Back to Journals » Neuropsychiatric Disease and Treatment » Volume 20
Added Clinical Value of Intraplaque Neovascularization Detection to Color Doppler Ultrasound for Assessing Ischemic Stroke Risk
Authors Cui L, Liu R, Zhou F, Liu Y, Tian B, Chen Y, Xing Y
Received 27 December 2023
Accepted for publication 9 April 2024
Published 23 April 2024 Volume 2024:20 Pages 899—909
DOI https://doi.org/10.2147/NDT.S456872
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Taro Kishi
Liuping Cui,1,2 Ran Liu,1 Fubo Zhou,1 Yumei Liu,1 Bing Tian,1 Ying Chen,2,* Yingqi Xing1,3,4,*
1Department of Vascular Ultrasound, Xuanwu Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Neurology, The First Hospital of Jilin University, Changchun, People’s Republic of China; 3Beijing Diagnostic Center of Vascular Ultrasound, Beijing, People’s Republic of China; 4Center of Vascular Ultrasound, Beijing Institute of Brain Disorders, Collaborative Innovation Center for Brain Disorders, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yingqi Xing, Department of Vascular Ultrasonography, Xuanwu Hospital, Capital Medical University, 45 Changchun Road, Beijing, Xicheng District, 100053, People’s Republic of China, Tel +86-18610047846, Email [email protected]
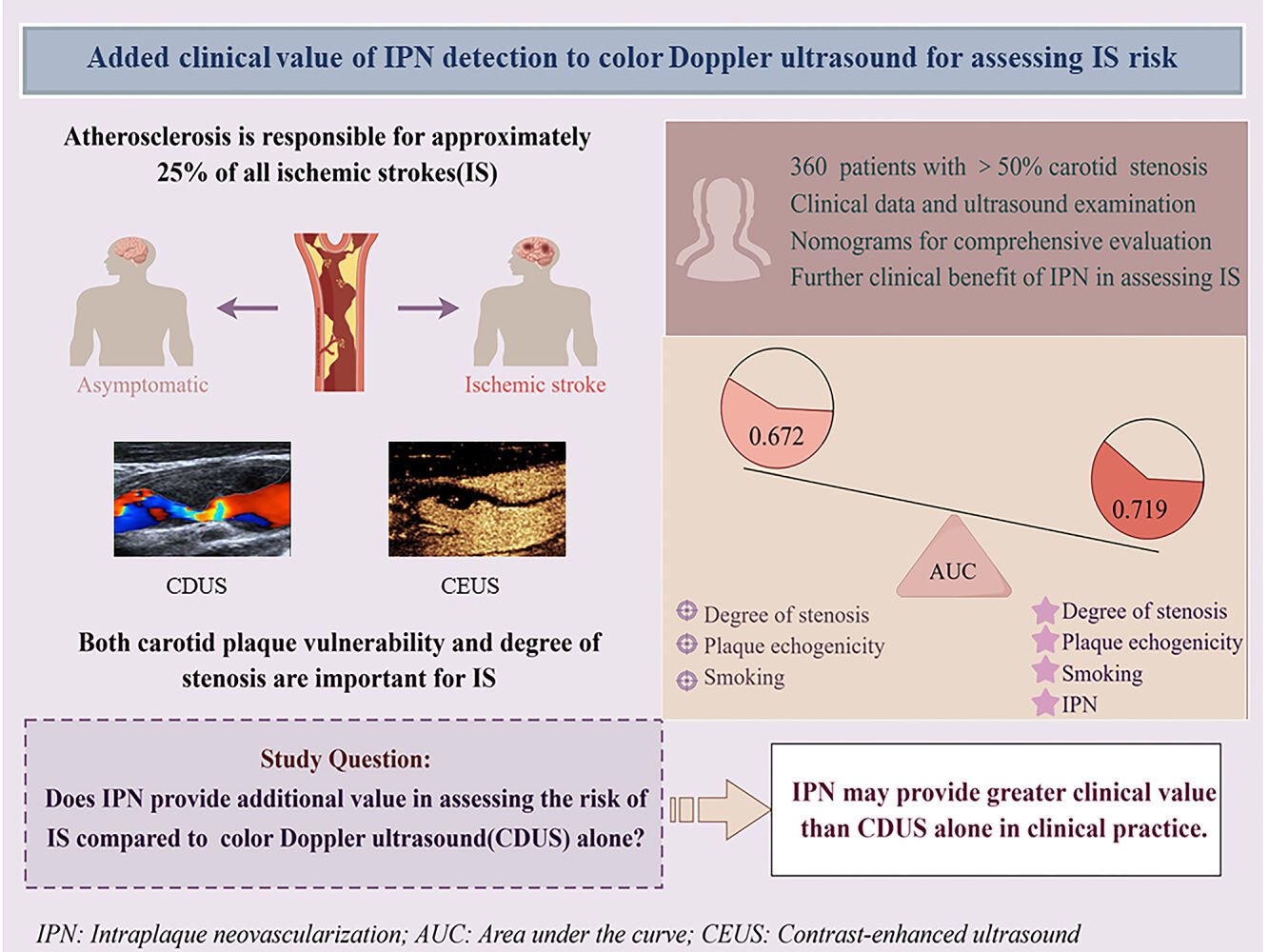
Purpose: Intraplaque neovascularization, assessed using contrast-enhanced ultrasound (CEUS), is associated with ischemic stroke. It remains unclear whether detection of intraplaque neovascularization combined with color Doppler ultrasound (CDUS) provides additional value compared with CDUS alone in assessing ischemic stroke risk. Therefore, we investigated the clinical value of combined CEUS, CDUS, and clinical features for ischemic stroke risk stratification.
Patients and Methods: We recruited 360 patients with ≥ 50% carotid stenosis between January 2019 and September 2022. Patients were examined using CDUS and CEUS. Covariates associated with ischemic stroke were identified using multivariate logistic regression analysis. The discrimination and calibration were verified using the C-statistic and Hosmer–Lemeshow test. The incremental value of intraplaque neovascularization in the assessment of ischemic stroke was analyzed using the Delong test.
