Back to Journals » Therapeutics and Clinical Risk Management » Volume 10
Therapeutic implications of Epstein–Barr virus infection for the treatment of nasopharyngeal carcinoma
Authors Hutajulu SH , Kurnianda J, Tan IB, Middeldorp JM
Received 16 March 2014
Accepted for publication 4 May 2014
Published 5 September 2014 Volume 2014:10 Pages 721—736
DOI https://doi.org/10.2147/TCRM.S47434
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Susanna Hilda Hutajulu,1 Johan Kurnianda,1 I Bing Tan,2,3 Jaap M Middeldorp4
1Department of Internal Medicine, Faculty of Medicine Universitas Gadjah Mada/Dr Sardjito General Hospital, Yogyakarta, Indonesia; 2Department of Ear, Nose and Throat, The Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital, Amsterdam, The Netherlands; 3Department of Ear, Nose and Throat, Faculty of Medicine Universitas Gadjah Mada/Dr Sardjito General Hospital, Yogyakarta, Indonesia; 4Department of Pathology, VU University Medical Center, Amsterdam, The Netherlands
Abstract: Nasopharyngeal carcinoma (NPC) is highly endemic in certain regions including the People's Republic of China and Southeast Asia. Its etiology is unique and multifactorial, involving genetic background, epigenetic, and environment factors, including Epstein–Barr virus (EBV) infection. The presence of EBV in all tumor cells, aberrant pattern of antibodies against EBV antigens in patient sera, and elevated viral DNA in patient circulation as well as nasopharyngeal site underline the role of EBV during NPC development. In NPC tumors, EBV expresses latency type II, where three EBV-encoded proteins, Epstein–Barr nuclear antigen 1, latent membrane protein 1 and 2 (LMP1, 2), are expressed along with BamH1-A rightward reading frame 1, Epstein–Barr virus-encoded small nuclear RNAs, and BamH1-A rightward transcripts. Among all encoded proteins, LMP1 plays a central role in the propagation of NPC. Standard treatment of NPC consists of radiotherapy with or without chemotherapy for early stage, concurrent chemoradiotherapy in locally advanced tumors, and palliative systemic chemotherapy in metastatic disease. However, this standard care has limitations, allowing recurrences and disease progression in a certain proportion of cases. Although the pathophysiological link and molecular process of EBV-induced oncogenesis are not fully understood, therapeutic approaches targeting the virus may increase the cure rate and add clinical benefit. The promising results of early phase clinical trials on EBV-specific immunotherapy, epigenetic therapy, and treatment with viral lytic induction offer new options for treating NPC.
Keywords: immunotherapy, epigenetic therapy, viral lytic induction therapy
Introduction
Nasopharyngeal carcinoma (NPC) is a malignancy that originates from the epithelial cells extending over the nasopharyngeal surface.1 The World Health Organization (WHO) has categorized NPC into three histopathological types including keratinizing squamous cell carcinoma (WHO type I), with varying degrees of differentiation; nonkeratinizing squamous cell carcinoma (WHO type II), retaining epithelial cell shape and growth pattern; and undifferentiated carcinoma (WHO type III), which does not produce keratin and lacks a distinctive growth pattern. For prognostic significance, WHO types II and III are considered together.2,3
Epidemiology studies on NPC show an uncommon geographical incidence. Even though it occurs sporadically in most countries throughout the world, NPC is prevalent in Southeast Asian countries and in native populations of the Arctic region, Northern Africa, and the Middle East. Furthermore, NPC is endemic in southern China, with annual incidence exceeding 20/100,000 population4–6 as well as in Sarawak, Malaysia, representing a regional hot spot with annual incidence of 30/100,000 population.7
The etiology of NPC involves genetic background and environmental factors. Chinese ethnicity is considered a significant susceptibility factor for NPC because communities living in, and migrated from, the southern part of the People’s Republic of China are known to have the highest NPC incidence in the world, which is retained by Chinese offspring settling in other countries.8,9 NPC family aggregation also indicates a strong genetic influence, with excess risk among individuals with a first-degree relative with NPC being four to ten-fold.10–13 Familial NPC has been linked to genetic predisposition such as human leukocyte antigen (HLA) genotypes and susceptibility loci on chromosome 9, 4, and 3.14–16 However, recent reports of genome-wide association studies indicated wider complexity of genetic factors.17,18 It is suggested that genetic factors and environmental exposures play a combined role in triggering NPC. Environmental determinants for NPC risk include food, tobacco smoke, alcohol consumption, occupational dust, inhalant, and Epstein–Barr virus (EBV) infection. The Cantonese-style salted fish containing nitrosamines,19–21 and preserved food containing butyrates,22,23 heavy smoking,24 and exposure to phorbol esters25 are carcinogenic items that have been consistently linked to NPC.
