-aged OSA patients.46
Our study suggested that the sleep spindle metrics serve as independent and sensitive indicators of executive function in young adult and middle-aged patients with OSA. In our study, we initially observed that young adult and middle-aged OSA patients with higher overall, fast and slow sleep spindle density, as well as longer duration of sleep spindles during N2 sleep time, exhibited reduced psychomotor functions and processing speed. We also found similar results of sleep spindle metrics during N2 sleep time in executive functions, including cognitive flexibility, divided attention, response monitoring, and task switching. Moreover, our study revealed a positive association between longer spindle duration during N3 sleep time and improvements in executive function, as well as working memory and inhibitory control, respectively. The sleep spindle oscillation is generated by the TRN in combination with the dorsal thalamus and is then transmitted to the cortex. In the cortex, the sleep spindles are synchronized and amplified, while thalamic-cortical connections maintain and regulate the duration of the sleep spindle oscillation.47,48 Therefore, individual characteristics of spindles, such as amplitude, density, and duration, reflect the integrity of the underlying thalamo-cortical network. Deficits in these parameters indicate dysfunctions in complementary thalamo-cortical neuronal circuits in psychiatric disorders.49 Although there is no direct evidence of impaired thalamo-cortical functional connectivity in patients with OSA, our findings supported the utility of sleep spindle metrics as useful executive function markers in this population. Consistent with previous studies in healthy sedentary middle-aged and older adults, we confirmed that higher overall and fast sleep spindle density were associated with better executive function and less cognitive decline.50 Additionally, in our study, we observed that a higher density of slow sleep spindles was significantly associated with better performance in the TMT-A and TMT-B test among patients. In contrast to a community-based cohort study in middle-aged and older adults,24 we did not observed an association between fast sleep spindle density and TMT-B test during N3 sleep time; instead, we observed that longer sleep spindle duration was significantly associated with better TMT-B performance in this period.
We conducted a comprehensive investigation of the relationship between sleep spindle metrics and executive functioning in young adult and middle-aged patients with OSA, and age and education were not negligible as important associations on cognition. The study utilized a novel U-Net-type neural network to detect sleep spindles from electroencephalogram (EEG) recordings. The main strength of our study was that we first focused on young and middle-aged adults with objectively measured sleep spindles and multiple standardized and validated cognitive tests. Sleep spindle metrics could be used to monitor the effectiveness of OSA interventions. Improvements in specific sleep spindle characteristics might serve as objective EEG indicators of OSA treatment. However, it is worth noting that our study design was cross-sectional, which limited our ability to determine causality. In addition, due to practical constraints, we performed only one night of PSG, although multiple nights of sleep testing would be desirable. Although our study focused on the link between executive function and sleep spindles in patients with OSA, cognitive deficits were observed in multiple domains. We need to further investigate the relationship between sleep spindles and other important cognitive functions.
Conclusion
In conclusion, our study investigated the associations between sleep spindle metrics and executive function and confirmed that sleep spindle metrics serve as independent sensitive indicators of executive function, although age and education play an irreplaceable role. We found that sleep spindle metrics, particularly higher overall density, fast density and slow density during N2 sleep time, and longer duration during N2 and N3 sleep time, were associated with better executive function. These findings highlight the importance of considering sleep spindle metrics and their impact on executive function in clinical practice and research. Future research should focus on investigating the underlying mechanisms linking sleep spindle metrics and executive function.
Abbreviations
OSA, obstructive sleep apnea; PFC, prefrontal cortex; MD, dorsomedial nucleus;
EEG, electroencephalogram; TRN, thalamic reticular nucleus; SDB, sleep-disordered
breathing; PSG, polysomnography; AHI, apnea-hypopnea index; ECG, electrocardiogram;
EMG, electromyography; EOG, electrooculogram; AASM, American Academy of Sleep
Medicine; ESS, Epworth Sleepiness Scale; EDS, excessive daytime sleepiness; TMT,
Trail Making Test A and B; SCWT, Stroop Color-Word Test; CWT, Continuous Wavelet
Transform; min-SpO2, minimum oxygen saturation; AI, arousal index; ODI, oxygen
desaturation index; BMI, body mass index; NC, neck circumference; CR, cognitive reserve.
Data Sharing Statement
The data underlying this article cannot be shared publicly for the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding authors.