Results: We analyzed the data of 162 symptomatic and 159 asymptomatic patients who satisfied the inclusion and exclusion criteria, respectively. Based on multivariate logistic regression analysis, we constructed a nomogram using intraplaque neovascularization, degree of carotid stenosis, plaque hypoechoicity, and smoking status, with a C-statistic of 0.719 (95% confidence interval [CI]: 0.666– 0.768) and a Hosmer–Lemeshow test p value of 0.261. The net reclassification index of the nomogram was 0.249 (95% CI: 0.138– 0.359), and the integrated discrimination improvement was 0.053 (95% CI: 0.029– 0.079). Adding intraplaque neovascularization to the combination of CDUS and clinical features (0.672; 95% CI: 0.617– 0.723) increased the C-statistics (p=0.028).
Conclusion: Further assessment of intraplaque neovascularization after CDUS may help more accurately identify patients at risk of ischemic stroke. Combining multiparametric carotid ultrasound and clinical features may help improve the risk stratification of patients with ischemic stroke with ≥ 50% carotid stenosis.
Plain Language Summary: We studied whether using contrast-enhanced ultrasound (CEUS) to detect intraplaque neovascularization could help better determine the risk of ischemic stroke. We compared the combined use of color Doppler ultrasound (CDUS) and CEUS with CDUS alone in patients with more than 50% carotid narrowing. Our findings showed that combining clinical details, CDUS, and CEUS was more effective (0.719 vs 0.672). This means that CEUS provides extra insight when gauging ischemic stroke risk compared with CDUS alone. This could help in accurately identifying patients at high risk of stroke. However, more extensive studies are needed to fully understand the role of these tests in the evaluation of stroke risk.
