Back to Journals » Clinical Ophthalmology » Volume 13
Three-year outcomes of combined trabecular micro-bypass and phacoemulsification in a predominantly Hispanic population with primary open-angle glaucoma
Authors Gallardo MJ , Supnet RA
Received 28 September 2018
Accepted for publication 19 February 2019
Published 28 May 2019 Volume 2019:13 Pages 869—879
DOI https://doi.org/10.2147/OPTH.S189071
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mark J Gallardo,1,2 Richard A Supnet1
1El Paso Eye Surgeons, PA, El Paso, TX, USA; 2Department of Ophthalmology, University of Texas Health Science Center at San Antonio, San Antonio, TX, USA
Purpose: To present long-term, real-world outcomes after implanting one trabecular micro-bypass stent with cataract surgery for primary open-angle glaucoma (POAG) in a predominantly Hispanic patient population.
Patients and methods: This retrospective, consecutive case series evaluated intraocular pressure (IOP), medications, and safety through 36 months after implanting one iStent®, during phacoemulsification cataract surgery. Eyes were stratified into 2 subgroups classified by preoperative IOP and surgical goal. The Controlled Group had IOP <18 mmHg on ≥1 medications, and goal to reduce medications. The Uncontrolled Group had IOP ≥18 mmHg and/or maximum tolerated medication load, and goal to reduce IOP. Assessments included IOP, medications, visual fields (VF), retinal nerve fiber layer thickness (RNFL), adverse events, and secondary surgeries.
Results: Of 168 total operated eyes, 87 eyes (49 Controlled, 38 Uncontrolled) completed 36 months of follow-up and comprise the Consistent Cohort in this report. At baseline, 79.6% (39/49) of Controlled eyes and 71.1% (27/38) of Uncontrolled eyes were from Hispanic patients. In the Controlled Group desiring medication reduction, mean medications were reduced by 77.3% (2.6 medications preoperatively vs 0.6 at 36 months; p<0.001. All Controlled eyes maintained or reduced medications versus preoperative; no eyes were on ≥3 medications (vs 61.2% preoperatively); and 58.3% were medication-free (vs 0% preoperatively). In the Uncontrolled Group desiring IOP reduction, mean IOP decreased by 31.2% (19.4 mmHg preoperatively vs 13.4 mmHg at 36 months; p<0.001), 91.7% of eyes achieved IOP ≤18 mmHg, 69.4% reached IOP ≤15 mmHg, and 77.8% decreased IOP ≥20% vs baseline. Uncontrolled eyes also experienced a 45.3% medication reduction (2.2 medications preoperatively vs 1.2 at 36 months; p<0.001). Favorable safety included no intraoperative complications, and stable VF and RNFL through 36 months.
Conclusion: In this predominantly Hispanic patient cohort, significant IOP and medication reductions were sustained safely through 36 months after iStent implantation during cataract surgery.
Keywords: glaucoma, trabecular micro-bypass, Hispanic, micro-invasive glaucoma surgery, IOP, long term, iStent®
Introduction
Glaucoma is a leading cause of blindness globally, and its incidence is known to vary by race.1–3 Several population-based studies have documented high rates of open-angle glaucoma in the Hispanic population; and from 2011 to 2050, Hispanic individuals are expected to become the largest US group affected by primary open-angle glaucoma (POAG).4–7 Treatments for glaucoma range from topical medication to laser procedures and incisional filtration surgeries such as trabeculectomy and tube implantation.
