Back to Journals » Clinical Ophthalmology » Volume 12
XEN glaucoma treatment system in the management of refractory glaucomas: a short review on trial data and potential role in clinical practice
Authors De Gregorio A, Pedrotti E , Stevan G, Bertoncello A , Morselli S
Received 11 January 2018
Accepted for publication 28 February 2018
Published 30 April 2018 Volume 2018:12 Pages 773—782
DOI https://doi.org/10.2147/OPTH.S146919
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
A De Gregorio,1 E Pedrotti,2 G Stevan,1 A Bertoncello,1 S Morselli1
1Ophthalmic Unit, San Bassiano Hospital, Bassano del Grappa, Italy; 2Eye Clinic, Department of Neuroscience, Biomedicine and Movement Sciences, University of Verona, Verona, Italy
Abstract: The recent development of new devices that are significantly less invasive, collectively termed minimally invasive glaucoma surgery, offers new perspective of intraocular pressure reduction with less risk, short operating times, and rapid recovery. The aim of this work is to provide a panoramic review of the currently published clinical data to assess the potential role of XEN gel stent (Allergan PLC, Irvine, CA, USA) in the management of glaucoma, which is the only filtering minimally invasive glaucoma surgery device that allows the subconjunctival filtration. The ab interno placement of the XEN gel stent offers an alternative for lowering intraocular pressure in refractory glaucoma as a final step, and in patients intolerant to medical therapy as an early surgical approach with minimum conjunctival tissue disruption, restricted flow to avoid hypotony, and long-term safety.
Keywords: glaucoma, gel stent, minimally invasive, surgery, subconjunctival drainage
Introduction
Glaucoma, which affects 64,300,000 people, 3.5% of the world’s population,1 is a heterogeneous group of optic neuropathies, which causes irreversible but potentially preventable vision loss, related in most form, of glaucoma to elevated intraocular pressure (IOP). In the healthy eye, aqueous humor (AH) flow against resistance generates an average IOP of ~15 mmHg, necessary to inflate the eye and maintain the proper shape and optical properties of the globe. There is an equilibrium between the production and drainage of AH, and impairment in outflow leads to IOP elevation. This basic concept is a central tenet of glaucoma pathology and treatment. Therefore, understanding AH dynamics and mechanisms is the challenge in the management of glaucoma. Lowering IOP through use of medication, laser treatments, or incisional surgery is currently the only means of preventing progression of glaucoma.2
While many patients may be controlled by medications, poor compliance to therapy and ocular toxicity are issues that lead to an early surgical approach. The 4 main approaches of IOP reduction include increasing trabecular outflow by bypassing juxtacanalicular trabecular meshwork (TM), increasing uveoscleral outflow via suprachoroidal pathways, reducing AH production from the ciliary body, or creating a subconjunctival drainage pathway from the anterior chamber (AC).3
Subconjunctival drainage of AH, resulting in bleb formation, has been a cornerstone of glaucoma surgery for more than a century4 and the superiority of external filtration surgery is unquestionable.
From the subconjunctival space, the AH has numerous potential drainage pathways, including diffusion through the conjunctiva, diffusion into the venous system of the sclera and conjunctiva, as well as potential lymphatic pathways;5,6 furthermore, subconjunctival drainage, bypassing the TM, Schlemm’s canal, and collector channels entirely, eliminates the risk of reducing the efficacy due to any other outflow obstruction. All other bleb-less drainage spaces (Schlemm’s canal and suprachoroidal pathways) have a limitation since AH outflow critically depends on the venous system.7
The recent development of new devices that are significantly less invasive, collectively termed minimally invasive glaucoma surgery (MIGS), offers new perspective of IOP reduction with less risk, short operating times, and rapid recovery.
MIGS has been defined as any glaucoma surgical procedure that avoids conjunctival dissection and thus approaches via ab interno incision (clear cornea wound), aiming to provide a safer and less invasive means of lowering IOP than traditional surgery, with the goal of reducing dependency on topical medication.3,8
Proven outflow mechanism of action in the subconjunctival space with a minimally invasive approach is an important goal; XEN gel stent (Allergan PLC, Irvine, CA, USA) is the only filtering MIGS device that allows subconjunctival filtration.
The ab interno placement of the XEN gel stent offers an alternative for lowering IOP with minimum conjunctival tissue disruption, restricted flow to avoid hypotony, and long-term safety.