Keywords: atherosclerosis, contrast-enhanced ultrasound, vulnerable plaque, carotid ultrasound
Graphical Abstract:
Introduction
Ischemic stroke contributes greatly to the declining quality of life in middle-aged and older adults. The TOAST classification highlights five etiologies of ischemic stroke, and large-artery atherosclerotic cerebral infarction and cardioembolic infarction are the acute ischemic stroke subtypes with the highest occurrence of early in-hospital mortality. Moreover, the short-term prognosis of patients with atherothrombotic or cardioembolic stroke is poor compared with that of patients with other acute ischemic stroke subtypes.1,2 The risk of subsequent adverse events remains high even in patients with minor ischemic stroke, especially in patients with large atherosclerotic arteries.3 Approximately 25% of ischemic stroke cases are caused by atherosclerosis, and current guidelines only consider the degree of carotid stenosis to determine treatment options.4,5 Compared with digital subtraction angiography, duplex ultrasound has a sensitivity of 89% and specificity of 84% for diagnosing stenosis, and 97% and 99% for diagnosing occlusion. However, clinical scientists are increasingly realizing that both carotid plaque features and the degree of stenosis are important parameters in ischemic stroke etiology.6,7 In addition to medical history, physical examination, and serological indicators, carotid artery imaging can provide crucial information for assessing stroke and overall cardiovascular risk.8
Ultrasound is the first-line modality used to screen for carotid atherosclerotic disease, and its value depends not only on its cost-effectiveness, wide availability, excellent safety, and reproducibility but also on its evolving multiparametric properties. The correlation between stenosis severity and plaque echogenicity, as measured by color Doppler ultrasound (CDUS), and the likelihood of ischemic stroke are well established.9 Intraplaque neovascularization (IPN) is associated with an increased risk of neovessel rupture, hemorrhage, and inflammation in response to plaque activity.10 Recent research indicates that contrast-enhanced ultrasound (CEUS) is a reliable method for identifying IPN and that IPN, as assessed by CEUS, is an independent risk factor for ischemic stroke.11–13 However, there is insufficient evidence to support the widespread clinical use of CEUS. Further research is needed to investigate whether a combination of CEUS and CDUS can provide a more accurate assessment of ischemic stroke in patients with ≥50% carotid stenosis.
Nomograms can be used to combine multiple parameters and visualize the probability of clinical events, thereby satisfying the need for a comprehensive clinical assessment. In this study, we aimed to construct nomograms to investigate the clinical value of a combination of CEUS, CDUS, and clinical features for stratifying the risk of ischemic stroke. We hypothesized that the addition of IPN detection to CDUS could help accurately identify individuals at an increased risk of ischemic stroke, thus helping clinicians determine the optimal treatment strategy.
Materials and Methods
Study Design and Population
This study was conducted prospectively at two centers and included 360 consecutive patients hospitalized between January 2019 and September 2022, all with ≥50% carotid stenosis. Among these patients, 240 were from the First Hospital of Jilin University, and the remaining 120 were from Xuanwu Hospital of Capital Medical University. Patients were categorized into symptomatic and asymptomatic groups based on the European Stroke Organization guidelines for endarterectomy and stenting for carotid stenosis. Symptomatic carotid stenosis was defined as an ischemic cerebrovascular event that had caused a transient ischemic attack or stroke within the previous 6 months. Asymptomatic carotid stenosis was defined as a stenosis unrelated to any ocular or cerebral ischemic event in the region of the ipsilateral carotid artery within the previous 6 months.14 The exclusion criteria were as follows: (1) other causes of carotid artery stenosis, such as carotid dissection and Takayasu’s arteritis; (2) ischemic stroke caused by cardioembolism; (3) poor-quality ultrasound; and (4) severe systemic diseases (Figure 1).
 |
Figure 1 Flow chart of the study design. |
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. All procedures adhered to institutional guidelines, and written informed consent was obtained from all patients before enrolment. The study protocol was approved by the Ethics Committee of the First Hospital of Jilin University (No. 2015–285).
Carotid Ultrasound Protocol
The common carotid artery, carotid bifurcation, and internal carotid artery were assessed both longitudinally and transversely using ultrasound equipment (Aplio500; Toshiba, Japan; Epiq7; Philips, Netherlands) equipped with a linear probe (4–11 MHz). The patient was placed in the supine position, and the head was gently rotated approximately 30–40° to the contralateral side for examination. Two sonographers (CY and LR), each with a decade of experience, independently analyzed all images.
The degree of carotid stenosis was categorized as mild (<50%), moderate (50–69%), or severe (≥70%) based on the standards published by the North American Radiological Society.15 Using the Gray–Weale scale, plaque echogenicity was categorized in longitudinal sections as follows: homogeneous echolucent (Type I), predominantly echolucent(Type II), predominantly echogenic plaque with limited areas of echolucency (Type III), homogeneously echogenic (Type IV), or displaying severe calcification (Type V). Types I and II, characterized by a higher proportion of echolucent plaques, were classified as hypoechoic plaques, whereas types III and IV were classified as non-hypoechoic plaques.16 Upon observing the plaque, its thickness, length, and echogenicity were meticulously recorded. In cases where multiple plaques were present, the thickest plaque was chosen as the primary target for analysis.
