Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 8
TriCalm® hydrogel is significantly superior to 2% diphenhydramine and 1% hydrocortisone in reducing the peak intensity, duration, and overall magnitude of cowhage-induced itch
Authors Papoiu A, Chaudhry H, Hayes E, Chan YH, Herbst K
Received 6 December 2014
Accepted for publication 27 January 2015
Published 24 April 2015 Volume 2015:8 Pages 223—229
DOI https://doi.org/10.2147/CCID.S78809
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Alexandru DP Papoiu,1 Hunza Chaudhry,2 Erin C Hayes,2 Yiong-Huak Chan,3 Kenneth D Herbst4
1Independent contractor, San Diego, CA, USA: 2Cosmederm Bioscience, Inc., San Diego, CA, USA; 3Biostatistics Unit, Yong Loo Lin School of Medicine, National University of Singapore, Singapore; 4Department of Medicine, University of California, San Diego, San Diego, CA, USA
Background: Itch is one of the most frequent skin complaints and its treatment is challenging. From a neurophysiological perspective, two distinct peripheral and spinothalamic pathways have been described for itch transmission: a histaminergic pathway and a nonhistaminergic pathway mediated by protease-activated receptors (PAR)2 and 4. The nonhistaminergic itch pathway can be activated exogenously by spicules of cowhage, a tropical plant that releases a cysteine protease named mucunain that binds to and activates PAR2 and PAR4.
Purpose: This study was conducted to assess the antipruritic effect of a novel over-the-counter (OTC) steroid-free topical hydrogel formulation, TriCalm®, in reducing itch intensity and duration, when itch was induced with cowhage, and compared it with two other commonly used OTC anti-itch drugs.
Study participants and methods: This double-blinded, vehicle-controlled, randomized, crossover study recorded itch intensity and duration in 48 healthy subjects before and after skin treatment with TriCalm hydrogel, 2% diphenhydramine, 1% hydrocortisone, and hydrogel vehicle, used as a vehicle control.
Results: TriCalm hydrogel significantly reduced the peak intensity and duration of cowhage-induced itch when compared to the control itch curve, and was significantly superior to the two other OTC antipruritic agents and its own vehicle in antipruritic effect. TriCalm hydrogel was eight times more effective than 1% hydrocortisone and almost six times more effective than 2% diphenhydramine in antipruritic action, as evaluated by the reduction of area under the curve.
Conclusion: TriCalm hydrogel has a robust antipruritic effect against nonhistaminergic pruritus induced via the PAR2 pathway, and therefore it could represent a promising treatment option for itch.
Keywords: antipruritic treatment, nonhistaminergic pruritus, head-to-head comparison
Introduction
Itch has been reported as the most frequent complaint in the dermatology clinic.1 In 1660, Hafenreffer defined itch as an “unpleasant sensation that elicits the desire or reflex to scratch.”2 For a long time, the neural mechanisms underlying the transmission of itch remained elusive. In the last decade, the pathophysiology of itch has been intensively investigated and significant progress has been made in understanding its cellular and molecular mechanisms. Itch is classified as acute or chronic. According to the International Forum for the Study of Itch, acute pruritus is defined as an itch lasting less than 6 weeks, while chronic pruritus is an itch persisting for 6 or more weeks.1,3 Chronic itch is a complex multifactorial phenomenon that may arise primarily from skin diseases, such as atopic dermatitis or psoriasis, but it is not confined solely to dermatological conditions.1,4 Pruritus is associated with a marked reduction in quality of life and has been reported to be as debilitating as chronic pain.5–8 If left untreated, pruritus can lead to skin damage through the self-perpetuating, counterproductive itch–scratch cycle, and to severe sleep impairment, mood disturbances, and poorer performance at work and/or school.9 Unfortunately, pruritus is particularly difficult to treat. To date, there are no target-specific treatments available for itch, and antipruritic therapies are only somewhat effective, since they do not always successfully address the underlying cause.1,9 Currently, the medications used to treat itch include antihistamines, opioid receptor agonists or antagonists, corticosteroids, and in severe cases, antidepressants. These agents provide some degree of itch relief; however, no complete resolution is attained in most cases.10 Corticosteroid therapy may be effective, but its use is limited due to its dose- and time-dependent side effects, including risk of adrenal axis suppression, skin atrophy, tachyphylaxis, striae, rosacea-like symptoms, perioral dermatitis, acne, and purpura.11–14 According to expert opinion, new substances and classes of antipruritic drugs are needed urgently.10