 | Figure 5 Mean IOP and medication burden through 36 months postoperative, Uncontrolled Group (goal to reduce IOP), Consistent Cohort (n=38). |
 | Figure 6 Proportional analysis of medication burden, Uncontrolled Group, Consistent Cohort (n=38). |
In recent years, the glaucoma treatment landscape has diversified to include micro-invasive glaucoma surgery (MIGS) procedures, as a complement or alternative to existing treatment modalities. The first MIGS device, the iStent® Trabecular Micro-Bypass (Glaukos Corporation, San Clemente, CA, USA), has amassed a sizeable and diverse evidence base regarding the long-term safety and utility of this device in glaucoma. Evaluations have been wide-ranging,8–25 including a variety of study designs (eg, randomized controlled trials, consecutive case series, nonrandomized controlled trials), clinical settings (eg, single site vs multicenter, real-world vs protocol-driven), countries (predominantly in North and South America, Europe, Asia, and Asia-Pacific), and emphases (eg, clinical studies, cost-effectiveness analyses, basic science/preclinical studies, meta-analyses). Studies also have evaluated different severities of glaucoma (including mild to refractory), different types of glaucoma (eg, POAG, pseudoexfoliative glaucoma, narrow-angle glaucoma, ocular hypertension, normotensive glaucoma), different lenticular status (phakic and pseudophakic), and different indications (eg, one or multiple stents, standalone or with cataract surgery, with or without concomitant glaucoma medication).8–25 To-date, however, relatively little evidence exists regarding MIGS procedures specifically in Hispanic populations. Given the aforementioned high incidence of glaucoma in Hispanic patients, and rising disease prevalence within the US in coming years, long-term outcomes of MIGS procedures in Hispanic patients is of particular interest.
Prior work by Gallardo et al described 12-month outcomes of combined trabecular micro-bypass and phacoemulsification in a predominantly Hispanic patient population with primary open-angle glaucoma.13 The authors reported favorable safety and meaningful reductions in IOP and medication through 12 months postoperative. In that evaluation, patients were categorized preoperatively into three groups based on IOP and surgical goal. Group 1 included eyes with IOP <18 mmHg on at least 1 medication and goal to reduce medication; Group 2 included eyes with IOP not controlled on ≤2 medications and goal to reduce IOP; and Group 3 included eyes with uncontrolled IOP and/or on ≥3 medications, and goal to avoid a filtering surgery by reducing IOP.
In the present report, the authors present longer-term outcomes (through 36 months) within this predominantly Hispanic patient population; they also stratify patients into two groups based on preoperative IOP and surgical goal (rather than three). The Controlled Group in this report (Group 1 in the prior publication) had IOP <18 mmHg on 1 or more medications, and goal to reduce medication burden; the Uncontrolled Group (Groups 2 and 3 in the prior publication) had IOP ≥18 mmHg and/or maximal topical medication load, and goal to reduce IOP. Postoperative outcomes including IOP, medications, and safety were evaluated in the context of each group’s respective surgical goal. To our knowledge, the present study is the first real-world documentation of MIGS outcomes in a predominantly Hispanic population through a sustained follow-up period of three years.
Patients and methods
Study design and subjects
Similar to the earlier work published by Gallardo et al, this study was a retrospective, consecutive case series performed by a single surgeon (MG) at an outpatient surgical facility in El Paso, Texas over a 2-year period (January 2013 through December 2014). Subjects underwent phacoemulsification cataract surgery with concurrent implantation of one iStent Trabecular Micro-Bypass stent. Subjects were required to have POAG, cataract requiring surgery, normal angle anatomy, and a need for IOP and/or medication reduction. Exclusionary factors were narrow or closed anterior chamber angle, ocular inflammation, or corneal opacities compromising gonioscopy. Pre- and postoperative assessments included IOP (by Goldmann applanation), medications, visual fields (VF, measured by Humphrey VF Analyzer using 24-2 SITA-Fast (Swedish Interactive Testing Algorithm) testing protocol), mean peripapillary retinal nerve fiber layer thickness (RNFL, measured by Optovue optical coherence tomography (OCT); Optovue, Inc., Fremont, CA), operative complications, postoperative adverse events, and secondary surgical interventions. Data collection methods abided by the tenets of the Declaration of Helsinki, and all patients signed an informed consent to allow for the retrospective evaluation of their clinical data. Ethics approval was obtained from the Surgical Center of El Paso Medical Executive Committee (El Paso, TX, USA). Patients were followed through 36 months postoperatively, and follow-up is ongoing.
Device description, surgical procedure, and perioperative medication
The iStent Trabecular Micro-Bypass is a heparin-coated, single-piece, “L”-shaped titanium stent designed to create a patent opening in the trabecular meshwork to reestablish normal physiologic aqueous outflow and thereby decrease IOP (Figure 1, Figure 2). The stent is pre-loaded on a single-use inserter which facilitates ab interno stent placement into the nasal portion of Schlemm’s canal. Postoperatively, patients received 1 week of topical antibiotic and 4 weeks of topical non-steroidal anti-inflammatory medication.