Among environmental factors, EBV infection has attracted the greatest attention, and its association to NPC is highly documented. Interestingly, EBV reactivation is triggered by the (co)carcinogenic agents mentioned above, suggesting a synergistic effect.25 The presence of viral DNA, RNA, and protein in all tumor cells, viral reactivation, and the aberrant antibody profiles against EBV antigens in patient sera highlight the role of EBV in NPC development.26,27 Moreover, the existence of EBV in all NPC tumor cells provides opportunities for the development of diagnostic and therapeutic approaches.
EBV infection and NPC pathogenesis
General features of EBV infection
EBV is a gamma herpes virus that infects most adults in the world.28 Humans are the only natural host for EBV, which transmits via salivary contact.29 Primary infection generally takes place in early childhood and causes no or only mild nonspecific symptoms. Infection during adolescence or adulthood may result in infectious mononucleosis from which most recover without any sequelae.30 More recently, EBV is implicated as the causal factor in several chronic and autoimmune disorders as well as cancer.31–33 In 1997, WHO officially declared EBV a class I human carcinogenic agent for its causal role in the pathogenesis of multiple distinct lymphomas and carcinomas.34
Upon infection, EBV enters a latent state in “immortalized” circulating B-lymphocytes in the peripheral blood, causing the infected individual to be an asymptomatic carrier for life.35–38 The basis for B-cell immortalization and latent persistence is formed by innate functions of a small number of viral gene products, which also drive the malignant phenotype of EBV-associated malignancies.27 Epithelial coinfection generally occurs parallel to B-cell infection, leading to persistent virus secretion in saliva.36,38 Latent EBV has no serious consequences in the vast majority of healthy individuals, as long as the immune system remains unaffected. However, in particular conditions, the virus or its infected host cell may be activated and subsequently plays a role in the pathogenesis of a wide spectrum of EBV-related disorders.31 The EBV-associated malignancies include epithelial tumors such as nasopharyngeal and gastric carcinomas, mesenchymal tumors such as follicular dendritic cell tumor/sarcoma, EBV-driven lymphoid malignancies including Burkitt’s lymphoma, acquired immunodeficiency syndrome (AIDS)-associated and immunodeficiency-associated lymphoproliferative disorders and lymphoma, extranodal natural killer (NK) cell/T-cell lymphoma, Hodgkin’s lymphoma, and B-cell lymphoma in elderly persons.27,31,39
Characteristics of EBV infection in NPC development
Following an initial infection, saliva which contains EBV virions is sampled by the tonsil, where infection occurs at the crypt of the tonsil.36 EBV passes over the epithelial barrier to reach submucosal naïve B-cells residing in the mantle zone. Thus, the incoming virus infects epithelial cells or infiltrating B-lymphocytes in the lymphoepithelium of the naso- or oropharyngeal mucosa. At those places, it establishes a primary focus of latent infection (transformation) and lytic replication. Virus released from EBV-infected epithelial cells or B-cells with lytic infection can be transmitted from host to host via saliva or by infecting other mucosal cells.40 The virus that enters resting B-lymphocytes will spread throughout the lymphoid tissue and express several latency programs to ensure genome maintenance and persistence.38 Upon B-cell infection, EBV drives the B-cell into immortalization and cellular proliferation in an efficient stepwise process while virus replication is suppressed by methylation, a situation mirrored in most EBV-positive lymphoblastoid cell lines in vitro.27 This latency is called latency type III, where EBV expresses the full spectrum of eleven latent gene products. These include Epstein–Barr nuclear antigens (EBNAs 1, 2, 3A, 3B, 3C, and EBNA-LP), three latent membrane proteins (latent membrane protein 1/LMP1, LMP2A, and LMP2B), two Epstein–Barr virus-encoded small nuclear RNAs (EBERs), and microRNAs (miRNAs) mapped to the BHRF1 and BART regions of the EBV genome, the latter recently found to comprise over 40 independent miRNA species.29 In vivo, virus-specific