The aim of this paper is to provide a review of the currently published clinical data to assess the potential role of XEN gel stent in the management of glaucoma.
XEN gel stent
XEN gel stent is a hydrophilic tube made of a porcine gelatin cross-linked with glutaraldehyde to achieve permanence in tissue. This material is used for a variety of medical applications because of its well-established biocompatibility, and it does not cause a foreign-body reaction.9 (Figure 1A).
  | Figure 1 (A) XEN gel stent; (B) preloaded injector and correct handling. |
The implant is hard when dry and becomes soft within 1–2 min when hydrated, adapting to the tissue shape, thus avoiding migration and potential erosion. It has been demonstrated that the gel stent is ~100 times more flexible than the silicon tubing used in traditional tube–shunt surgery.4 The implant is housed in a disposable preloaded handheld inserter designed specifically for an ab interno surgical implantation (Figure 1B).
It decreases IOP by creating a permanent outflow pathway from the AC to the subconjunctival space through a scleral channel of 2–4 mm. The first implants proposed by Aquesys Inc. (Aliso Viejo, CA, USA) were of 3 different diameters (45, 63, and 140 μm) for varying levels of IOP control.
The smallest one, XEN45, the only one currently available, has an inside diameter of ~45 μm, an outside diameter of 150 μm, and is 6 mm long.
It was designed from principles of laminar fluid dynamics (Hagen–Poiseuille equation) to avoid early postoperative hypotony as demonstrated by recent experimental study.10 Indeed, the rate of AH turnover is estimated to be 1.0%–1.5% of the AC volume per min, which is 2.4±0.6 μL/min (mean ± SD, daytime measurements in adults aged 20–83 years)11 and the XEN45 gives a flow of 1.2 μL/min (at 5 mmHg pressure gradient), providing ~6–8 mmHg flow resistance, which reduces the risk of hypotony.4
Surgical technique
Lewis first described the surgical procedure,4 which was revised further by other authors. The procedure can be performed under local or topical anesthesia. Mitomycin C (MMC) is generally used in a concentration of 0.1–0.2 mg/mL (absolute dose of 10–20 μg). It is injected with a 30-gauge needle in the subconjunctival supero-temporal quadrant space to obtain a bubble that is gently rolled toward the supero-nasal quadrant. This induces a hydroexpansion, which reduces tissue resistance, preparing the space for the implant and supporting the bleb formation (Figure 2). Blood vessels should be avoided during the introduction of the needle into the subconjunctival space as bleeding may compromise visibility required for the stent implantation.
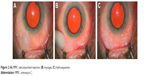  | Figure 2 (A) MMC subconjunctival injection; (B) massage; (C) hydroexpansion. |
Cataract surgery, if planned, may be performed after this step using miotic drugs after intraocular lens (IOL) implantation and ophthalmic viscosurgical device (OVD) removal. The intended area of placement in the supero-nasal quadrant, which is 3 mm from the limbus, is marked. The AC is filled with standard cohesive OVD and an infero-temporal 1.8 mm clear corneal incision is created through which the preloaded inserter needle (double-beveled 27 gauge) is directed across the AC to the opposite side to penetrate the angle. The needle passes through the sclera and emerges in the subconjunctival space ~3.0 mm posterior to the limbus, as previously marked, in the target supero-nasal quadrant. Once the tip of the needle is visible in the subconjunctival space, it is rotated toward the 12 o’clock position and the stent is gently delivered by advancing the sliderat.
The needle housing the implant is retracted without drawing the implant back. During this step, to stabilize the eye, a straight micro-manipulator is used in the side port corneal incision to maintain contact between the needle sleeve and the angle. (Figure 3) De Gregorio et al described a “three hands technique” in which the sliderat is pushed by a third hand; this allows reduction of the inserter movements, stabilizing the contact during stent delivery.12 The ideal stent placement should leave 2.0 mm of exposed implant in the subconjunctival space (preferentially in a more superficial layer than the sub-Tenon space), 1.0 mm in the AC, and 3.0 mm tunneled through sclera (Figure 4).