It has become increasingly clear that current antihistamines, which are of benefit in treating itch of allergic causes (such as immediate hypersensitivity reactions), are mostly ineffective in controlling the itch associated with diseases such as atopic eczema or psoriasis.15,16 This suggests that nonhistaminergic mechanisms are predominantly involved in pruritus. Current neurobiological models of itch transmission recognize the existence of distinct peripheral and central neural pathways that transduce histamine-based and nonhistamine-based forms of itch.15–19
One of the nonhistaminergic pathways that has been elucidated recently is the cowhage itch pathway.20 As postulated from the pioneering work of Shelley and Arthur almost 60 years ago,21 the cowhage plant releases a cysteine protease named mucunain, which binds to and activates protease-activated receptors PAR2 and PAR4.20 The PAR2–mediated itch pathway has been implicated in the pathophysiology of pruritus in atopic eczema.19,22 Therefore, cowhage itch has been proposed as a valuable experimental model for itch in humans.23–26 The cowhage itch model, using cowhage spicules, has been employed to study the efficacy of various topical antipruritic formulations.26–28 In a study performed in healthy subjects, as well as in patients with atopic dermatitis, we have demonstrated that, in comparison with the classical model using histamine, cowhage itch is a robust, reliable, and reproducible experimental model that is highly effective to induce an acute and transient itch.23 Typically, after the application of spicules, cowhage itch has an onset time of about 30–90 seconds, peaks after 90–120 seconds, and lasts for approximately 6–8 minutes. In a previous study, we utilized cowhage itch to compare the antipruritic efficacy of TriCalm® hydrogel with two other antipruritic topical agents: 2% diphenhydramine and 1% hydrocortisone.26
This study was conducted to test the antipruritic effect of the steroid-free topical hydrogel formulation, TriCalm hydrogel, in reducing itch intensity and duration, when itch was induced with cowhage spicules. TriCalm hydrogel is an over-the-counter (OTC) topical formulation designed to alleviate skin irritation (itching, burning, or stinging sensations). We compared the ability of this topical agent to relieve skin irritation (its antipruritic efficacy) with topical 2% diphenhydramine and 1% hydrocortisone, and with its own hydrogel vehicle.
Materials and methods
Study design and subjects
This study employed a double-blinded, vehicle-controlled, randomized, crossover design. Adult men and women between 18 and 50 years of age were included in this study if they were in general good health and had no disease that could impair evaluation of itch or pain perception (such as peripheral neuropathy). The exclusion criteria included current or recent use (within 1 week prior to study initiation) of oral or topical analgesics (such as antihistamines, opioids, and neuroleptics) or other medications known to interfere with itch or pain perception; use of emollients on the forearms on the day of the study, or use of medicated topical preparations on the forearms for the week prior to the study; history of neuropathy-causing diseases, such as uremia; history of uncontrolled thyroid disease; uncontrolled diabetes mellitus or diabetic neuropathy; and allergy to any of the study medications.
The study was performed in accordance with the current version of the Declaration of Helsinki (52nd WMA General Assembly, Edinburgh, Scotland, 2000). The trial was conducted in agreement with the International Conference on Harmonisation (ICH) guidelines on Good Clinical Practices (GCP). All subjects provided written informed consent to participate in the study.