Acknowledgments
The authors appreciate the contribution of the study participants, otolaryngologists, neurologists and technologists at the Department of Otolaryngology Head and Neck Surgery, Beijing Tongren Hospital.
Funding
This research was funded by the National Natural Science Foundation of China (81970866) and Beijing Dongcheng District Outstanding Talent Funding Project (2023-dchrcpyzz-10).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. doi:10.1016/j.smrv.2016.07.002
2. Cunningham J, Hunter M, Budgeon C, et al. The prevalence and comorbidities of obstructive sleep apnea in middle-aged men and women: the Busselton healthy ageing study. J Clin Sleep Med. 2021;17(10):2029–2039. doi:10.5664/jcsm.9378
3. Torres G, Sánchez-de-la-torre M, Barbé F. Relationship between osa and hypertension. Chest. 2015;148(3):824–832. doi:10.1378/chest.15-0136
4. Bradley TD, Floras JS. Obstructive sleep apnea and its cardiovascular consequences. Lancet. 2009;373(9657):82–93. doi:10.1016/S0140-6736(08)61622-0
5. Bubu OM, Andrade AG, Umasabor-Bubu OQ, et al. Obstructive sleep apnea, cognition and alzheimer’s disease: a systematic review integrating three decades of multidisciplinary research. Sleep Med Rev. 2020;50:101250. doi:10.1016/j.smrv.2019.101250
6. Beaudin AE, Raneri JK, Ayas NT, et al. Cognitive function in a sleep clinic cohort of patients with obstructive sleep apnea. Ann Am Thorac Soc. 2021;18(5):865–875. doi:10.1513/AnnalsATS.202004-313OC
7. Nemeth D, Csábi E, Janacsek K, Várszegi M, Mari Z. Intact implicit probabilistic sequence learning in obstructive sleep apnea. J Sleep Res. 2012;21(4):396–401. doi:10.1111/j.1365-2869.2011.00983.x
8. Stranks EK, Crowe SF. The cognitive effects of obstructive sleep apnea: an updated meta-analysis. Arch Clin Neuropsychol. 2016;31(2):186–193.
9. Bucks RS, Olaithe M, Eastwood P. Neurocognitive function in obstructive sleep apnoea: a meta-review. Respirology. 2013;18(1):61–70. doi:10.1111/j.1440-1843.2012.02255.x
10. Parnaudeau S, Bolkan SS, Kellendonk C. The mediodorsal thalamus: an essential partner of the prefrontal cortex for cognition. Biol Psychiatry. 2018;83(8):648–656. doi:10.1016/j.biopsych.2017.11.008
11. Halassa MM, Sherman SM. Thalamocortical circuit motifs: a general framework. Neuron. 2019;103(5):762–770. doi:10.1016/j.neuron.2019.06.005
12. Giraldo-Chica M, Rogers BP, Damon SM, Landman BA, Woodward ND. Prefrontal-thalamic anatomical connectivity and executive cognitive function in schizophrenia. Biol Psychiatry. 2018;83(6):509–517. doi:10.1016/j.biopsych.2017.09.022
13. Fernandez LMJ, Lüthi A. Sleep spindles: mechanisms and functions. Physiol Rev. 2020;100(2):805–868. doi:10.1152/physrev.00042.2018
14. Baran B, Karahanoğlu FI, Mylonas D, et al. Increased thalamocortical connectivity in schizophrenia correlates with sleep spindle deficits: evidence for a common pathophysiology. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(8):706–714. doi:10.1016/j.bpsc.2019.04.012
15. Carvalho DZ, Gerhardt GJ, Dellagustin G, et al. Loss of sleep spindle frequency deceleration in obstructive sleep apnea. Clin Neurophysiol. 2014;125(2):306–312. doi:10.1016/j.clinph.2013.07.005