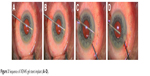  | Figure 3 Sequence of XEN45 gel stent implant (A–D). |
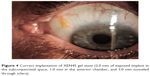  | Figure 4 Correct implantation of XEN45 gel stent (2.0 mm of exposed implant in the subconjunctival space, 1.0 mm in the anterior chamber, and 3.0 mm tunneled through sclera). |
The use of a mirrored gonioscope to verify placement through the angle and avoid iris root trauma is not always necessary, and is used at the discretion of the surgeon. The OVD is then washed, allowing the implant, when correctly positioned and patent, to immediately begin shunting fluid from the AC to the subconjunctival space. The initial bleb extends further into the non-dissected conjunctiva due to a gentle diffusion of the AH.
Better bleb morphology and function may be obtained by forced infusion of the fluid through paracentesis at the end of the procedure. All corneal incisions are sutured or hydro-sutured.
Antibiotic drug prophylaxis of the surgeon’s choice is generally continued for the first 2 weeks and it is associated with a topical corticosteroid 4 times each day for a month followed by a slow taper over the second month.13,14
Indication: inclusion and exclusion criteria
For inclusion criteria, indications differ slightly in Europe and in the USA.
Europe indication: XEN gel stent is intended to reduce IOP in patients with primary open-angle glaucoma (OAG) who have failed previous medical treatments.
US indication: Management of refractory glaucomas, including cases where previous surgical treatment has failed, cases of primary OAG, and pseudoexfoliative or pigmentary glaucomas with open angle that are unresponsive to maximum-tolerated medical therapy.
The XEN gel stent is generally contraindicated under the following circumstances or conditions: angle-closure glaucoma (gonioscopically, irido-corneal angle should be Shaffer 3 or 4 as narrow angles may cause blockage of the AC portion of the XEN implant by the iris; patients with Shaffer 2 or less could be selected provided the lens extraction effectively opened the irido-corneal angle and widened the AC); previous glaucoma shunt/valve in the target quadrant; presence of conjunctival scarring, prior conjunctival surgery or other conjunctival pathologies (eg, pterygium) in the target quadrant; active inflammation (eg, blepharitis, conjunctivitis, keratitis, and uveitis); active iris neovascularization or neovascularization of the iris within 6 months of the surgical date; AC IOL; presence of intraocular silicone oil; vitreous present in the AC; impaired episcleral venous drainage (eg, Sturge–Weber or nanophthalmos or other evidence of elevated venous pressure); known or suspected allergy or sensitivity to drugs required for the surgical procedure or any of the device components (eg, porcine products or glutaraldehyde); and history of dermatologic keloid formation.
Clinical data results
Several preclinical studies have been performed using animal models (rabbit and canine) and demonstrated that the glutaraldehyde cross-linked porcine gelatin did not induce significant intraocular inflammation and tissue reaction, not undergoing structural change or degradation after 1 year.10
Lewis described a case study in which an implant misplaced during early-stage pilot surgery was explanted 6 months postoperatively and analyzed. No tissue growth on the outside or inside was observed, and no signs of fibrosis around the implant was reported.4
All published clinical results and complications are briefly reported in Tables 1 and 2, respectively.
  | Table 2 Postoperative complications correlated to XEN45 implant reported in published studies |
In the first clinical human study, the implantation of 2 models of XEN gel stent (XEN140 and XEN63) was performed in 37 eyes (though it is not specified how many XEN140 and XEN63 have been implanted) with OAG at the time of cataract surgery without intraoperative use of MMC. Twelve months postoperatively, the mean IOP was significantly reduced to 15.4±3.0 mmHg from 22.4±4.2 mmHg preoperatively (32.25% mean IOP reduction), with a statistically significant reduction of medication classes (from 2.5±1.4 to 0.9±1.0; 50% of patients completely off medication). Despite this study not being designed to address safety, the authors reported no major or vision-threatening complications; the postoperative needling rate (with MMC or 5-fluorouracil) was 32%, although the same authors suspected that this rate would be lower if the procedure was performed with MMC at the time of implantation.13
Sheybani et al14 published further results of a multicenter, nonrandomized and prospective cohort trial on the XEN140 stand-alone surgical implantation without intraoperative MMC use in 49 eyes with refractory OAG. It is interesting to note that 71% (35 of 49 eyes) of the implanted eyes had prior failed glaucoma procedures/surgeries: 21 eyes had prior trabeculectomy with MMC, 2 eyes had prior tube shunt surgery, 3 eyes had prior external trans-scleral cyclophotocoagulation, and 9 eyes had laser trabeculoplasty. Only 45 eyes completed 12 months of follow-up (3 patients required additional glaucoma surgeries), with a mean IOP reduction of 36.4% from baseline and a decrease in number of medications from 3.0 to 1.3 (42% of patients off medications completely). No serious adverse events were reported in this study, although 9% of patients needed AC fills with OVD in the first postoperative week. None of the patients developed choroidal detachment, chronic hypotony, or hypotony maculopathy during the study period. Approximately 43% of patients underwent needling, a higher rate compared with current trabeculectomy studies. However, considering that more than 50% of patients had previous failed glaucoma surgery with conjunctival tissue disruption, the success of the device would have been higher and/or the needling rate lower if the eyes have had a conjunctival sparing surgery and if MMC had been used at the time of implantation.