Demographics and characteristics of study participants
Forty-eight subjects, including 20 men and 28 women between 19 and 50 years of age (mean ± standard deviation: 35.0±10.1 years) were tested. Of a total of 49 subjects screened, one participant did not fulfill study criteria. Subjects were free to withdraw from the study at any time without giving a reason; however, no subjects withdrew from the study. The results were analyzed in 47 healthy subjects, as one data set from one subject was not included in the analysis due to an exceedingly atypical, prolonged itch response (in excess of 40 minutes).
Cowhage itch induction
Itch was induced by the application of 40–45 cowhage spicules, which were counted under a magnifying lens and then were rubbed into the subject’s skin by a study investigator, as we described previously.23 The spicules were rubbed gently in a circular motion within a 16 cm2 square area of skin, for 45 seconds. A sterile gauze perforated in a rectangular shape matching the 4×4 cm treatment area was used to cover the surrounding skin and prevent the spicules from stimulating the skin outside the designated area. At the conclusion of each experiment, when itch had subsided, the spicules were removed using adhesive tape (Scotch®; 3M, St Paul, MN, USA).
Antipruritic agents tested
Treatments administered consisted of TriCalm hydrogel; hydrogel vehicle of TriCalm; 2% diphenhydramine; and Cortizone-10®, containing 1% hydrocortisone. TriCalm hydrogel is an OTC product manufactured by Cosmederm Bioscience, Inc. (San Diego, CA, USA) containing as the active ingredient aluminum acetate 0.2%. Cortizone-10 (Rite Aid, Camp Hill, PA, USA) was purchased as an OTC product from a local Rite Aid pharmacy. A lotion containing 2% diphenhydramine was compounded by La Vita Pharmacy (San Diego, CA, USA) to avoid the alcohols used in the formulation of a commercially available OTC Benadryl® (diphenhydramine), and other, flavored, ingredients such as menthol, that could affect the “blinding” process and may have interfered with the study’s outcome (since these ingredients are counterirritants that can have an effect on itch perception). A pretreatment design was used, ie, topical agents were applied to the skin and left in place for 15 minutes prior to itch induction in order to minimize differences in penetration rates and pharmacokinetics for the agents tested.
Application of cowhage spicules and topical antipruritic agents
Cowhage spicules and topical antipruritic agents were applied in the following sequence. First, itch was induced with cowhage as a control in the absence of any treatment, on the upper side of the volar aspect of the right forearm, on a 16 cm2 (4×4 cm) square area. Then, randomized applications of topical 2% diphenhydramine, 1% hydrocortisone, TriCalm hydrogel, and hydrogel vehicle were performed on four other rectangular areas of the same size, two each on the right and left forearms. After 15 minutes of pretreatment with the test agents, the excess (unabsorbed) amount was removed using a paper towel. Cowhage spicules were subsequently applied in the same fashion as for the control, in four distinct experiments. The assignment of treatment on the four skin areas followed the crossover randomization scheme known as the Williams single, wherein the four sequences of applications were repeated in every four subjects in such a way that every test site received a topical agent just once, and the order of application was changed for the next participant, with a repetition period of 4. This design was selected to avoid order effects and a potential pairing selection bias of the same test area with the same agent. Each experiment using a different topical agent was separated from the subsequent one by a break of 20 minutes, counted from the moment when itch subsided in the previous step.
Cowhage itch was recorded for its full duration (in seconds) until it resolved completely. The starting point (t0) for collection of ratings coincided with the moment when cowhage application was stopped. Using a numerical 10 cm visual analog scale (VAS), from 0 (no itch) to 10 (maximum unbearable itch), itch intensity was assessed every 15 seconds until itch sensation had completely subsided for all conditions. Spicules were left in place until experiments were concluded and itch ratings completely and stably returned to 0 (until three consecutive zeros were recorded). Area under the curve (AUC) was calculated from the temporal profile curves of itch ratings as a function of time.
Statistical analysis
All statistical analyses were performed using PASW® 18.0 software (SAS Institute Inc., Cary, NC, USA) with statistical significance threshold set at P<0.05. The peak VAS itch ratings, AUC, and the overall duration for each treatment arm and the cowhage itch control were compared using a mixed model and were post hoc corrected by the Bonferroni method.