Data analyses
Paired t-tests were used to compare preoperative versus Month 36 IOP, medications, VF mean deviation, and RNFL thickness. Results were considered significant for p-values of <0.05. Proportional analyses were completed to show percentages of eyes on 0, 1, 2, 3, or 4 medications; changes in medication number at 36 months versus preoperative; eyes with IOP ≤18 mmHg or IOP ≤15 mmHg; and eyes achieving ≥20% IOP reduction at 36 months versus preoperative. Data from eyes that had secondary glaucoma surgery during follow-up were excluded from subsequent IOP and medication analyses.
Results
Subject accountability, demographics, and preoperative ocular parameters
A total of 168 eyes of 128 subjects with POAG and visually significant cataract underwent phacoemulsification cataract surgery with implantation of a single iStent trabecular micro-bypass stent. A total of 87 eyes completed 36 months of follow-up and comprise the cohort summarized in this report (Table 1).
 | Table 1 Subject accountability at 36 months postoperative |
As shown in Table 2, the majority of eyes were from Hispanic patients (39/49 or 79.6% of Controlled eyes, and 27/38 or 71.1% of Uncontrolled eyes), with mean age of approximately 73 years old. Both groups included subjects with history of prior ocular surgery that could impact the IOP endpoint, including laser procedures as well as incisional surgeries. Consistent with its category’s definition, Controlled eyes (with controlled IOP but substantial medication burden) had preoperative mean IOP of 13.5±1.8 mmHg on 2.6±0.8 medications, while Uncontrolled eyes (with uncontrolled IOP regardless of medication burden) had preoperative mean IOP of 19.4±3.7 mmHg on 2.2±1.2 medications.
 | Table 2 Demographics and preoperative ocular characteristics, Consistent Cohort (n=87) |
IOP and medication use
In the Controlled Group aiming for medication reduction, mean medication number was reduced by 77.3% (from 2.6±0.8 medications preoperatively to 0.6±0.8 medications at 36 months; p<0.001) (Figure 3). All Controlled eyes maintained or reduced their medication burden versus preoperative; no eyes were on ≥3 medications (vs 61.2% preoperatively), and 58.3% of eyes were medication-free (versus 0% preoperatively) (Table 3, Figure 4). Meanwhile, IOP also decreased significantly (13.5±1.8 mmHg preoperatively vs 12.6±2.6 mmHg at 36 months; p=0.028) (Figure 3).
 | Table 3 Proportions of eyes with decreased, maintained, or increased medication burden versus preoperative, Controlled Group (n=49) |
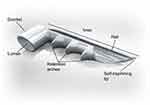 | Figure 1 iStent® Trabecular Micro-Bypass. |
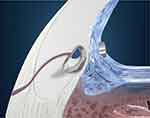 | Figure 2 iStent® implantation location. |
In the Uncontrolled Group desiring IOP reduction, mean IOP decreased by 31.2% (19.4±3.7 mmHg preoperatively vs 13.4±2.0 mmHg at 36 months; p<0.001) (Figure 5). Most eyes (91.7%) achieved IOP ≤18 mmHg and 69.4% of eyes reached IOP ≤15 mmHg; 77.8% of Uncontrolled eyes achieved a clinically significant reduction in IOP, defined as ≥20% decrease compared to baseline. Although medication reduction was not expected to accompany IOP reduction, a 45.3% medication reduction did occur (2.2±1.2 medications preoperatively vs 1.2±1.0 at 36 months; p<0.001) (Figure 5). Additionally, 94.4% of eyes maintained or reduced their medication burden versus preoperative, including a greater than 90% decrease (57.9% to 5.6%) in the proportion of eyes with high medication burden (≥3 medications) (Figure 6, Table 4).
