Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 8
Vitamin D status and cholecalciferol supplementation in chronic kidney disease patients: an Italian cohort report
Authors Cupisti A, Vigo V, Baronti ME, D'Alessandro C, Ghiadoni L, Egidi MF
Received 23 June 2015
Accepted for publication 11 August 2015
Published 19 November 2015 Volume 2015:8 Pages 151—157
DOI https://doi.org/10.2147/IJNRD.S90968
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Pravin Singhal
Adamasco Cupisti, Valentina Vigo, Maria Enrica Baronti, Claudia D'Alessandro, Lorenzo Ghiadoni, Maria Francesca Egidi
Nephrology, Transplant and Dialysis Division, AOUP, Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy
Abstract: This study investigated the factors associated with hypovitaminosis D, in a cohort of 405 prevalent patients with chronic kidney disease (CKD) stages 2–4, living in Italy and followed-up in tertiary care. The effect of cholecalciferol 10,000 IU once-a-week for 12 months was evaluated in a subgroup of 100 consecutive patients with hypovitaminosis D. Vitamin D deficiency was observed in 269 patients (66.4%) whereas vitamin D insufficiency was found in 67 patients (16.5%). In diabetic patients, 25-hydroxyvitamin D deficiency was detected in 80% of cases. In patients older than 65 years, the prevalence of hypovitaminosis D was 89%. In the univariate analysis, 25-hydroxyvitamin D was negatively related to age, parathyroid hormone (PTH), proteinuria, and Charlson index, while a positive relationship has emerged with hemoglobin level. On multiple regression analysis, only age and PTH levels were independently associated with 25-hydroxyvitamin D levels. No relationship emerged between vitamin D deficiency and renal function. Serum levels of 25-hydroxyvitamin D or prevalence of hypovitaminosis D did not differ between patients on a free-choice diet and on a renal diet, including low-protein, low-phosphorus regimens. Twelve-month oral cholecalciferol administration increased 25-hydroxyvitamin D and reduced PTH serum levels. In summary, hypovitaminosis D is very prevalent in CKD patients (83%) in Italy, and it is similar to other locations. PTH serum levels and age, but not renal function, are the major correlates of hypovitaminosis D. Implementation of renal diets is not associated with higher risk of vitamin D depletion. Oral cholecalciferol administration increased 25-hydroxyvitamin D and mildly reduced PTH serum levels. Oral cholecalciferol supplementation should be recommended as a regular practice in CKD patients, also when serum 25-hydroxyvitamin D determination is not available or feasible.
Keywords: CKD, vitamin D, cholecalciferol, calcifediol, hypovitaminosis, PTH, renal disease, CKD-MBD
Introduction
Disorders of vitamin D metabolism are frequent in chronic kidney disease (CKD) and represent a crucial physiopathological mechanism of CKD and mineral bone disorders. Vitamin D deficiency is a highly prevalent abnormality in CKD patients,1,2 as well as the reduction of the active form, calcitriol – 1,25-hydroxyvitamin D. Sun exposure is crucial to meet the requirements for vitamin D. Thus, factors such as climate, location, aging, lifestyle, and skin pigmentation may affect the endogenous production of vitamin D.
However, dietary intake of cholecalciferol (from animal sources) or ergocalciferol (from plant sources) plays a role and represents nearly 20% of the 25-hydroxyvitamin D supply. It has been suggested that renal diets targeted to reducing dietary phosphorus intake can contribute to the development of hypovitaminosis D in CKD patients, especially in dialysis patients.3
Vitamin D deficiency is defined as 25-hydroxyvitamin D serum levels lower than 20 ng/mL, whereas vitamin D insufficiency is defined as 25-hydroxyvitamin D serum levels ranging 21–30 ng/mL.4 Vitamin D deficiency causes bone and mineral abnormalities and may also play a role in several diseases including infection, cardiovascular diseases, malignancy, insulin resistance, diabetes, autoimmune disease, and impaired physical functioning.4 In CKD patients, vitamin D deficiency is associated with poorer outcomes.5,6
The prevalence of vitamin D insufficiency/deficiency status is similar in people without or with CKD who do not require dialysis.7 Major determinants of vitamin D status include age, sex, body mass index, obesity, and diabetes.8
Instead, the level of residual renal function is not a direct major determinant of vitamin D status.7 However, certain conditions associated with CKD such as protein losses or inadequate food intake can predispose CKD patients to severe hypovitaminosis D.