Results
Effect of treatments on peak itch intensity, itch duration, and total itch perceived (AUC)
Data were obtained from the cowhage itch arm alone in the absence of any treatment (control) and with TriCalm hydrogel, topical 2% diphenhydramine, 1% hydrocortisone, and hydrogel vehicle on four other rectangular areas of the same size, two each on the right and left forearms. The cowhage itch model demonstrated highly reproducible characteristics, showing amplitude and temporal features very similar to those obtained in the double-blind, vehicle-controlled study we conducted previously in 32 healthy volunteers.26 Figure 1 shows the time-course curve of itch intensity. Among the three antipruritic agents tested, TriCalm hydrogel produced the highest reduction of itch, in all three parameters: intensity (VAS scale 0–10), itch duration (seconds), and total itch (AUC). AUC integrates the overall duration and instantaneous itch intensity at equally spaced sampling time points. Data for these endpoints are provided in Figure 2.
The antipruritic effect of treatments versus cowhage itch
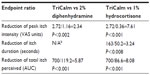
Compared with the control cowhage itch, TriCalm effectively reduced peak itch intensity (± standard error of the mean [SEM]) by 2.72±0.42 VAS units, equivalent to a 41.3% reduction (P<0.001); duration of itch (± SEM) by 163±33 seconds, equivalent to a 39.8% reduction (P<0.001); and total itch perceived (AUC) by 50.5% (P<0.001) (Figure 3). In contrast, 2% diphenhydramine reduced peak itch intensity by 1.16±0.42 VAS units, equivalent to a 17.6% reduction (P=0.057, not significant [ns]); did not reduce the duration of itch, which was in fact prolonged on average by 24.9 seconds; and reduced total itch perceived (AUC) by 8.6% (ns) (Figure 3). The 1% hydrocortisone reduced peak itch intensity by 0.36±0.42 VAS units, equivalent to a 5.5% reduction (ns); duration of itch by 50.2±33 seconds, equivalent to a 12.3% reduction (ns); and total itch perceived (AUC) by 6.2% (ns) (Figure 3).
TriCalm hydrogel performance versus its own hydrogel vehicle (vehicle control)
In the vehicle control group, the peak itch intensity (± SEM) was 5.54±0.39 VAS units, itch duration was 368±22.7 seconds, and total itch perceived (AUC) was 1,134±132 units. TriCalm hydrogel significantly reduced peak itch intensity when compared to its own hydrogel vehicle by 1.67±0.42 VAS units, equivalent to a 30.1% reduction (P<0.001); reduced duration of itch (± SEM) by 122±33 seconds, equivalent to a 33% reduction (P<0.001); and reduced total itch perceived (AUC) by 40% (P<0.02).
Comparative efficacy of TriCalm versus 2% diphenhydramine and 1% hydrocortisone
TriCalm hydrogel was significantly superior to all the other treatments, 2% diphenhydramine and 1% hydrocortisone, in reduction of peak itch intensity (P<0.002 versus diphenhydramine; P<0.001 versus hydrocortisone), itch duration (P<0.008 versus hydrocortisone [note: diphenhydramine did not reduce itch duration]), and total itch perceived (AUC, P<0.001 for TriCalm hydrogel versus each comparator). Comparing the therapeutic effects in reduction of AUC in comparison to the control itch curve, TriCalm hydrogel was almost six times more effective than 2% diphenhydramine in antipruritic action and over eight times more effective than 1% hydrocortisone, both commonly used as OTC topical antipruritic agents (Table 1).