cytotoxic T-cells (CTLs) will eliminate these proliferating EBV-infected cells.41 However, a few cells may escape the immune response and generate resting memory B-cells, where viral antigen expression is mostly suppressed by methylation. This latency is typically called latency 0 (true latency), with only noncoding EBERs and BARTs being expressed, or latency I, when EBNA1 is coexpressed to secure genome maintenance in dividing cells.42 EBV-infected memory B-cells preferentially home to the nasal–oral mucosal lymphoid tissues of the Waldeyer ring, where they can differentiate into plasma cells and produce EBV progeny.43 Otherwise, EBV-infected memory B-cells persist in the peripheral blood and indefinitely serve as viral reservoir and can switch to an activated state of latency that ensures immune evasion and mimic a germinal center reaction through well-regulated coexpression of EBNA1, LMP1, and LMP2, which is called the default program or latency II.44–46 When triggered by antigen, EBV-infected B-cells become plasma cells that support viral replication close to the mucosal epithelium and provide a source of infectious virions for other B-cells or local epithelial cells.43
EBV infection has been shown to be an early event in the development of NPC. The undifferentiated form, WHO type III NPC, shows the most consistent worldwide link with EBV.47,48 This type of tumor is characterized by the presence of carcinoma cells and a prominent lymphocytic infiltrate. Interaction between tumor cells and lymphocytes seems to be decisive for the continued growth of the malignant component.27 Unlike many other cancers, namely cervical or breast cancer, early premalignant lesion of NPC such as dysplasia and carcinoma in situ (CIS) is uncommon. Rarity of lesions without associated carcinoma (3%) and a rapid development of invasive carcinoma strongly implies a swift growth sequence of the initiated cell from dysplasia to CIS and invasive malignancy. This contrasts with human papillomavirus-associated cancers in which CIS may remain for years.48,49 Despite infrequent premalignant lesions, high grade dysplasia and isolated CIS show the presence of EBV. All cases of CIS expressed LMP1 and EBERs, a hallmark of latent EBV infection, and contained clonal EBV genomes,48,49 suggesting that the preinvasive tissue represents the outgrowth of a single EBV-infected progenitor cell.50 Conversely, normal nasopharyngeal epithelium or low-grade dysplasia lesions do not show EBV infection.51,52
The pathophysiological link and molecular mechanisms of EBV-mediated carcinogenesis in NPC are not fully understood. Consistent expression of specific viral genes in every cell of NPC and in premalignant lesions underline the important role of EBV in disease development.47 Gene expression is mainly restricted to the EBNA1, LMP1, LMP2, plus BARF1 and the noncoding EBERs and BARTs, classified as type II latency.27 Functional aspects of these genes have been recently reviewed in detail.53–59 Among the latent genes expressed in NPC, LMP1 is considered the primary viral oncoprotein. The expression of LMP1 is important for EBV to facilitate tumor cell growth and survival advantages, and thus keep the malignant phenotype.27,60,61 LMP1 has pivotal effects on cellular gene expression of multiple genes including the promotion of cell growth, antiapoptotic functions, and enhancement in cell motility.61,62 LMP1 plays a role in the invasive and metastatic property of NPC by inducing matrix metalloproteinase 9, upregulating the expression of mucin 1, and downregulating cell–cell adhesion.63–65 It also associates with exosomes, secreted endosome-derived vesicles that carry proteins, mRNAs, and miRNAs to adjacent or distant cells to modulate immune function, angiogenesis, cell proliferation, tumor invasion, and intercellular communication.66–68 By this association, LMP1 may regulate its transforming capacity and modulate the cellular microenvironment to escape immune recognition.69–72 In epithelial cells, LMP1 activates transcription of the epidermal growth factor receptor (EGFR), which is also detected at high levels in NPC.73 However, its expression in NPC tissues is highly variable in frequency (20%–90% of cases) and level48,68,74,75 in contrast to other EBV-related malignancies such as Hodgkin lymphoma and nasal NK/T-cell lymphoma, which mostly express clearly detectable levels of LMP1.76,77