Pérez-Torregrosa et al15 and De Gregorio et al12 published the first 2 clinical prospective studies on XEN45 gel stent implantation with adjunctive MMC combined with cataract surgery.
The first study was performed on 30 eyes with a diagnosis of mild/moderate OAG and cataract, demonstrating an IOP reduction of 29.34% at 12 months with a 94.57% medication decrease (from 3.07±0.69 to 0.17±0.65). Both intra- and postoperative complications were relatively minor, often inherent to the surgical maneuvers and spontaneously solved.
Indeed, a critical point reported by the authors is the correct final placement of the stent, emphasizing that the best pathway would be 2 mm subconjunctival to avoid extrusion, 3 mm intra-scleral to increase resistance to reduce excessive drainage, and 1 mm in the AC to limit contact with the corneal endothelium. This induced the authors to relocate 6 implants intraoperatively and reimplant in 1 case. During the follow-up, no needling was performed and the authors reported only 1 case of fibrosis with encapsulated bleb after 5 months, treated only with hypotensive medications.15
In the second study published by De Gregorio et al,12 XEN45 was implanted in 41 eyes with OAG in combination with microincisional cataract surgery. Outcomes of this study are that XEN45 implant is statistically effective in reducing IOP and medications even after 12 months, with an IOP reduction of 41.82%, and 80.4% of patients off medication. Only 1 patient had previous incisional glaucoma surgery (deep sclerectomy). Fifteen (36.6%) patients had allergies to anti-hypertensive drugs. All cataract surgeries were uneventful and no major complications during implantation surgery were experienced except for transient bleedings (subconjunctival and/or in the AC).
It is interesting to note that postoperatively, no patients needed AC refilling with OVD presumably due to the smaller inner diameter of the stent compared with those implanted in the first studies. All patients completed 12 months of follow-up, excluding 1 patient who needed a trabeculectomy after 1 month for a stent failure due to a presumed obstruction; in 1 case, the implant migrated completely in the AC (Figure 5). The implant was explanted and replaced with a new one. This study reported only 1 case of bleb fibrosis requiring needling (2.4%). The use of subconjunctival MMC in presence of surgical untouched conjunctival tissue can explain the different needling rate respect to the first pilot studies.
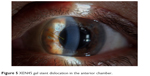  | Figure 5 XEN45 gel stent dislocation in the anterior chamber. |
An international multicenter retrospective study has recently been published that compares the efficacy, safety, and risk factors for failure of standalone XEN45 gel stent implantation versus trabeculectomy, both with adjunctive MMC.16
In this study, 354 eyes with uncontrolled glaucoma and no prior incisional filtering surgery underwent microstent implantation (n=185) or trabeculectomy (n=169) in 4 academic ophthalmology centres, providing a large database. The results demonstrated that there was no difference in efficacy, risk of failure, and safety profile between the 2 surgical procedures.
The most impressive risk factors for failure was the presence of diabetes in both kind of interventions. Nonwhite patients had statistically significant higher failure rates for trabeculectomy but it was not significant for eyes receiving microstent. Other statistically significant differential interactions between microstent and trabeculectomy were observed regarding preoperative visual acuity and IOP. Eyes with preoperative vision better than 0.4 logMAR showed statistically significantly better results with microstents, whereas those with worse vision tended to do better with trabeculectomies; eyes with preoperative IOP of >21 mmHg tended to do better with microstents, whereas those with ≤21 mmHg tended to do better with trabeculectomies. One-quarter of the microstent eyes and one-third of the trabeculectomy eyes were receiving glaucoma medications at the last follow-up; more subjects in the trabeculectomy group had post-surgical intervention, mostly by laser suture lysis (49.7%), although there were likely more needlings (43.2%) in the microstent group. The trabeculectomy group had more transient complications, mostly driven by leaks or dehiscences, though there were no cases of long-term complications from hypotony in either group.