 | Table 4 Proportions of eyes with decreased, maintained, or increased medication burden versus preoperative, Uncontrolled Group (n=38) |
 | Figure 3 Mean medication burden and IOP through 36 months postoperative, Controlled Group (goal to reduce medications), Consistent Cohort (n=49). |
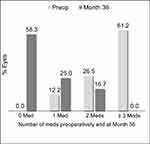 | Figure 4 Proportional analysis of medication burden through 36 months postoperative, Controlled Group, Consistent Cohort (n=49). |
Safety assessment
No intraoperative complications occurred in either study group, including no corneal injury, iris damage, hypotony, hyphema, or failure to implant the stent. Postoperatively, adverse events consisted of 6 cases of IOP elevation ≥10 mmHg versus preoperative IOP, all of which resolved with treatment within one week and resulted in no sequelae. Specifically, 4 eyes had Day 1 IOP elevated ≥10 mmHg versus preoperative IOP. These cases were treated with topical or oral antiglaucomatous medication, resulting in IOP normalization without sequelae by Week 1. In 1 eye, topical steroid (prednisolone acetate) was prescribed at Day 1 due to 1+ anterior chamber cell. IOP increase ≥10 mmHg vs baseline was observed at Week 1, and was presumed to be steroid-induced as there was no indication of another etiology. The case was treated with topical antiglaucomatous medication and rapid taper of steroid, resulting in IOP normalization without sequelae by the 2-week visit. Finally, 1 eye had IOP elevated ≥10 mmHg versus preoperative IOP at Day 1 and Week 1; this elevation was attributed to red blood cell aggregation extending from the iStent. The aggregation was cleared with YAG laser at the 1-week visit, resulting in IOP normalization by Week 2. No other postoperative adverse events occurred throughout the remainder of the 3-year follow-up period.
VF mean deviation and RNFL thickness remained stable in both groups through three years postoperative compared to preoperative levels (Tables 5 and 6). Secondary glaucoma surgery was undertaken in 1 Controlled subject (trabeculectomy with express shunt after the 12-month visit) and 2 Uncontrolled subjects (trabeculectomy with express shunt after the 12-month visit, and canaloplasty at 17 months postoperative). No other secondary surgeries occurred, including no laser glaucoma procedures.
 | Table 5 Visual field mean deviation (MD) through 36 months postoperative, Eyes with available data at each visit |
 | Table 6 Mean peripapillary RNFL thickness through 36 months postoperative, Eyes with available data at each visit |
Discussion
The present report shows real-world outcomes through 36 months following iStent implantation with cataract surgery in a predominantly Hispanic patient population. Consistent with the prior 1-year publication for this study, patients demonstrated meaningful reductions in IOP and medications over time, while maintaining favorable safety. Results were consistent with numerous prior studies of iStent implantation in conjunction with cataract surgery for mild to severe glaucoma.8–25
Given that the Controlled and Uncontrolled groups had distinct surgical goals, this study sought to evaluate postoperative outcomes in the context of those goals. The Controlled Group already had controlled IOP (<18 mmHg) and aimed to decrease medication burden. Postoperative performance for this parameter included a clinically and statistically significant (77.3%) reduction in medications, with 100% of eyes either maintaining or reducing their medication burden versus preoperative, and the mean per-patient medication burden reducing by approximately 2 full medications. The patient-level benefit of reducing the number of drops must not be underestimated. Multiple studies have shown poor medication adherence due to side effects, costs, instillation difficulties, multiple dosing regimens, and patient comorbidities;26–28 Additionally, adherence is known to decline dramatically when the number of topical medications is increased from one to multiple drops.29 This can have long-term consequences, as poor medication adherence is a well-known risk factor for glaucoma progression.30–32
In the Uncontrolled Group with IOP ≥18 mmHg and/or maximum tolerated topical medications, the priority of treatment was to reduce IOP; if medications were reduced as well, this would be seen as an additional (though not required) benefit. Postoperative performance for IOP included a significant (31.2%) reduction in IOP, with nearly all eyes achieving IOP ≤18 mmHg and a substantial portion reaching IOP ≤15 mmHg. In addition, medications were reduced by 45.3%, with the mean per-patient medication burden reducing by approximately 1 full medication.