Guidelines recommend to treat hypovitaminosis D and suggest an optimal 25-hydroxyvitamin D serum levels >30 ng/mL, even if this cutoff is not based on strong evidence. In recent years, vitamin D supplementation practices are increased significantly and have achieved greater serum 25-hydroxyvitamin D levels.9,10
In the present study, we examined the prevalence of vitamin D deficiency in CKD patients living in our county, and the correlates for vitamin D insufficiency/deficiency including dietary regimens. The effects of a once-a-week cholecalciferol supplementation schedule in vitamin D depleted CKD patients have been examined, as well.
Patients and methods
This population-based, prospective study includes CKD outpatients, free from dialysis, on tertiary care. Patients were included if aged >18 years, if were in stable clinical conditions, and if in CKD stages 2–4. Exclusion criteria were malignancy, acute illness, hospital admission in the previous 3 months, systemic inflammatory diseases, hepatic insufficiency, inflammatory bowel disease, pancreatitis, hypercalcemia, hyperphoshatemia, and parathyroid hormone (PTH) serum levels >500 pg/mL. Kidney transplant recipients or patients taking any vitamin D product (calcitriol, calcifediol, paracalcitol, ergocalciferol, and cholecalciferol) were excluded as well.
We finally included 405 prevalent outpatients: 260 males (age 68.3±12.3 years) and 145 females (age 68.9±13.0 years).
All the patients underwent an assessment for 25- hydroxyvitamin D serum levels. PTH, calcium, phosphorus, serum albumin, lipid panel as well as serum creatinine, urea, electrolytes, uric acid, and hemoglobin were determined in overnight fasting blood samples. Body mass index and Charlson comorbidity index were calculated as well.
A subgroup of 100 consecutive patients with hypovitaminosis D (<30 ng/mL), who received oral supplementation of cholecalciferol (10,000 IU/week) and who were evaluated at baseline and after 12 months of treatment, were retrospectively selected. The evaluation at 12 months was assumed to exclude the bias due to seasonal variability in 25-hydroxyvitamin D levels.
25-Hydroxyvitamin D serum levels were determined using an high performance liquid chromatography-UV method (Eureka Lab Division, Chiaravalle, Ancona, Italy). Serum 1-84 PTH was measured using a second generation assay (Liaison N-tact PTH II, DiaSorin, Saluggia, Italy), in all the cases. All the other biochemical analysis was performed by routinely used laboratory methods. Glomerular filtration rate was estimated (eGFR) by the 4-variable Modification of Diet in Renal Disease formula.1 This study was in adherence with the Declaration of Helsinki and was approved by the Ethics Committee of the Institutional Review Board at Pisa University Hospital.
Statistical analysis
Descriptive statistics is given as mean ± standard deviation. Statistical analysis was performed by Student’s t-test for unpaired and paired samples.
Simple and multiple regression analyses were performed to examine univariate and independent correlations of 25-hydroxyvitamin D serum levels.
Differences were considered as statistically significant when P<0.05.
Results
Table 1 shows the demographic and laboratory characteristics of the entire cohort of patients, according to CKD stages.
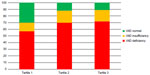
As a whole, 25-hydroxyvitamin D serum levels resulted 17.5±11.2 ng/mL. No differences were observed between males and females (18.2±11.0 and 16.4±11.6 ng/mL, respectively). Vitamin D deficiency was observed in 269 patients (66.4%), whereas vitamin D insufficiency was found in 67 patients (16.5%). Hence a cumulative detection of hypovitaminosis D was observed in 82.9% of our CKD population. Figure 1 shows the prevalence of vitamin D deficiency and insufficiency by tertiles of age. In the second and third tertiles, the prevalence of hypovitaminosis was significantly higher than that in the first tertile. It derives that CKD patients older than 65 years have a 90% risk of hypovitaminosis D.
As expected, 25-hydroxyvitamin D serum levels were higher when blood collection was taken in summer period (Figure 2).
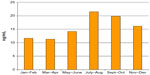  | Figure 2 Bimonthly course of the median values of 25-hydroxyvitamin D serum levels in the studied cohort. |
Table 2 shows the characteristics of the studied patients, according to the 25-hydroxyvitamin D serum levels.