Analysis of Intraplaque Neovascularization
After recording the CDUS data, the targeted plaques were simultaneously visualized in 2D grayscale and CEUS. Specifically, this examination was conducted using a linear probe (5–8 MHz) with the gain appropriately adjusted while maintaining a mechanical index of 0.16 to avoid disrupting the contrast microbubbles. A suspension of the contrast agent (SonoVue, Bracco, Milan, Italy) and saline (5 mL) was injected through the peripheral vein. The optimal longitudinal section of the target plaque was selected, recorded for 10–20 s at baseline, and observed for a minimum of 120 s after the contrast microbubbles reached the carotid artery. Raw data were stored on hard drives for subsequent analysis.
IPN was graded according to the extent and location of microbubbles, with Grade 0 indicating no visible microbubbles within the plaque, Grade 1 indicating mild microbubbles limited to the shoulder and/or adventitial side of the plaque, and Grade 2 indicating widespread microbubbles throughout the plaque (Figure 2).17 IPN grading was independently conducted by two CEUS sonographers, CY and LR. In cases of inconsistent grading, a third experienced sonographer acted as an adjudicator. The sonographers were blinded to both the clinical information and results provided by their counterparts.
Clinical Features
A trained investigator who was blinded to the patients’ ultrasound data assessed the clinical features of the patients using a standardized questionnaire. The questionnaire was used to collect the following information: age and sex; history of hypertension, diabetes, and coronary artery disease (CAD); smoking and drinking statuses; family history of cardiovascular disease; and total cholesterol, triglyceride, high-density lipoprotein, low-density lipoprotein, and fasting blood glucose levels.
Statistical Analysis
For continuous variables, normally distributed data are expressed as mean ± standard deviation and were analyzed using Student’s t-test; non-normally distributed data are expressed as median and interquartile range and were analyzed using a non-parametric rank-sum test. Categorical variables are expressed as frequencies and percentages (%) and were analyzed using the chi-squared test. Univariate and multivariate logistic regression analyses were used to identify the risk factors for ischemic events, which were used to create nomograms. The Delong test was used to compare different receiver-operating characteristic (ROC) curves (with or without IPN). Intra- and interobserver agreements were assessed using Cohen’s kappa coefficient.
For the established nomogram, we assessed discriminability and calibration using the area under the ROC (AUC) and calibration curves. Bootstrapping (resampling=1000) was used for internal validation. Finally, the net reclassification index (NRI) and integrated discrimination improvement (IDI) indices were used to illustrate the favorable effect of IPN on the assessment of ischemic events.
Statistical analyses were performed using IBM SPSS Statistics (Version 26.0; IBM, Armonk, NY) and R version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria). The above tests were two-tailed (p<0.05).
Results
Baseline Clinical Features
Of the 360 consecutive patients enrolled, 39 were excluded after applying the inclusion criteria, and 321 patients were finally included in the study. Their mean age was 64.3 ± 7.5 years, and 279 (86.9%) were men. The cohort was divided into symptomatic and asymptomatic groups comprising 162 and 159 patients, respectively.
There were no significant differences in age, sex, or serological indices between the groups. Compared with the asymptomatic group, the symptomatic group had more smokers (p=0.001), alcohol consumers (p=0.084), and patients with diabetes (p=0.016). Other vascular risk factors, including hypertension, family history of cardiovascular disease, and history of CAD, were not significantly different between the two groups (Table 1).
 |
Table 1 Patient Baseline Features (n=321) |
Plaque Features
Patients in the symptomatic group showed a higher degree of severe stenosis, hypoechoic plaques, and Grade 2 IPN than those in the asymptomatic group (p<0.05). The plaque length and thickness did not differ significantly between the two groups (Table 1). For IPN assessment, we found significant intra- (0.775) and interobserver (0.725) reliability.
Multivariable Analysis and ROC Curve Analysis
Significant variables identified in the univariate analyses were included in the multivariate analyses. These analyses revealed that smoking (odds ratio [OR]: 1.716; 95% confidence interval [CI]: 1.056–2.787), the degree of stenosis (OR: 2.440; 95% CI: 1.442–4.130), plaque echogenicity (OR: 1.948; 95% CI: 1.207–3.262), and IPN grading (OR: 2.684; 95% CI: 1.654–4.352) were all significantly associated with ischemic stroke (Supplementary Table 1).