The ability of this treatment modality to achieve two distinct surgical goals, and in two different clinical populations (eyes with Controlled and Uncontrolled glaucoma) is noteworthy. Additionally, these postoperative improvements were sustained consistently through 36 months after surgery, with no indication of waning effect such as that seen with laser trabeculoplasty33–35 and/or with customary transient post-phacoemulsification IOP reduction.36–38 These iStent results are consistent with the evidence base of studies showing durable IOP and medication reductions after iStent implantation for a variety of glaucoma severities and clinical settings.8–25
Given that glaucoma progression occurs over time, the long-term VF and RNFL data through 3 years are of particular interest. Each of these measures remained stable throughout follow-up versus preoperative values, indicating preservation of structure and function in this disease. Other safety parameters were similarly favorable, including no intraoperative complications, postoperative events limited to the immediate postoperative period only, and no adverse event-related sequelae.
Limitations of this study include its design as an unmasked, single-arm, retrospective case series. Pre- and postoperative medication washouts were not completed, since they are not part of routine clinical practice and possibly could place patients at risk of glaucomatous damage. There was no formal control group to which to compare the treatment group; however the numerical values for patients’ preoperative IOP and medications were considered viable comparators for the postoperative values. Mean diurnal IOP measurements were not completed, allowing for possible regression to the mean. As with any combined surgical intervention, it was not possible to separate the IOP effect of each procedure (stent implantation versus phacoemulsification); however since the observed IOP reduction was greater, and more longstanding, than the reduction expected after cataract surgery,36–38 it is probable that stent implantation was the primary (or likely the sole) cause of the observed 3-year improvements in IOP and medications. As is the case in most studies from real-world patient populations, the accountability of this cohort does not compare with a formal randomized controlled trial. Incomplete follow-up, like medication non-adherence, can be problematic for a large number of glaucoma patients, and thus is an inherent difficulty in real-world clinical practice and clinical studies. This difficulty highlights the benefit of using a more permanent, less compliance-dependent intervention (such as a micro-invasive surgical implant) to achieve IOP reduction. Future evaluations from this cohort could include analyses past 36 months of follow-up, and/or stratification by type of prior glaucoma surgery, number of preoperative glaucoma medications, or racial background.
Conclusion
In summary, this series demonstrated favorable safety and significant reductions in both IOP and medication burden through 36 months after iStent implantation with cataract surgery in a real-world clinical setting. Importantly, these favorable outcomes were observed in two subgroups (Controlled and Uncontrolled) having different degrees of IOP control, and with regard to both surgical goals (medication reduction and IOP reduction, respectively). Finally, this report provides data on long-term outcomes of MIGS surgery in Hispanic patients, a population that heretofore has had relatively limited representation in MIGS studies.
Acknowledgments
No financial support was given for this study. Editorial assistance was provided by Dana M. Hornbeak, MD, MPH (Glaukos Corporation) and Maureen Johnson, RN (ClinReg Consulting Services, Inc.). Glaukos Corporation covered journal publication fees. Meeting Presentation: Presented in part at the 2018 annual meeting of the American Society of Cataract and Refractive Surgery (ASCRS).
Disclosure
Mark J. Gallardo. M.D. is a consultant and speaker for Glaukos Corporation and for Ellex Corporation. Dr Mark J Gallardo reports non-financial support from Glaukos during the conduct of the study and outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Sommer A, Tielsch JM, Katz J, et al. Racial differences in the cause-specific prevalence of blindness in east Baltimore. N Engl J Med. 1991;325:1412–1417. doi:10.1056/NEJM199111143252004
2. Tielsch JM, Sommer A, Katz J, Royall RM, Quigley HA, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma: the Baltimore Eye Survey. JAMA. 1991;266:369–374.
3. Gordon MO, Beiser JA, Brandt JD, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720; discussion 829–830.
4. Varma R, Ying-Lai M, Francis BA,
5. Quigley HA, West SK, Rodriguez J, Munoz B, Klein R, Snyder R. The prevalence of glaucoma in a population-based study of Hispanic subjects: proyecto VER. Arch Ophthalmol. 2001;119:1819–1826.