The 25-hydroxyvitamin D levels were lower in diabetic than in nondiabetic patients (13.6±9.5 vs 19.4±11.5 ng/mL, P<0.001): accordingly, the prevalence of 25-hydroxyvitamin D deficiency was higher among the 129 diabetic patients than in the nondiabetic cohort (79.8% vs 60.5%, P<0.01).
A total of 225 patients underwent renal dietician counseling and were on renal diets consisting of protein- (<0.8 g/kg/day) and phosphate-restricted regimens, which supply quite low amount of vitamin D (median 0.36 μg/day, range 0.1–0.6 μg/day); 180 patients were not on a dietary counseling program and followed a free-choice diet that presumably contained higher amount of vitamin D (median 1.3 μg/day, range 0.3–4.0 μg/day). Serum 25-hydroxyvitamin D did not differ between these two groups (16.5±10.2 vs 18.2±11.8 ng/mL, respectively), and no relationship emerged between urinary urea excretion and 25-hydroxyvitamin D levels. Within the group of renal diet patients, no difference in serum 25-hydroxyvitamin D levels resulted between patients (n=55) on a low-protein diet (0.6 g/kg/day) and those (n=170) on a protein prescription of 0.8 g/kg/day (17.1±9.8 vs 16.9±10.5 ng/mL, respectively); therefore, no difference emerged regarding the prevalence of hypovitaminosis D (65% vs 73%, respectively).
The univariate analysis showed an inverse relationship between 25-hydroxyvitamin D levels and age (r=−0.259, P<0.001), PTH (r=−0.312, P<0.001), Charlson index (r=−0.236, P<0.001), whereas a positive relationship with hemoglobin (r=0.210, P<0.001). In the cohort of 76 patients with urine protein excretion >0.3 g/day, 25- hydroxyvitamin D serum levels were inversely related to urine protein excretion (r=−0.315, P<0.05).
On multiple regression analysis, only age (R2=0.014) and PTH levels (R2=0.060) were independently associated with 25-hydroxyvitamin D levels (R2=0.15) (Table 3).
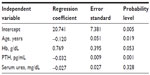  | Table 3 Simple regression analyses examining univariate and independent correlations of 25-hydroxyvitamin D serum levels |
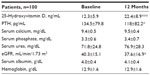
In the 100 CKD patients receiving oral supplementation of cholecalciferol (10,000 IU/week), we observed a twofold increase in 25-hydroxyvitamin D serum levels (Table 4) and the prevalence of 25-hydroxyvitamin D deficiency lowered from 76% to 33% of patients. A mild but significant reduction of PTH level occurred despite a mild reduction of eGFR. Although serum 25-hydroxyvitamin D nearly doubled in all the CKD stages, a significant reduction of PTH occurred in stage 3b patients (139±66 vs 117±57 pg/mL, P<0.05) only. No changes regarding calcium and phosphorus serum levels were observed (Table 4).
Discussion
The present study confirms the high prevalence of 25-hydroxyvitamin D depletion in a cohort of CKD patients living in a temperate climate, namely Tuscany (Italy). These data are well in agreement with others from different regions and reported in the paper by Cuppari and Garcia-Lopes.11 In most of the published reports, 25-hydroxyvitamin D serum levels were lower than 20 ng/mL, so the prevalence of 25-hydroxyvitamin D deficiency or insufficiency ranged from 70% to nearly 100%.11 A significantly higher average level of 25-hydroxyvitamin D has been reported in CKD patients living in southern Italy: this is well in accordance with the more sunny climate of Sicily than Tuscany.5
Patients older than 65 years showed a very high prevalence of hypovitaminosis D, approaching 90%. Hypovitaminosis D was also associated with higher Charlson comorbidity index, a finding that is in keeping with the well-known relationship between hypovitaminosis D and morbidity/mortality risk.
As already reported, hypovitaminosis D was independent from the different stages of CKD. At variance with the literature, we did not find a higher risk of hypovitaminosis D in women.
A negative relationship emerged between 25- hydroxyvitamin D and urine protein excretion. Moreover, proteinuric patients can develop vitamin D deficiency by urinary loss of vitamin D-binding protein, which binds most of the serum calcifediol.