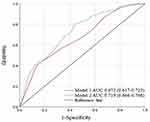
We compared the accuracy of assessing ischemic stroke with and without IPN using a combination of the following variables: Model 1 (smoking, degree of stenosis, and plaque echogenicity) and Model 2 (smoking, degree of stenosis, plaque echogenicity, and IPN grading). The Delong test demonstrated a significant difference between Model 1, with an AUC of 0.672 (95% CI: 0.617–0.723), and Model 2, with an AUC of 0.719 (95% CI: 0.666–0.768) (p=0.028) (Figure 3).
Nomogram Creation and Validation
We constructed a nomogram containing four independent risk factors (Model 2), which was internally validated with an AUC of 0.710 (95% CI: 0.559–0.859), indicating good discrimination. The Hosmer–Lemeshow test revealed no significant difference between the prediction and actual observation, and the calibration curve showed good agreement between the predicted probabilities and the actual observation (Figure 4 and Supplementary Figure 1).
Subsequent analysis demonstrated that, in comparison to Model 1 (excluding IPN grading), the NRI of the nomogram was 0.249 (95% CI: 0.138–0.359), and the IDI was 0.053 (95% CI: 0.029–0.079). Both values showed a statistically significant difference, indicating that the nomogram exhibited a superior discriminatory power and risk reclassification ability (Table 2).
 |
Table 2 Discrimination and Calibration of Models 1 and 2 |
Discussion
In this study, we developed a nomogram that incorporated CEUS, CDUS, and clinical features to stratify the risk of ischemic stroke in patients with carotid stenosis ≥50%. Our results indicated that the assessment of IPN using CEUS after CDUS could better identify vulnerable plaques. This nomogram showed a higher accuracy in assessing ischemic stroke than the model based only on clinical and CDUS parameters. This implies that the nomogram created using multiparametric ultrasound and clinical features may more accurately identify subgroups at high risk of ischemic stroke.
Atherosclerosis can lead to ischemic stroke through two underlying pathological mechanisms. The first involves carotid artery stenosis arising from the thickening of atherosclerotic plaques, which disrupts normal blood flow dynamics and results in insufficient distal arterial perfusion. The second mechanism entails an escalation in carotid plaque buildup accompanied by a thin fibrous cap, which can trigger plaque rupture and the release of plaque content into the bloodstream. This, in turn, can lead to embolism within distal arteries, ultimately culminating in cerebral ischemia.18 Contemporary guidelines emphasize the importance of considering carotid imaging parameters that offer insights into plaque morphology and composition. This is crucial for a more precise assessment of the risk of ischemic stroke.19,20
CDUS can assess the degree of carotid stenosis and the compositional and morphological features associated with vulnerable plaques.16 Although the scanning accuracy may vary widely among laboratories, the performance criteria and accuracy levels can be set using standardized protocols. Hypoechoic plaques are more prone to causing ipsilateral ischemic strokes than hyperechoic plaques. Nevertheless, the exact mechanisms behind this phenomenon remain incompletely understood.21 This phenomenon is presently ascribed to the higher presence of a lipid-rich necrotic core (LRNC) and intraplaque hemorrhage (IPH).22 Although it is difficult to identify plaque components using CDUS, a precise distinction may not be clinically important because both an LRNC and IPH increase the risk of ischemic stroke.23
IPN is a notable characteristic of early atherosclerotic plaques, signifying their vulnerability. Studies based on carotid plaque histology have shown that inflammatory cells such as macrophages, T cells, and mast cells co-localize in the microvessels within the plaque, revealing a positive correlation between local inflammatory infiltration and neovascularization. Furthermore, owing to the structural incompleteness of pathological neovascularization and endothelial dysfunction caused by local hypoxia in plaques, neovascularization is prone to extravasation, resulting in IPH.24 CEUS is an advanced imaging technique capable of detecting microvascular perfusion and possesses distinctive advantages when it comes to assessing IPN. Research has found that IPN assessed using CEUS is strongly associated with cardiovascular events.25
He et al employed superb microvascular imaging (SMI) to identify IPN and utilized a combination of plaque hardness and the extent of carotid stenosis for risk stratification in patients with asymptomatic carotid stenosis. They found that multimodal ultrasound had higher accuracy than conventional ultrasound.26 However, SMI can be performed using only specific ultrasound systems, and the effects of clinical features on ischemic stroke occurrence were not considered. In this study, we analyzed the patient clinical features and found that smoking was an independent risk factor for ischemic stroke, which is consistent with the findings of previous studies.27 Age has an important effect on atherosclerosis and ischemic stroke occurrences. However, in our study, the mean age of patients in both groups was 64 years, and the age difference between groups was not significant. Demographic features and risk factors of patients with ischemic stroke are known to be different in the subgroup of elderly patients aged ≥85 years.28 Future studies should evaluate the diagnostic value of IPN in patients at high risk for stroke at various age groups.