6. Jung JL, Cg I-L, Lazcano-Gomez G, Jr S, My K. Intraocular pressure control after trabeculectomy, phacotrabeculectomy and phacoemulsification in a hispanic population. J Curr Glaucoma Pract. 2014;8(2):67–74. doi:10.5005/jp-journals-10008-1164
7. Vajaranant TS, Wu S, Torres M, Varma R. The changing face of primary open-angle glaucoma in the United States: demographic and geographic changes from 2011 to 2050. Am J Ophthalmol. 2012;154:303–314. doi:10.1016/j.ajo.2012.02.024
8. Samuelson TW, Katz LJ, Wells JM, Duh YJ, Giamporcaro JE. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118:459–467. doi:10.1016/j.ophtha.2010.07.007
9. Craven ER, Katz LJ, Wells JM, Giamporcaro JE. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012;38:1339–1345. doi:10.1016/j.jcrs.2012.03.025
10. Arriola-Villalobos P, Martinez-de-la-Casa J, Diaz-Valle D, et al. Combined iStent trabecular micro-bypass stent implantation and phacoemulsification for coexistent open-angle glaucoma and cataract: a long-term study. Br J Ophthalmol. 2012;96:645–649. doi:10.1136/bjophthalmol-2011-300218
11. Ferguson TJ, Berdahl JP, Schweitzer JA, Sudhagoni RG. Clinical evaluation of a trabecular micro-bypass stent with phacoemulsification in patients with open-angle glaucoma and cataract. Clin Ophthalmol. 2016;10:1767–1773. doi:10.2147/OPTH.S114306
12. Neuhann TH. Trabecular micro-bypass stent implantation during small-incision cataract surgery for open-angle glaucoma or ocular hypertension: long-term results. J Cataract Refract Surg. 2015;41:2664–2671. doi:10.1016/j.jcrs.2015.06.032
13. Gallardo M, Supnet R, Giamporcaro JE, Hornbeak DM. Outcomes of combined trabecular micro-bypass and phacoemulsification in a predominantly hispanic patient population. Clin Ophthalmol. 2016;10:1931–1937. doi:10.2147/OPTH.S117403
14. Fea AM, Consolandi G, Zola M, et al. Micro-bypass implantation for primary open-angle glaucoma combined with phacoemulsification: 4-year follow-up. J Ophthalmol. 2015;2015:Article ID 795357, 4 pages. doi:10.1155/2015/795357
15. Belovay GW, Naqi A, Chan BJ, Rateb M, Ahmed II. Using multiple trabecular micro-bypass stents in cataract patients to treat open-angle glaucoma. J Cataract Refract Surg. 2012;38(11):1911–1917. doi:10.1016/j.jcrs.2012.07.017
16. Ferguson TJ, Swan R, Ibach M, Schweitzer J, Sudhagoni R, Berdahl JP. Trabecular microbypass stent implantation with cataract extraction in pseudoexfoliation glaucoma. J Cataract Refract Surg. 2017;43(5):622–626. doi:10.1016/j.jcrs.2017.02.029
17. Ferguson T, Swan R, Ibach M, Schweitzer J, Sudhagoni R, Berdahl JP. Evaluation of a trabecular microbypass stent with cataract extraction in severe primary open-angle glaucoma. J Glaucoma. 2018;27(1):71–76. doi:10.1097/IJG.0000000000000825
18. Ferguson T, Berdahl J, Schweitzer J, Sudhagoni R. Evaluation of a trabecular micro- bypass stent in pseudophakic patients with open-angle glaucoma. J Glaucoma. 2016;25:896–900. doi:10.1097/IJG.0000000000000529
19. Jay Katz L, Erb C, Guillamet AC, et al. Long-term titrated IOP control with one, two, or three trabecular micro-bypass stents in open-angle glaucoma subjects on topical hypotensive medication: 42-month outcomes. Clin Ophthalmol. 2018;12:255–262. doi:10.2147/OPTH.S152268
20. Chang DF, Donnenfeld ED, Katz LJ, et al. Efficacy of two trabecular micro-bypass stents combined with topical travoprost in open-angle glaucoma not controlled on two preoperative medications: 3-year follow-up. Clin Ophthal. 2017;11:523–528. doi:10.2147/OPTH.S121041