A very high prevalence of vitamin D deficiency was observed in diabetic CKD patients. This is of a real concern since hypovitaminosis D was associated with an increased risk of kidney disease progression and mortality.12,13
In our series, we found a relationship between hematocrit or hemoglobin and 25-hydroxyvitamin D. These findings are in agreement with other authors who reported that in the early CKD 25-hydroxyvitamin D and 1,25-hydroxyvitamin D deficiencies were independently associated with low hemoglobin levels.14 However, the reasons accounting for this relationship are not yet known, as it is not proven the favorable effect of vitamin D supplementation in improving anemia in CKD patients.15
Renal diets consist of low-protein, low-phosphate regimens including avoidance of dairy products and yolk (for the very high phosphate content) and limitation of meat and fish. Hence these diets supply lower amount of vitamin D than mixed free-choice diets. Nevertheless, we did not find any association between renal diet regimens and 25-hydroxyvitamin D deficiency. Namely, the prevalence of hypovitaminosis D or 25-hydroxyvitamin D serum levels did not differ between patients on low-protein, low-phosphorus diets16,17 and those on free-choice diets. This is not surprising, since dietary supply represents a minor component for determining vitamin D status.
The 25-hydroxyvitamin D serum levels were higher when blood collection was taken in summer, to confirm the seasonal variability of calcifediol (Figure 1). This is also the reason why the effect of cholecalciferol administration was assessed at 12 months and not at 3 or 6 months.
Multivariate analysis showed that age and PTH explained 1.5% and 4.5%, respectively, of 25-hydroxyvitamin D variance. It is noteworthy that the maximum linearity occurs in stage 3b CKD patients.
Evidence exists that not only the reduction of calcitriol, but also lower levels of calcifediol can contribute to the development of secondary hyperparathyroidism. Therefore, vitamin D repletion using ergocalciferol or cholecalciferol is recommended to obtain adequate vitamin D status in CKD.11 K-DIGO guidelines suggest that vitamin D deficiency and insufficiency in patients with CKD stages 3–5D should be corrected as it occurs in the general population. However, this recommendation is based on low quality of evidence (2c stage).3
Then, supplementation of cholecalciferol we proposed was addressed to patients with hypovitaminosis D and the effects were measured at 12-month follow-up, to avoid the changes linked to the potential changes of lifestyle and sun exposure related to seasonal-climate changes. In our treated patients, serum levels of 25-hydroxyvitamin D almost doubled, together with a mild decrease of PTH despite a decrease of renal function. This occurred without any change in serum levels of phosphate and calcium. This suggests that cholecalciferol supplementation may be effective and safe in the early-stage CKD patients with hypovitaminosis D.
As already discussed, the inverse relationship between PTH and 25-hydroxyvitamin D is well known, and it occurs in the earlier stages of CKD, ie, when more chances of 1,25-hydroxyvitamin D synthesis happen.11 To confirm, studies of ergocalciferol or cholecalciferol supplementation have demonstrated PTH reductions (even if quite modest) that are more likely to occur in stage 3 CKD, while in stages 4–5 CKD this beneficial effect was not observed.18–20
The attenuation of secondary hyperparathyroidism by ergocalciferol or cholecalciferol was not confirmed in other series.21,22
Given that the majority of CKD patients, independently on the stage, have a condition of hypovitaminosis D, the cholecalciferol administration may safely contribute to ameliorate calcium–phosphate abnormalities in CKD. Oral cholecalciferol at the dosage of 10,000 IU once-a-week should be recommended as a regular practice in CKD care management even when determinations of 25-hydroxyvitamin D serum levels are not feasible. This is true, especially in diabetic, elderly, or proteinuric patients.
Major limitation of the study consists in the observational, single-center, retrospective design. Conversely, the number of patients is quite larger than other published studies, and it allows analysis of the different CKD stages too. Although the cholecalciferol supplementation was performed with a nonrandomized retrospective design, the 12-month follow-up was longer than in other studies and may prevent the bias due to seasonal variability.