Researchers studying the incremental value of IPN assessed using CEUS in predicting CAD found that, beyond stress echocardiography and history of CAD, the presence of IPN increases the accuracy of CAD prediction.29 To the best of our knowledge, only a few studies have investigated the additional role of CEUS-assessed IPN beyond CDUS in ischemic stroke risk stratification in the real world. Our findings indicate that, in contrast to CDUS features, assessing the IPN through CEUS offers supplementary and pertinent information regarding pathological mechanisms, which could potentially be more valuable in distinguishing individuals at a heightened risk of ischemic stroke.
Exploring the relationship between the imaging features of atherosclerotic disease and the risk of ischemic stroke to guide optimal management and prevention is a growing area of research.30 Previous studies have shown that the use of statins reduces and stabilizes atherosclerotic plaques.31 Additionally, good adherence to statin treatment has been found to contribute to ischemic stroke prevention.32,33 Therefore, the effect of medication use and medication adherence on the relationship between plaque vulnerability and ischemic stroke occurrence should be considered in future studies. Furthermore, plaque vulnerability features such as plaque ulceration and intraplaque motion detected by ultrasound may provide information on ischemic stroke risk.34 The vulnerability traits of carotid atherosclerotic plaques are as crucial for predicting ischemic events as the degree of stenosis itself. Our findings will promote the inclusion of multiparametric ultrasound features in assessing carotid atherosclerotic disease.
This study had some limitations. First, only two centers were included in the study. Before this nomogram can be clinically implemented, randomized clinical trials and cost-effectiveness assessments are required to determine the exact role of carotid plaque vulnerability features in ischemic stroke risk stratification. Second, the proportion of male patients was relatively high, which is related to the higher incidence of ischemic stroke in men. In the future, more attention should be paid to the effect of sex on ischemic stroke. Third, we only analyzed the thickest plaque, which may have introduced potential spectrum bias. Furthermore, future studies should emphasize the clinical significance of IPN, plaque ulceration, and intraplaque motion in relation to ischemic stroke. Finally, we excluded patients with severe plaque calcification, which could have affected the accuracy of the IPN analysis.
Conclusion
The nomogram combining multiparametric carotid ultrasound and clinical features allows for more accurate risk stratification of patients with ischemic stroke with ≥50% carotid artery stenosis. CEUS when used along with CDUS may provide greater clinical value than CDUS alone in clinical practice. Larger multicenter studies, including randomized clinical trials and cost-effectiveness assessments, should be performed to determine the exact role of carotid plaque vulnerability features in ischemic stroke risk stratification.
Abbreviations
CEUS, contrast-enhanced ultrasound; CDUS, color Doppler ultrasound; IPN, intraplaque neovascularization; CAD, coronary artery disease; IQR, interquartile range; ROC, receiver operating characteristic; AUC, area under the ROC; OR, odds ratio; CI, confidence interval; NRI, net reclassification index; IDI, integrated discrimination improvement; LRNC, lipid-rich necrotic core; IPH, intraplaque hemorrhage; SMI, superb microvascular imaging.
Data Sharing Statement
Data are available on reasonable request. All supporting data within the article will be made available by the corresponding author.
Acknowledgments
The authors thank all staff involved in this study and the patients and their families for their participation and cooperation. The graphical abstract was drawn by Figdraw.
Funding
This study was supported by the National Key Research and Development Projects (2022YFC3602400) and Beijing Hospitals Authority Youth Programme (QML20230814) and the National Natural Science Foundation of China (Grant No. 82102066), and the Key Technology R&D for Social Development in Jilin Province (20240304065SF).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang YJ, Li ZX, Gu HQ. China stroke statistics: an update on the 2019 report from the national center for healthcare quality management in neurological diseases, China national clinical research center for neurological diseases, the Chinese stroke association, national center for chronic and non-communicable disease control and prevention, Chinese center for disease control and prevention and institute for global neuroscience and stroke collaborations. N Engl J Med. 2022;7:415–450.