21. Donnenfeld ED, Solomon KD, Voskanyan L, et al. A prospective 3-year follow-up trial of implantation of two trabecular microbypass stents in open-angle glaucoma. Clin Ophthalmol. 2015;9:2057–2065. doi:10.2147/OPTH.S91732
22. Vold SD, Voskanyan L, Tetz M, et al. Newly diagnosed primary open-angle glaucoma randomized to 2 trabecular bypass stents or prostaglandin: outcomes through 36 months. Ophthalmol Ther. 2016;5(2):161–172. doi:10.1007/s40123-016-0065-3
23. Buffet J, Brasnu E, Baudouin C, Labbé A. Efficacy of two trabecular micro-bypass stents during phacoemulsification for mild to advanced primary open-angle glaucoma controlled with topical hypotensive medications. J Glaucoma. 2017;26(12):1149–1154. doi:10.1097/IJG.0000000000000808
24. Brown RH, Gibson Z, Zhong L, Lynch MG. Intraocular pressure reduction after cataract surgery with implantation of a trabecular microbypass device. J Cataract Refract Surg. 2015;41(6):1318–1319. doi:10.1016/j.jcrs.2015.01.015
25. Myers JS, Masood I, Hornbeak DM, et al. Prospective evaluation of two iStent® trabecular stents, one iStent Supra® suprachoroidal stent, and postoperative prostaglandin in refractory glaucoma: 4-year outcomes. Adv Ther. 2018;35(3):395–407. doi:10.1007/s12325-018-0666-4
26. Nordstrom BL, Friedman DS, Mozaffari E, Quigley HA, Walker AM. Persistence and adherence with topical glaucoma therapy. Am J Ophthalmol. 2005;140:598–606. doi:10.1016/j.ajo.2005.04.051
27. Friedman DS, Quigley HA, Gelb L, et al. Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the Glaucoma Adherence and Persistency Study (GAPS). Invest Ophthalmol Vis Sci. 2007;48:5052–5057. doi:10.1167/iovs.07-0290
28. Schwartz GF, Reardon G, Mozaffari E. Persistency with latanoprost or timolol in primary open-angle glaucoma suspects. Am J Ophthalmol. 2004;137:S13–S16.
29. Robin AL, Novack GD, Covert DW, Crockett RS, Marcic TS. Adherence in glaucoma: objective measurements of once-daily and adjunctive medication use. Am J Ophthalmol. 2007;144:533–540. doi:10.1016/j.ajo.2007.06.012
30. Rossi GC, Pasinetti GM, Scudeller L, Radaelli R, Bianchi PE. Do adherence rates and glaucomatous visual field progression correlate? Eur J Ophthalmol. 2011;21(4):410–414. doi:10.5301/EJO.2010.6112
31. Stewart WC, Chorak RP, Hunt HH, Sethuraman G. Factors associated with visual loss in patients with advanced glaucomatous changes in the optic nerve head. Am J Ophthalmol. 1993;116:176–181.
32. Granstrom PA. Progression of visual field defects in glaucoma. Relation to compliance with pilocarpine therapy. Arch Ophthalmol. 1985;103:529–531.
33. Juzych MS, Chopra V, Banitt MR, et al. Comparison of long-term outcomes of selective laser trabeculoplasty versus argon laser trabeculoplasty in open-angle glaucoma. Ophthalmology. 2004;111:1853–1859. doi:10.1016/j.ophtha.2004.04.030
34.
35. Chung PY, Schuman JS, Netland PA, Lloyd-Muhammad RA, Jacobs DS. Five-year results of a randomized, prospective, clinical trial of diode vs argon laser trabeculoplasty for open-angle glaucoma. Am J Ophthalmol. 1998;126:185–190.
36. Vizzeri G, Weinreb RN. Cataract surgery and glaucoma. Curr Opin Ophthalmol. 2010;21(1):20–24. doi:10.1097/ICU.0b013e328332f562
37. Poley BJ, Lindstrom RL, Samuelson TW. Long-term effects of phacoemulsification with intraocular lens implantation in normotensive and ocular hypertensive eyes. J Cataract Refract Surg. 2008;34(5):735–742. doi:10.1016/j.jcrs.2007.12.045
38. Shingleton BJ, Gamell LS, O’Donoghue MW, Baylus SL, King R. Long-term changes in intraocular pressure after clear corneal phacoemulsification: normal patients versus glaucoma suspect and glaucoma patients. J Cataract Refract Surg. 1999;25(7):885–890.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