Despite the large sample size, the multicenter design, and the long follow-up (30 months) allowing for reasonable external generalizability, the authors emphasized that there were several limitations to the study. It was a retrospective study and had loss to follow-up, which could over- or underestimate the success rate; in addition, it was still underpowered for safety considerations.
The multicenter prospective study published by Grover et al reported the results at 12 months on performance/safety of XEN45 implant following MMC pretreatment in 65 eyes with refractory glaucoma (failed prior filtering/cilioablative procedure or uncontrolled IOP on maximum-tolerated medical therapy).17
In an analysis that excluded patients with missing data (n=4) and those who required a glaucoma-related secondary surgical intervention (n=9), mean IOP change from baseline was >27% at all postoperative visits, reaching −9.1 mmHg (35.6% IOP reduction) at 12 months. Compared with baseline, 36 (69.2%) patients required fewer topical medications, 16 (30.8%) required the same number, and no patients required more (or oral medications). Overall, mean medication use decreased from 3.5 at baseline (n=65) to 1.7 at 12 months; in the subgroup analysis specified previously (n=52), 38.5% of patients did not require any medications.
Intraoperative complications and postoperative adverse events reported were mostly mild/moderate and transient, resolving without sequelae, and none were unexpected in this population of patients with refractory glaucoma. The postoperative complication most commonly reported in this study was bleb fibrosis requiring needling.
The surgical technique used in this study differs from the previous reported for subconjunctival pretreatment with MMC. The target area was treated with sponges saturated (0.2 mg/mL) for 2 min, assuming a conjunctival incisional approach requiring final conjunctiva closure with suture. That is the main reason for the observed needling rate (32.3%) according to the authors, who underline that the conjunctival opening required for the application of MMC may have induced scar formation. Although surgeons who use the gelatin stent in other countries have regularly administered MMC as a subconjunctival injection prior to the surgery, MMC is not Food and Drug Administration-approved for subconjunctival injection in the USA.
In a further prospective interventional study recently published by Galal et al18 13 eyes with primary OAG and no previous trabeculectomy surgery underwent XEN45 implantation with subconjunctival 0.01% MMC. Of those eyes, 3 were pseudophakic and 10 underwent simultaneous phacoemulsification and XEN implantation. At the end of the follow-up (12 months), patients achieved a mean IOP reduction of 29.4% (from 16±4mmHg pre-op to 12±3mmHg, p=0.01) with a decrease in medication number of 94.57% (from 1.9±1 preoperatively to 0.3±0.49, p=0.003); 42% of eyes achieved complete success (IOP reduction ≥20% without any glaucoma medications) and 66% qualified success (IOP reduction of ≥20% with medications). Complications included 2 transient hypotony with choroidal detachment that was resolved with medical therapy, 1 implant extrusion that required repositioning and conjunctival sutures, and 2 eyes needed further surgical intervention (trabeculectomy) due to inadequately controlled IOP. The rate of needling reported in this study was of 30.7%.
It is interesting to highlight that, in all previously mentioned studies, baseline mean IOP reported was always on therapy and no washout was performed before surgery. Consequently, the IOP reduction was calculated comparing IOP on therapy before, and IOP mostly off medication after, surgery.
Although postoperative adverse events reported in all these studies were mostly mild/moderate and transient, it is important to mention a case of suprachoroidal bleeding 2 days after XEN45 implantation reported by Prokosch-Willing et al.19
In this case report, an 84-year-old female patient with pseudoexfoliation glaucoma was correctly implanted with XEN45 without complications. On the first postoperative day, the patient presented an IOP of 4mmHg without choroidal detachment, a functioning bleb, and a deep AC. On the second postoperative day, she experienced a sudden strong pain and developed suprachoroidal bleeding with an increased IOP (54 mmHg). A wait-and-see strategy was followed and the bleeding resolved spontaneously with medical therapy after 6 weeks.