There is abundant expression of BARTs and BARF1 mRNA in NPC samples, suggesting their crucial role in the pathogenesis.56,78,79 Multiple EBV-encoded miRNAs have been detected in NPC, encoded in the BART region.80 The virus actively employs its miRNAs to manipulate various viral and cellular functions. Some miRNAs may inhibit viral replication by targeting viral DNA polymerase for degradation.81 Cluster one BART miRNAs can downregulate the expression of the viral LMP1.82 Other miRNAs associate with viral replication, modulate host cell homeostasis and immune responses, and promote host cell survival.59,83,84
EBV reactivation from latency requires expression of viral immediate early transactivators, Zta and Rta, which drive further lytic gene expression including thymidine kinase, protein kinase, and the EBV-encoded DNA polymerase. These gene products are essential to create new viral genomes.62,85,86 As indicated above, EBV may sporadically reactivate to a lytic state in epithelial cells, where newly expressed viral proteins become an antigenic stimulus that induce characteristic immune responses.50,87,88 High titers of immunoglobulin A (IgA) against EBV replicative antigens suggest an increased viral lytic replication at early stages and are shown to precede the development of NPC clinical presentation.89,90 Elevated IgA titers may also reflect enhanced epithelial infection.91 Epithelial cells carrying EBV may be more susceptible to DNA damage92,93 and allow for genetic changes induced by other environmental carcinogens.94 Further, genetic changes may contribute to, and facilitate, latent infection or interact with EBV transforming proteins during the tumor propagation.47
Immune responses against EBV and viral immune evasion in NPC
Normally, virus infection elicits host innate and adaptive immune responses; the latter involving diverse antibody (humoral) and T-cell-based (cellular) reactions to multiple EBV antigens.41,95 To manipulate immune recognition and survive destruction by the immune system, EBV has evolved variable strategies of immune evasion.96,97 EBV is in fact a highly immunogenic virus, as shown by the strong response generated in infectious mononucleosis at the time of primary contact.98–100 During lytic replication, the virus downmodulates HLA I and HLA II to escape CD4 and CD8 T-cell recognition. It also interferes with the effector T-cell action through the viral interleukin (IL)-10 homologue encoded by the BCRF1 gene. During latency in memory B-cells, EBV markedly reduces the expression of the most immunogenic latent proteins, such as members of the EBNA3 family. It restricts gene expression to only LMP-2 and/or EBNA1.27 The EBNA1 protein is crucial for the maintenance of the EBV episome in the dividing cells through sequence-specific binding at OriP and the chromosome.101 The presence of a Gly-Ala repeat domain in its sequence allows EBNA1 to escape the recognition of CD8 T-cells by preventing it from proteasomal degradation and presentation to major histocompatibility complex (MHC) class I.102–104 The detection of BARF1- and LMP1-containing exosomes in the circulation of NPC cases indicates that EBV continuously intervenes with the immune system during malignant development. Secreted exosomes have been observed to associate with LMP1 to silence local T-cells or cell regulatory molecules such as galectin-9.69,70,105,106 In addition, exosomes carry viral miRNAs that can modulate host immune cells.68 The secreted BARF1 protein has immunomodulating potential via colony stimulating factor-1 binding, leading to altered behavior of tumor-associated macrophages.57,107
The vast majority of NPC patients demonstrate aberrant immunity against EBV. EBV-specific antibody responses in NPC are more robust and more variable than those in healthy EBV carriers, and therefore have diagnostic significance.88 Arising from the nasopharyngeal mucosal epithelium, NPC is marked by infiltrating lymphocytes that secrete various immunomodulatory cytokines such as transforming growth factor β, IL-5, IL-6, and IL-10, which influence antibody class switching and lead to the generation of IgA.108,109 Clinical onset of NPC is generally associated with high titers of IgA antibodies, especially against tumor-derived latent EBNA1 and EBV lytic cycle antigens, suggesting that lytic virus reactivation accompanies the process of malignant growth.88,110 Elevated IgA-viral capsid antigen/EBNA1 antibody titer may be detectable long before NPC clinical appearance and thus has clinical relevance for NPC diagnostic and screening.89,111–113 In contrast to EBNA1 and viral lytic antigens that evoke a strong humoral immune response during tumor propagation, tumor-associated membrane proteins such as LMP1, LMP2, and BARF1 barely induce a significant antibody response.114–117