16. Teh JZ, Grummitt L, Haroutonian C, et al. Overnight declarative memory consolidation and NREM sleep EEG oscillations in older adults with obstructive sleep apnea. Sleep. 2023;46(6):zsad087. doi:10.1093/sleep/zsad087
17. Shetty M, Perera A, Kadar M, et al. The effects of sleep-disordered breathing on sleep spindle activity in children and the relationship with sleep, behavior, and neurocognition. Sleep Med. 2023;101:468–477. doi:10.1016/j.sleep.2022.11.028
18. Djonlagic I, Mariani S, Fitzpatrick AL, et al. Macro and micro sleep architecture and cognitive performance in older adults. Nat Hum Behav. 2021;5(1):123–145. doi:10.1038/s41562-020-00964-y
19. Lafortune M, Gagnon JF, Martin N, et al. Sleep spindles and rapid eye movement sleep as predictors of next morning cognitive performance in healthy middle-aged and older participants. J Sleep Res. 2014;23(2):159–167. doi:10.1111/jsr.12108
20. Vermeulen MCM, Van der Heijden KB, Swaab H, Van Someren EJW. Sleep spindle characteristics and sleep architecture are associated with learning of executive functions in school-age children. J Sleep Res. 2019;28(1):e12779. doi:10.1111/jsr.12779
21. Joo EY, Jeon S, Kim ST, Lee JM, Hong SB. Localized cortical thinning in patients with obstructive sleep apnea syndrome. Sleep. 2013;36(8):1153–1162. doi:10.5665/sleep.2876
22. Roy B, Sahib AK, Kang D, Aysola RS, Kumar R. Brain tissue integrity mapping in adults with obstructive sleep apnea using T1-weighted and T2-weighted images. Ther Adv Neurol Disord. 2022;15:17562864221137505. doi:10.1177/17562864221137505
23. Parker JL, Melaku YA, D’Rozario AL, et al. The association between obstructive sleep apnea and sleep spindles in middle-aged and older men: a community-based cohort study. Sleep. 2022;45(3):zsab282. doi:10.1093/sleep/zsab282
24. Parker JL, Appleton SL, Adams RJ, et al. The association between sleep spindles and cognitive function in middle-aged and older men from a community-based cohort study. Sleep Health. 2023;9(5):774–785. doi:10.1016/j.sleh.2023.03.007
25. Yeager BE, Bruss J, Duffau H, et al. Central precuneus lesions are associated with impaired executive function. Brain Struct Funct. 2022;227(9):3099–3108. doi:10.1007/s00429-022-02556-0
26. Furst T, Massaro A, Miller C, Williams BT, LaMacchia ZM, Horvath PJ. β-alanine supplementation increased physical performance and improved executive function following endurance exercise in middle aged individuals. J Int Soc Sports Nutr. 2018;15(1):32. doi:10.1186/s12970-018-0238-7
27. Hwang JY, Kim N, Kim S, et al. Stroop task-related brain activity in patients with insomnia: changes after cognitive-behavioral therapy for insomnia. Behav Sleep Med. 2019;17(5):621–633. doi:10.1080/15402002.2018.1435546
28. D’Rozario AL, Dungan GC 2nd, Banks S, et al. An automated algorithm to identify and reject artefacts for quantitative EEG analysis during sleep in patients with sleep-disordered breathing. Sleep Breath. 2015;19(2):607–615. doi:10.1007/s11325-014-1056-z
29. Ronneberger O, Fischer P, Brox T. U-net: convolutional networks for biomedical image segmentation. In: Navab N, Hornegger J, Wells W, Frangi A, editors. Medical Image Computing and Computer-Assisted Intervention – MICCAI 2015. Lecture Notes in Computer Science. 9351. Cham: Springer; 2015. 234–241—.