Discussion
There is an unmet need for an efficacious targeted treatment of pruritus. Data from randomized, controlled trials of antipruritic agents used for treatment of itch are limited, and treatments currently used in clinical practice have variable and, in most cases, suboptimal effectiveness.5 Currently available antihistamines, which are commonly used as first-line therapy, are not effective in reducing itch in various acute or chronic systemic conditions.29 This may be because histamine is not the principal mediator of itch in the majority of conditions characterized by persistent itch.22 In fact, proteases such as cathepsin S, mast cell tryptase, and kallikrein, which act on the PAR2 receptor, may play an important role in the pathophysiology of itch; therefore, an exogenous PAR2 ligand, such as the mucunain released by cowhage spicules, represents a relevant itch stimulus and model for studying itch.22,23 PAR2 receptors are important mediators of neuroimmune communication that underlies the mechanism of itch generation in acute and chronic settings, such as skin reactions induced by contact with allergens, chemical-induced irritation, immune response-mediated inflammation, and neurogenic inflammation.30
While topical steroids have been effective in the treatment of inflammatory dermatologic diseases, their utility in treating itch specifically is relatively limited. Moreover, the long-term use of corticosteroids can result in a number of side effects, including risk of adrenal axis suppression, skin atrophy, tachyphylaxis, striae, rosacea-like symptoms, perioral dermatitis, acne, and purpura.9,11–14 Additional side effects that occur less frequently with topical steroids include hypertrichosis, pigmentation alterations, delayed wound healing, and exacerbation of skin infections.12 TriCalm hydrogel is a steroid-free, OTC formulation with demonstrated antipruritic efficacy in the nonhistaminergic form of cowhage itch.23 The exact antipruritic mechanism of TriCalm is not yet elucidated, but it can be attributed (at least in part) to the astringent action of its active ingredient, aluminum acetate.
This is the fourth double-blind, vehicle-controlled, crossover trial using the cowhage itch model for testing antipruritic medications.26–28 The present study confirms and strengthens the results of the previous double-blind, vehicle-controlled, crossover study using the cowhage itch model for testing TriCalm hydrogel and other antipruritic medications in 32 healthy volunteers.26 The results of this study show that the performance of TriCalm hydrogel was superior to the other tested treatments, 2% diphenhydramine and 1% hydrocortisone (two commonly used OTC topical agents currently marketed as antipruritics in the United States), in all categories, including reduction in peak itch intensity, total itch perceived, and itch duration, as compared with the cowhage itch control. TriCalm hydrogel was over eight times more effective than 1% hydrocortisone and almost six times more effective than 2% diphenhydramine in antipruritic action (AUC reduction). In addition, TriCalm hydrogel was well tolerated, and no safety issues related to the applied treatments were observed. Considering the demonstrated antipruritic efficacy of TriCalm hydrogel, additional studies to further investigate the clinical benefit of this agent in pruritic conditions would be valuable. This study also confirms the validity of the cowhage model for studying itch and, implicitly, of the PAR2 pathway for testing topical antipruritic preparations.
Acknowledgment
The authors thank Advanced Clinical Concepts LLC for assistance with writing and editorial support.
Disclosure
KDH and ADPP serve as consultants for Cosmederm Bioscience, Inc. The authors report no other conflicts of interest in this work.