Quite the opposite of humoral response, the EBV-specific cellular responses are normal or suppressed compared to those in healthy EBV carriers. In fact, during lytic viral replication, the expression of all viral genes, including lytic proteins and over 80 viral gene products, elicit many T-cell antigens that give rise to a significant response of CD4 and CD8 T-lymphocytes.41 Despite abundant cellular response to latent and lytic gene products, EBV can successfully replicate. Multiple viral gene products interfere with the antigen processing machinery and the MHC molecule expression in infected cells. These include the expression of BNLF2a, which prevents peptide-loading of MHC class I molecules through inhibition of the transporter associated with antigen processing, leading to reduced presentation of viral antigens.118 Another viral lytic protein, BGLF5, blocks the synthesis of new MHC class I molecules and modulates the expression of MHC class II molecules.119 Viral BILF1 downregulates MHC class I molecules that present at the cell surface.120 Viral IL-10 homologue encoded by the BCRF1 gene interferes with the effector T-cell action.41,121 All together, EBV lytic proteins effectively interfere with CD8 and CD4 T-cell surveillance, allowing EBV to continue generating viral progeny.97 A reduced T-cell response occurs at the level of tumor-infiltrating lymphocytes, suggesting a local tumor-induced immune evasion despite host immune competence.122 Specifically, NPC tumor containing EBV uses resistance to apoptosis and local T-cell silencing as its strategy to evade T-cell surveillance and responses.69–71,122–125
Epigenetic mechanisms in NPC
Epigenetics describes the molecular processes that controls gene transcription, independent of DNA sequence. Mechanisms involved in epigenetic regulation include DNA methylation, histone deacetylation, and RNA interference. Among these, DNA methylation is the major modification most widely studied in NPC. DNA methylation refers to a post replication modification in which a methyl group is covalently added to the 5-carbon of cytosine bases that are located in cytosine-guanosine dinucleotides (generally named CpGs).126 The contributions of CpG methylation in NPC pathogenesis include the silencing of EBV immunodominant antigens and various tumor suppressor genes (TSGs).127,128
Epigenetic regulation of EBV gene expression
EBV uses the host DNA methylation mechanism for viral persistence, either in normal or neoplastic tissue. By epigenetic modulation, EBV controls its own promoters and restricts expression of latent, episomal genomes. Tight latency is characterized by absence of virus production and only a limited set of viral promoters being expressed, allowing EBV to contribute to NPC development. Gene expression in latency is regulated mostly through various modes of promoter utilization to control the expression of viral genes associated with growth and transformation. DNA methylation suppresses the expression of Wp and Cp, which are the initial promoters of transcripts encoding nuclear antigens EBNA1–6 at early stages of B-cell infection and transformation.129 Epigenetic mechanisms also silence the promoters of LMP1, LMP2A, and LMP2B and suppress expression of transmembrane proteins. However, DNA methylation does not control Qp, a major latent promoter for EBNA1 transcripts. By using Qp, EBV expresses the indispensable viral protein EBNA1 when all other EBV proteins are switched off, including the immunodominant antigens (EBNA2, 3A, 3B, 3C).130 A fourth promoter driving EBNA1 expression is Fp, an early lytic promoter activated when EBV switches to the lytic cycle. Fp is nearly silenced in latency I and II tumors, including NPC.127 Interestingly, the promoter for viral BARF1, a viral gene universally expressed in NPC and considered to contribute to tumor cell growth and immune evasion, is highly methylated and appears to be regulated in latency by deltaNp63, a transcription factor essential for maintenance of the undifferentiated state in NPC tumor cells (Hoebe dissertation, unpublished data, VU University, Amsterdam, 2014).57