30. Kaulen L, Schwabedal JTC, Schneider J, Ritter P, Bialonski S. Advanced sleep spindle identification with neural networks. Sci Rep. 2022;12(1):7686. doi:10.1038/s41598-022-11210-y
31. Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613–619. doi:10.1001/jama.2011.1115
32. Shieu MM, Dunietz GL, Paulson HL, Chervin RD, Braley TJ. The association between obstructive sleep apnea risk and cognitive disorders: a population-based study. J Clin Sleep Med. 2022;18(4):1177–1185. doi:10.5664/jcsm.9832
33. Olaithe M, Bucks RS, Hillman DR, Eastwood PR. Cognitive deficits in obstructive sleep apnea: insights from a meta-review and comparison with deficits observed in COPD, insomnia, and sleep deprivation. Sleep Med Rev. 2018;38:39–49. doi:10.1016/j.smrv.2017.03.005
34. Crawford-Achour E, Dauphinot V, Martin MS, et al. Protective effect of long-term CPAP therapy on cognitive performance in elderly patients with severe osa: the proof study. J Clin Sleep Med. 2015;11(5):519–524. doi:10.5664/jcsm.4694
35. D’Rozario AL, Hoyos CM, Wong KKH, et al. Improvements in cognitive function and quantitative sleep electroencephalogram in obstructive sleep apnea after six months of continuous positive airway pressure treatment. Sleep. 2022;45(6):zsac013. doi:10.1093/sleep/zsac013
36. Taillard J, Sagaspe P, Berthomier C, et al. Non-REM sleep characteristics predict early cognitive impairment in an aging population. Front Neurol. 2019;10:197. doi:10.3389/fneur.2019.00197
37. Dunietz GL, Chervin RD, Burke JF, Conceicao AS, Braley TJ. Obstructive sleep apnea treatment and dementia risk in older adults. Sleep. 2021;44(9):zsab076. doi:10.1093/sleep/zsab076
38. Li Y, Owens RL, Sands S, et al. The effect of donepezil on arousal threshold and apnea-hypopnea index. a randomized, double-blind, cross-over study. Ann Am Thorac Soc. 2016;13(11):2012–2018. doi:10.1513/AnnalsATS.201605-384OC
39. Borghesani PR, Madhyastha TM, Aylward EH, et al. The association between higher order abilities, processing speed, and age are variably mediated by white matter integrity during typical aging. Neuropsychologia. 2013;51(8):1435–1444. doi:10.1016/j.neuropsychologia.2013.03.005
40. Hadar L, Trope Y, Ben-David BM. Aging impairs inhibitory control over incidental cues: a construal-level perspective. Psychol Sci. 2021;32(9):1442–1451. doi:10.1177/0956797621998316
41. Jia J, Wang F, Wei C, et al. The prevalence of dementia in urban and rural areas of China. Alzheimers Dement. 2014;10(1):1–9. doi:10.1016/j.jalz.2013.01.012
42. Jia F, Li Y, Li M, Cao F. Subjective cognitive decline, cognitive reserve indicators, and the incidence of dementia. J Am Med Dir Assoc. 2021;22(7):1449–1455. doi:10.1016/j.jamda.2020.08.005
43. Olaithe M, Pushpanathan M, Hillman D, et al. Cognitive profiles in obstructive sleep apnea: a cluster analysis in sleep clinic and community samples. J Clin Sleep Med. 2020;16(9):1493–1505. doi:10.5664/jcsm.8564
44. Stern Y, Habeck C, Moeller J, S, et al. Brain networks associated with cognitive reserve in healthy young and old adults. Cereb Cortex. 2005;15(4):394–402. doi:10.1093/cercor/bhh142
45. Ferreira D, Machado A, Molina Y, et al. Cognitive variability during middle-age: possible association with neurodegeneration and cognitive reserve. Front Aging Neurosci. 2017;9:188. doi:10.3389/fnagi.2017.00188
46. Lavrencic LM, Richardson C, Harrison SL, et al. Is there a link between cognitive reserve and cognitive function in the oldest-old?. J Gerontol a Biol Sci Med Sci. 2018;73(4):499–505. doi:10.1093/gerona/glx140
47. Bonjean M, Baker T, Lemieux M, Timofeev I, Sejnowski T, Bazhenov M. Corticothalamic feedback controls sleep spindle duration in vivo. J Neurosci. 2011;31(25):9124–9134. doi:10.1523/JNEUROSCI.0077-11.2011
48. Pratt JA, Morris BJ. The thalamic reticular nucleus: a functional hub for thalamocortical network dysfunction in schizophrenia and a target for drug discovery. J Psychopharmacol. 2015;29(2):127–137. doi:10.1177/0269881114565805
49. Ferrarelli F, Tononi G. Reduced sleep spindle activity point to a TRN-MD thalamus-PFC circuit dysfunction in schizophrenia. Schizophr Res. 2017;180:36–43. doi:10.1016/j.schres.2016.05.023
50. Guadagni V, Byles H, Av T, et al. Association of sleep spindle characteristics with executive functioning in healthy sedentary middle-aged and older adults. J Sleep Res. 2021;30(2):e13037. doi:10.1111/jsr.13037
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