XEN45 “bleb” characteristics and management
The presence of subconjunctival fluid upon implantation confirms the connection between the AC and subconjunctival space. An initial medium or high bleb appearance could be present during the immediate postoperative stages. During the first week, this bleb gradually reduces in volume, and in later stages, the morphology of an established and functioning bleb differs from the blebs seen after traditional filtering surgeries, being lower lying (Figure 6) due to the diffuse dispersion of AH over wide areas in the non-dissected Tenon’s and subconjunctival space.7
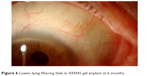  | Figure 6 Lower-lying filtering bleb in XEN45 gel implant at 6 months. |
In the early postoperative period, if the subconjunctival AH filtration finds an obstacle to its diffusion (in particular, the lower lid margin), a wide-pooling bleb can be produced. As reported by some authors,20 this hypertrophic bleb should be managed to avoid a mechanical ectropion. The same authors described the “Dry Lake” technique to drain the bleb through a conjunctival incision, treated afterward with fibrin tissue adhesive to stick planes. In our experience (data not published), a simple needling is generally sufficient to remove the subconjunctival fibrotic border in the lower margin of the bleb rehabilitating the diffuse AH dispersion over all the subconjunctival space (Figure 7).
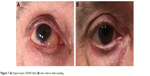  | Figure 7 (A) Hypertrophic XEN45 bleb; (B) after inferior bleb needling. |
One of the keys to successful filtering surgery is the development of a bleb in the postoperative period. Morphologic changes to the developing filtering bleb after surgery may help to predict early treatment failure, and guide bleb revision and management. An early study by Addicks et al21 demonstrated that failed trabeculectomy blebs had dense collagenous connective tissue in their walls, while in functioning blebs, the subepithelial connective tissue was loosely arranged and contained histologically clear spaces. Fea et al22 provided a macro- and microscopic analyses of bleb morphology in a prospective 12-month study on 12 eyes with primary OAG implanted with XEN45 gel stent either alone or combined with a cataract surgery. Biomicroscopy, in vivo confocal microscopy (IVCM) and anterior segment-optical coherence tomography (AS-OCT) were used to assess bleb morphology.
In this study, AS-OCT showed that bleb wall reflectivity was significantly higher in the failure group; IVCM revealed that stromal density was significantly lower in the success group. Microcysts within the bleb wall epithelium, first detected by Labbè et al23 following successful trabeculectomy, were significantly increased in density and area at the 6-month follow-up visit, suggesting a progressive aqueous percolation after stent implantation (Figure 8). Additionally, at 6 months, stromal reflectivity was significantly lower in the whole superior bulbar conjunctiva compared with previous observations, suggesting a slower manifestation of tissue rearrangement in deeper layers. On comparing successful and failed blebs at 12 months, the stromal density was significantly lower in the success group. Increased microcysts and loosely arranged connective tissue/low stromal reflectivity are suggestive of new or increased alternative AH outlow induced by the stent implantation.
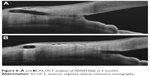  | Figure 8 (A and B) AS-OCT analysis of XEN45 bleb at 6 months. |
Although the manufacturer has provided some postoperative guidance, there is generally limited experience with regard to the assessment and postoperative management of a “XEN45 bleb”. Simply applying our usual bleb management in trabeculectomy may not be suitable for the XEN45 implant. Further studies should establish whether a less aggressive postoperative bleb management approach, in order to respect the concept of minimally invasive conjunctival sparing surgery, produces similar or better results.
Conclusion
XEN gel stent is an effective MIGS for controlling IOP in early, moderate, advanced, or refractory glaucoma patients. This surgical approach bypasses all trabecular and scleral resistance to create outflow but, unlike other scleral full-thickness procedures, obviates conjunctival dissection and provides sufficient resistance flow through the tube to avoid flat chambers or clinically significant hypotony. This conjunctiva-sparing ab interno approach with a safe profile gives the ophthalmologists a new tool to reach the target IOP in refractory glaucoma, as a final step, and in patients intolerant to medical therapy, as an early surgical treatment.
Disclosure
The authors report no conflicts of interest in this work.