The EBV lytic cycle is initiated by expression of immediate early genes, Zta and Rta, that are driven from the early gene promoters, Zp and Rp. The tight regulation of Zp and Rp is equally important for viral persistence as for Wp and Cp. Zp and Rp are hypermethylated in nearly all latency I and II EBV Burkitt’s lymphoma cell lines. In EBV-associated tumors, including NPC, Zta and Rta transcripts are barely detectable because Zp and Rp promoters are found to be heavily methylated.131
Epigenetics of tumor suppressor genes
NPC is marked by the number of genes targeted for silencing by promoter methylation. Well-established TSGs such as p53 and Rb, which are altered in many tumors, are rarely mutated in NPC. Studies on activated oncogenes or inactivated TSGs did not demonstrate specific translocations, p53 gene mutations, or Rb gene alterations, nor activating Ras mutations.132–134 EBV gene products have alternative functions that directly or indirectly affect these pathways. However, as indicated above, the intrinsic properties of EBNA1 may permit additional genetic changes to develop during malignant progression and contribute to tumor growth and metastasis. In contrast to genetic changes, epigenetic abnormalities of various TSGs are commonly detected in NPC. Aberrant epigenetic mechanisms disrupt multiple normal cellular regulatory and signaling pathways through DNA methylation of promoter CpG islands and/or histone modifications. These activities can provide cell growth and survival advantage and may be involved in the initiation and progression of NPC pathogenesis.128 The Ras-association family 1 gene (RASSF1A), which has a role in Ras signaling, cell cycle arrest, apoptosis, and DNA repair, together with p16, a cell cycle regulation gene, were two of the first TSGs observed to be hypermethylated in NPC.135,136 Polymerase chain reaction screening in NPC samples detected frequent loss of heterozygosity, indicating specific loss of DNA sequences involving the p16 cyclin dependent kinase inhibitor at 9p21 and the RASSF1A gene at 3p21. In vitro studies demonstrated that reintroduction of the RASSF1A gene into an NPC cell line inhibited cell growth. This suggests that inactivation of RASSF1A can be an important contributor to NPC development, as it does in many cancer types.135,136 More extensive studies demonstrated that high levels of CpG methylation spread throughout the cellular genome in EBV-associated NPC, and many TSGs were aberrantly methylated in their 5′ CpG islands. Multistep oncogenesis may involve TSGs that function in apoptosis, cell cycle and mitotic checkpoint regulation, intracellular adhesion, DNA damage repair, cytoskeleton organization, Wnt-signaling pathway, tumor invasion, and metastasis.128,137,138 Furthermore, the frequent hypermethylation of multiple TSGs has potential value for diagnostic purposes and early detection in NPC.139–141
Besides silencing the viral gene promoters, EBV also modifies the host genome methylation pattern. Such altered methylation profiles in key cancer-related genes may contribute to pivotal mechanisms during NPC pathogenesis.142 EBV-encoded LMP1 upregulates DNA methyltransferases (DNMTs) via the JNK/AP1-signaling pathway, inducing aberrant promoter methylation and reduced expression of certain cellular genes.138,143,144 Treatment with demethylating drugs in LMP1 expressing epithelial cells has been observed to reupregulate the expression of the corresponding gene.145 Elevated expression of DNMTs and other epigenetic modifiers, polycomb repressive complexes, are found in various tumors including NPC. In addition to DNMTs, activated polycomb repressive complexes could also modulate multiple cellular signaling pathways through EBV-encoded proteins.146,147 All together, this suggests that LMP1 can regulate both maintenance and de novo methylation. Using epigenetic strategies, EBV alters host gene expression to facilitate its existence.