2. Arboix A, Oliveres M, Massons J, et al. Early differentiation of cardioembolic from atherothrombotic cerebral infarction: a multivariate analysis. Eur J Neurol. 1999;6:677–683. doi:10.1046/j.1468-1331.1999.660677.x
3. Lee KJ, Shin DW. Risk of subsequent events in patients with minor ischemic stroke or high-risk transient ischemic attack. J Korean Med Sci. 2022;37:e254.
4. Heck D, Jost A. Carotid stenosis, stroke, and carotid artery revascularization. Prog Cardiovasc Dis. 2021;65:49–54. doi:10.1016/j.pcad.2021.03.005
5. Aboyans V, Ricco JB, Bartelink MEL, et al. 2017 esc guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European society for vascular surgery (esvs): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries endorsed by: the European stroke organization (eso)the task force for the diagnosis and treatment of peripheral arterial diseases of the European society of cardiology (esc) and of the European society for vascular surgery (esvs). Eur Heart J. 2018;39:763–816. doi:10.1093/eurheartj/ehx095
6. Murgia A, Balestrieri A, Francone M, et al. Plaque imaging volume analysis: technique and application. Cardiovasc Diagn Ther. 2020;10:1032–1047. doi:10.21037/cdt.2020.03.01
7. Naylor R, Rantner B, Ancetti S, et al. Editor’s choice - European society for vascular surgery (esvs) 2023 clinical practice guidelines on the management of atherosclerotic carotid and vertebral artery disease. Eur J Vasc Endovasc Surg. 2023;65:7–111. doi:10.1016/j.ejvs.2022.04.011
8. Saba L, Antignani PL, Gupta A, et al. International union of angiology (iua) consensus paper on imaging strategies in atherosclerotic carotid artery imaging: from basic strategies to advanced approaches. Atherosclerosis. 2022;354:23–40. doi:10.1016/j.atherosclerosis.2022.06.1014
9. Li H, Xu X, Luo B, et al. The predictive value of carotid ultrasonography with cardiovascular risk factors-a “spider” promoting atherosclerosis. Front Cardiovasc Med. 2021;8:706490. doi:10.3389/fcvm.2021.706490
10. Gu SY, Zhang LN, Chen J, et al. Associations of plaque morphology and location with intraplaque neovascularization in the carotid artery by contrast-enhanced ultrasound imaging. Front Neurol. 2023;14:1097070. doi:10.3389/fneur.2023.1097070
11. Huang R, Abdelmoneim SS, Ball CA, et al. Detection of carotid atherosclerotic plaque neovascularization using contrast enhanced ultrasound: a systematic review and meta-analysis of diagnostic accuracy studies. J Am Soc Echocardiogr. 2016;29:491–502. doi:10.1016/j.echo.2016.02.012
12. Song Y, Dang Y, Dang LL, et al. Association between intraplaque neovascularization assessed by contrast-enhanced ultrasound and the risk of stroke. Clin Radiol. 2020;75:70–75. doi:10.1016/j.crad.2019.08.019
13. Cui L, Xing Y, Zhou Y, et al. Carotid intraplaque neovascularisation as a predictive factor for future vascular events in patients with mild and moderate carotid stenosis: an observational prospective study. Ther Adv Neurol Disord. 2021;14:17562864211023992. doi:10.1177/17562864211023992
14. Bonati LH, Kakkos S, Berkefeld J, et al. European stroke organisation guideline on endarterectomy and stenting for carotid artery stenosis. Eur Stroke J. 2021;6:I–xlvii.