References
Tham Yc, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. | ||
Goel M, Picciani RG, Lee RK, Bhattacharya S. Aqueous humor dynamics: a review. Open Ophthalmol J. 2010;4:52–59. | ||
Richter GM, Coleman AL. Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmol. 2016;10:189–206. | ||
Lewis RA. Ab interno approach to the subconjunctival space using a collagen glaucoma stent. J Cataract Refract Surg. 2014;40(8):1301–1306. | ||
Singh D. Conjunctiva lymphatic system. J Cataract Refract Surg. 2003;29(4):632–633. | ||
Yu DY, Morgan WH, Sun X, et al. The critical role of the conjunctiva in glaucoma filtration surgery. Prog Retin Eye Res. 2009;28(5):303–328. | ||
Vera V, Horvath C. XEN gel stent: the solution designed by AqueSys®. In: Samples JR, Ahmed IIK, editors. Surgical Innovations in Glaucoma. New York, NY, USA: Springer; 2014. | ||
Saheb H, Ahmed II. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23(2):96–104. | ||
Shute TS, Dietrich UM, Baker JF, et al. Biocompatibility of a novel microfistula implant in nonprimate mammals for the surgical treatment of glaucoma. Invest Ophthalmol Vis Sci. 2016;57(8):3594–3600. | ||
Sheybani A, Reitsamer H, Ahmed II. Fluid dynamics of a novel micro-fistula implant for the surgical treatment of glaucoma. Invest Ophthalmol Vis Sci. 2015;56(8):4789–4795. | ||
Johnson M, Erickson K. Mechanisms and routes of aqueous humor drainage. In: Albert DM, Jakobiec FA, editors. Principles and Practice of Ophthalmology. Philadelphia: WB Saunders; 2000. | ||
De Gregorio A, Pedrotti E, Russo L, Morselli M. Minimally invasive combined glaucoma and cataract surgery: clinical results of the smallest Ab interno gel stent. Int Ophtalmol. Epub 2017 May 29. | ||
Sheybani A, Lenzhofer M, Hohensinn M, Reitsamer H, Ahmed II. Phacoemulsification combined with a new ab interno gel stent to treat open-angle glaucoma: Pilot study. J Cataract Refract Surg. 2015;41(9):1905–1909. | ||
Sheybani A, Dick B, Ahmed II. Early clinical results of a novel Ab interno gel stent for the surgical treatment of open-angle glaucoma. J Glaucoma. 2016;25(7):e691–e696. | ||
Pérez-Torregrosa VT, Olate-Pérez Á, Cerdà-Ibáñez M, et al. [Combined phacoemulsification and XEN45 surgery from a temporal approach and 2 incisions.] Arch Soc Esp Oftalmol. 2016;91(9):415–421. Spanish. | ||
Schlenker MB, Gulamhusein H, Conrad-Hengerer I, et al. Efficacy, safety, and risk factors for failure of standalone Ab interno gelatin microstent implantation versus standalone trabeculectomy. Ophthalmology. 2017;124(11):1579–1588. | ||
Grover DS, Flynn WJ, Bashford KP, et al. Performance and safety of a new Ab interno gelatin stent in refractory glaucoma at 12 months. Am J Ophthalmol. 2017;183:25–36. | ||
Galal A, Bilgic A, Eltanamly R, Osman A. XEN glaucoma implant with mitomycin C 1-year follow-up: result and complications. J Ophthalmol. 2017;2017:5457246. | ||
Prokosch-Willing V, Vossmerbaeumer U, Hoffmann E, Pfeiffer N. Suprachoroidal bleeding after XEN gel implantation. J Glaucoma. 2017;26(12):e261–e263. | ||
Fernández-García A, Romero C, Garzón N. [“Dry Lake” technique for the treatment of hypertrophic bleb following XEN(®) gel s placement]. Arch Soc Esp Oftalmol. 2015;90(11):536–538. Spanish. | ||
Addicks EM, Quigley HA, Green WR, Robin AL. Histologic characteristics of filtering blebs in glaucomatous eyes. Arch Ophthalmol. 1983;101(5):795–798. | ||
Fea AM, Spinetta R, Cannizzo PML, et al. Evaluation of Bleb morphology and reduction in IOP and glaucoma medication following implantation of a novel gel stent. J Ophthalmol. 2017;2017:9364910. | ||
Labbé A, Dupas B, Hamard P, Baudouin C. In vivo confocal microscopy study of blebs after filtering surgery. Ophthalmology. 2005;112(11):1979. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