References
Grundmann S, Ständer S. Chronic pruritus: clinics and treatment. Ann Dermatol. 2011;23(1):1–11. | |
Hafenreffer S. Nosodochium, in quo cutis, eique adaerentium partium, affectusomnes, singulari methodo, et cognoscendi e curandi fidelisime traduntur. Ulmae (Westphalia) Kühnen; 1660:98–102. Latin. | |
Ständer S, Weisshaar E, Mettang T, et al. Clinical classification of itch: a position paper of the International Forum for the Study of Itch. Acta Derm Venereol. 2007;87:291–294. | |
Ständer S, Weisshaar E, Luger TA. Neurophysiological and neurochemical basis of modern pruritus treatment. Exp Dermatol. 2008;17:161–169. | |
Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med. 2013;368:1625–1634. | |
Kini SP, DeLong LK, Veledar E, McKenzie-Brown AM, Schaufele M, Chen SC. The impact of pruritus on quality of life: the skin equivalent of pain. Arch Dermatol. 2011;147(10):1153–1156. | |
Zachariae R, Lei U, Haedersdal M, Zachariae C. Itch severity and quality of life in patients with pruritus: preliminary validity of a Danish adaptation of the itch severity scale. Acta Derm Venereol. 2012;92:508–514. | |
Carr CW, Veledar E, Chen SC. Factors mediating the impact of chronic pruritus on quality of life. JAMA Dermatol. 2014;150(6):613–620. | |
Ständer S, Grundmann SA. Chronic pruritus. G Ital Dermatol Venereol. 2012;147:161–169. | |
Ständer S, Weisshaar E. Medical treatment of pruritus. Expert Opin Emerg Drugs. 2012;17(3):335–345. | |
du Vivier A. Tachyphylaxis to topically applied steroids. Arch Dermatol. 1976;112:1245–1248. | |
Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006;54:1–15. | |
Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26 Suppl 3:47–51. | |
Viegas LP, Ferreira MB, Kaplan AP. The maddening itch: an approach to chronic urticaria. J Investig Allergol Clin Immunol. 2014;24(1):1–5. | |
Johanek LM, Meyer RA, Hartke T, et al. Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J Neurosci. 2007;27(28):7490–7497. | |
Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ Jr. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27(37):10007–10014. | |
Namer B, Carr R, Johanek LM, Schmelz M, Handwerker HO, Ringkamp M. Separate peripheral pathways for pruritus in man. J Neurophysiol. 2008;100(4):2062–2069. | |
Johanek LM, Meyer RA, Friedman RM, et al. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci. 2008;28(30):7659–7669. | |
Papoiu AD, Coghill RC, Kraft RA, Wang H, Yosipovitch G. A tale of two itches. Common features and notable differences in brain activation evoked by cowhage and histamine induced itch. Neuroimage. 2012;59(4):3611–3623. | |
Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci. 2008;28(17):4331–4335. | |
Shelley WB, Arthur RP. Mucunain, the active pruritogenic proteinase of cowhage. Science. 1955;122:469–470. | |
Steinhoff M, Neisius U, Ikoma A, et al. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci. 2003;23(15):6176–6180. | |
Papoiu AD, Tey HL, Coghill RC, Wang H, Yosipovitch G. Cowhage-induced itch as an experimental model for pruritus. A comparative study with histamine-induced itch. PLoS One. 2011;6(3):e17786. | |
LaMotte RH, Shimada SG, Green BG, Zelterman D. Pruritic and nociceptive sensations and dysesthesias from a spicule of cowhage. J Neurophysiol. 2009;101(3):1430–1443. | |
Ringkamp M, Schepers RJ, Shimada SG, et al. A role for nociceptive, myelinated nerve fibers in itch sensation. J Neurosci. 2011;31(42):14841–14849. | |
Papoiu AD, Valdes-Rodriguez R, Nattkemper LA, Chan YH, Hahn GS, Yosipovitch G. A novel topical formulation containing strontium chloride significantly reduces the intensity and duration of cowhage-induced itch. Acta Derm Venereol. 2013;93(5):520–526. | |
Gibson RA, Robertson J, Mistry H, et al. A randomised trial evaluating the effects of the TRPV1 antagonist SB705498 on pruritus induced by histamine, and cowhage challenge in healthy volunteers. PLoS One. 2014;9(7):e100610. | |
Hawro T, Fluhr JW, Mengeaud V, et al. Polidocanol inhibits cowhage- but not histamine-induced itch in humans. Exp Dermatol. 2014;23(12):922–923. | |
Hassan I, Haji ML. Understanding itch: an update on mediators and mechanisms of pruritus. Indian J Dermatol Venereol Leprol. 2014;80(2):106–114. | |
Kempkes C, Buddenkotte J, Cevikbas F, Buhl T, Steinhoff M. Role of PAR-2 in neuroimmune communication and itch. In: Carstens E, Akiyama T, editors. Itch: Mechanisms and Treatment. Boca Raton, FL: CRC Press; 2014:193–212. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.