15. Grant EG, Benson CB, Moneta GL, et al. Carotid artery stenosis: gray-scale and Doppler us diagnosis--society of radiologists in ultrasound consensus conference. Radiology. 2003;229:340–346. doi:10.1148/radiol.2292030516
16. Alexandratou M, Papachristodoulou A, Li X, et al. Advances in noninvasive carotid wall imaging with ultrasound: a narrative review. J Clin Med. 2022;11:6196. doi:10.3390/jcm11206196
17. Cui L, Xing Y, Wang L, et al. Carotid intraplaque neovascularization and future vascular events in patients with asymptomatic carotid stenosis. Front Pharmacol. 2022;13:804810. doi:10.3389/fphar.2022.804810
18. Savastano L, Mousavi H, Liu Y. Unifying theory of carotid plaque disruption based on structural phenotypes and forces expressed at the lumen/wall interface. Stroke Vasc Neurol. 2022;7:465–475. doi:10.1136/svn-2021-001451
19. Mantella LE, Liblik K, Johri AM. Vascular imaging of atherosclerosis: strengths and weaknesses. Atherosclerosis. 2021;319:42–50. doi:10.1016/j.atherosclerosis.2020.12.021
20. Gottsäter A. The European society for vascular surgery (esvs) 2023 clinical practice guidelines on antithrombotic therapy for vascular diseases: an indispensable resource in vascular care. Eur J Vasc Endovasc Surg. 2023;65:621–622. doi:10.1016/j.ejvs.2023.03.032
21. Jashari F, Ibrahimi P, Bajraktari G, et al. Carotid plaque echogenicity predicts cerebrovascular symptoms: a systematic review and meta-analysis. Eur J Neurol. 2016;23:1241–1247. doi:10.1111/ene.13017
22. Spanos K, Tzorbatzoglou I, Lazari P, et al. Carotid artery plaque echomorphology and its association with histopathologic characteristics. J Vasc Surg. 2018;68:1772–1780. doi:10.1016/j.jvs.2018.01.068
23. Baradaran H, Gupta A. Carotid vessel wall imaging on CTA. AJNR Am J Neuroradiol. 2020;41:380–386. doi:10.3174/ajnr.A6403
24. Kashiwazaki D, Koh M, Uchino H, et al. Hypoxia accelerates intraplaque neovascularization derived from endothelial progenitor cells in carotid stenosis. J Neurosurg. 2018;131:884–891. doi:10.3171/2018.4.JNS172876
25. Yan H, Wu X, He Y, et al. Carotid intraplaque neovascularization on contrast-enhanced ultrasound correlates with cardiovascular events and poor prognosis: a systematic review and meta-analysis. Ultrasound Med Biol. 2021;47:167–176. doi:10.1016/j.ultrasmedbio.2020.10.013
26. Li Y, Zheng S, Zhang J, et al. Multimodal ultrasound parameters aided carotid plaque risk stratification in patients with asymptomatic carotid stenosis. ACTA radiol. 2022;63:278–286. doi:10.1177/0284185121989189
27. Reitsma MB, Fullman N, Ng M. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the global burden of disease study 2015. Lancet. 2017;389:1885–1906. doi:10.1016/S0140-6736(17)30819-X
28. Arboix A, García-Eroles L, Massons J, et al. Lacunar infarcts in patients aged 85 years and older. Acta Neurol Scand. 2000;101:25–29. doi:10.1034/j.1600-0404.2000.00005.x
29. Huang R, DeMarco JK, Ota H, et al. Prognostic value of intraplaque neovascularization detected by carotid contrast-enhanced ultrasound in patients undergoing stress echocardiography. J Am Soc Echocardiogr. 2021;34:614–624. doi:10.1016/j.echo.2020.12.016
30. Saba L, Saam T, Jäger HR, et al. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol. 2019;18:559–572. doi:10.1016/S1474-4422(19)30035-3
31. Cui E, Kersche G, Grubic N, et al. Effect of pharmacologic anti-atherosclerotic therapy on carotid intraplaque neovascularization: a systematic review. J Clin Lipidol. 2023;17:315–326. doi:10.1016/j.jacl.2023.04.009
32. Vitturi BK, Gagliardi RJ. Effects of statin therapy on outcomes of ischemic stroke: a real-world experience in Brazil. Arq Neuropsiquiatr. 2020;78:461–467. doi:10.1590/0004-282x20200027
33. Vitturi BK, Gagliardi RJ. The influence of statin withdrawal and adherence on stroke outcomes. Neurol Sci. 2021;42:2317–2323. doi:10.1007/s10072-020-04790-y
34. Brinjikji W, Rabinstein AA, Lanzino G, et al. Ultrasound characteristics of symptomatic carotid plaques: a systematic review and meta-analysis. Cerebrovasc Dis. 2015;40:165–174. doi:10.1159/000437339
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.