In summary, hypovitaminosis D is very prevalent in CKD patients in Italy, and it is similar to other locations. PTH serum levels and age, but not renal function, are the major correlates of hypovitaminosis D. Implementation of renal diets, including low-protein diets, is not associated with higher risk of vitamin D depletion. Oral cholecalciferol administration increased 25-hydroxyvitamin D levels and mildly reduced PTH serum levels. Oral cholecalciferol supplementation should be recommended as a regular practice in CKD patients, also when 25-hydroxyvitamin D determination is not available or feasible.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. | |
Mehrotra R, Kermah D, Budoff M, et al. Hypovitaminosis D in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1144–1151. | |
Krassilnikova M, Ostrow K, Bader A, Heeger P, Mehrotra A. Low dietary intake of vitamin D and vitamin D deficiency in hemodialysis patients. J Nephrol Ther. 2014;4(3). | |
Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. | |
Santoro D, Gitto L, Ferraro A, Satta E, Savica V, Bellinghieri G. Vitamin D status and mortality risk in patients with chronic kidney disease. Ren Fail. 2011;33(2):184–191. | |
Zheng Z, Shi H, Jia J, Li D, Lin S. Vitamin D supplementation and mortality risk in chronic kidney disease: a meta-analysis of 20 observational studies. BMC Nephrol. 2013;14:199. | |
Guessous I, McClellan W, Kleinbaum D. Comparisons of serum vitamin D levels, status, and determinants in populations with and without chronic kidney disease not requiring renal dialysis: a 24-hour urine collection population-based study. J Ren Nutr. 2014; 24(5):303–312. | |
Figuiredo-Dias V, Cuppari L, Garcia-Lopes MG, de Carvalho AB, Draibe SA, Kamimura MA. Risk factors for hypovitaminosis D in nondialyzed chronic kidney disease patients. J Ren Nutr. 2012; 22(1):4–11. | |
Mazzaferro S, Goldsmith D, Larsson TE, Massy ZA, Cozzolino M. Vitamin metabolites and/or analogs: which D for which patient? Curr Vasc Pharmacol. 2014;12:339–349. | |
Mariani LH, White MT, Shults J. Increasing use of vitamin D supplementation in the chronic renal insufficiency cohort study. J Ren Nutr. 2014;24(3):186–193. | |
Cuppari L, Garcia-Lopes MG. Hypovitaminosis D in chronic kidney disease patients: prevalence and treatment. J Ren Nutr. 2009;19(1):38–43. | |
Lucisano S, Buemi M, Passantino A, Aloisi C, Cernaro V, Santoro D. New insights on the role of vitamin D in the progression of renal damage. Kidney Blood Press Res. 2013;37(6):667–678. | |
Fernandez-Juarez G, Luno J, Vicente Barrio V, et al. 25(OH) vitamin D levels and renal disease progression in patients with type 2 diabetic nephropathy and blockade of the renin-angiotensin system. Clin J Am Soc Nephrol. 2013;8:1870–1876. | |
Patel NM, Gutierrez OM, Andress DL, Coyne DW, Levin A, Wolf M. Vitamin D deficiency and anemia in early chronic kidney disease. Kidney Int. 2010;77:715–720. | |
Lucisano S, Di Mauro E, Montalto G, Cernaro V, Buemi M, Santoro D. Vitamin D and anemia. J Ren Nutr. 2014;24:61–62. | |
Piccoli GB, Deagostini MC, Vigotti FN, et al. Which low-protein diet for which CKD patient? An observational, personalized approach. Nutrition. 2014;30:992–999. | |
Aparicio M, Bellizzi V, Chauveau P, et al. Do keto analogues still have a role in delaying dialysis initiation in CKD predialysis patients? Semin Dial. 2013;26:714–719. | |
DeVille J, Thorp ML, Tobin L, Gray E, Johnson ES, Smith DH. Effect of ergocalciferol supplementation on serum parathyroid hormone and serum 25-hydroxyvitamin D in chronic kidney disease. Nephrology (Carlton). 2006;11:555–559. | |
Chandra P, Binongo JN, Ziegler TR, et al. Cholecalciferol (vitamin D3) therapy and vitamin D insufficiency in patients with chronic kidney disease: a randomized controlled pilot study. Endocr Pract. 2008;14: 10–17. | |
Kim SM, Choi HJ, Lee JP, et al. Prevalence of vitamin D deficiency and effects of supplementation with cholecalciferol in patients with chronic kidney disease. J Ren Nutr. 2014;24:20–25. | |
Al-Aly Z, Qazi RA, González EA, Zeringue A, Martin KJ. Changes in serum 25-hydroxyvitamin D and plasma intact PTH levels following treatment with ergocalciferol in patients with CKD. Am J Kidney Dis. 2007;50:59–68. | |
Zisman AL, Hristova M, Ho LT, Sprague SM. Impact of ergocalciferol treatment of vitamin D deficiency on serum parathyroid hormone concentrations in chronic kidney disease. Am J Nephrol. 2007;27:36–43. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.