Therapeutic implications
Standard of NPC treatment
NPC is highly radiosensitive and therefore radiotherapy remains the standard treatment for all stages of nondisseminated disease.148 Cases of stage I are treated by radiotherapy alone while stage III, IVA, and IVB disease, according to the American Joint Committee on Cancer 2010, are treated by radiotherapy with concurrent chemotherapy.149,150 For stage II, the combination of chemotherapy and radiotherapy is recommended to prevent distant failures, although randomized-controlled evidence is lacking.151 The standard care for stage I disease can achieve 5-year local control and a survival rate of 90%.152 In nonmetastatic diseases, the standard management can achieve 3 years disease-free and an overall survival of 82%–89%.153,154 However, disease control is often associated with radiation- or chemotherapy-related toxicities, resulting in a decreased quality of life.155 Furthermore, about 10% of cases experience recurrence, either local or regional. In cases of local recurrence, the best management remains to be determined. Treatment options include brachytherapy,156 photodynamic therapy,157 stereotactic radiosurgery,158 and nasopharyngectomy.159 For regional recurrence, the optimal treatment method is a neck dissection.160 Survival after recurrences is variable, depending on previous strategy, duration of the disease-free interval, and retreatment approach.161,162 Cases with metastatic NPC (stage IVC) can be treated with palliative therapy only. When patients are chemonaïve, platinum-based regimens give the best results.163 A recent study showed the benefit of a combination with radiation for locoregional disease in cases of distant metastases at diagnoses.164 However, when the above mentioned strategies have failed, limited options are available. The best response rates are found with gemcitabine, capecitabine, or docetaxel, which result in median survival of 9.5–15 months.165 Additional to the poor outcome, combination chemotherapy in metastatic NPC may relate to an unavoidable increased toxicity.166 Overall, these limitations urge the development of additional strategies to overcome the primary challenges in NPC treatment, which include reducing toxicity, maintaining rates of good local control, and decreasing rates of distant metastasis in locoregional disease.155
New targeted therapies
As tumor biology is highly explored, the role of targeted therapy brings hope for tailored treatment for all types of cancer, including NPC. The advances in molecular targeted therapy and personalized medicine have provided grounds for more specific treatment in NPC and have become the focus of recent research and development. More focused therapy targeting disease etiology may increase cure rates since standard modalities using radiation with or without chemotherapy cannot achieve it. Results from studies combining targeted therapy in NPC with current treatments have shown some clinical benefit and require further trials to determine their advantages. These treatments include drugs targeting EGFR,167,168 vascular endothelial growth factor (VEGF),169 antimammalian target of rapamycin,170 and tumor hypoxia.171
Overexpression of the EGFR has been detected in a high proportion of NPC patient tumors.172 The EBV oncoprotein LMP1 is known to activate transcription of the gene,73 underlining the importance of EGFR signaling in NPC pathogenesis. A chimeric anti-EGFR immunoglobulin G1 monoclonal antibody, cetuximab, has been developed and tested in a Phase II study in combination with carboplatin. In 60 recruited NPC cases with recurrent or metastatic disease, this trial demonstrated some clinical responses (11.7% of partial response and 48.3% of stable disease). Toxicities of grade 3–4 leukopenia and thrombocytopenia occurred in only 5% and 10% cases, respectively.173 Cetuximab was also tested in a Phase II trial in combination with cisplatin and intensity-modulated radiotherapy involving 30 patients with stage III/IV NPC. Although achieving 86.5% of 2-year progression-free survival, this protocol was associated with a high incidence of grade 3–4 mucositis and 20% grade 2 radiotherapy-related dermatitis.174 Two Phase II clinical studies have used another EGFR inhibitor, gefitinib, in patients with recurrent or metastatic NPC but failed to demonstrate any clinical response.167,168 Angiogenesis is another promising treatment target, and the expression of VEGF has been observed to significantly associate with angiogenesis and metastases in NPC.175 Bevacizumab, a chimeric monoclonal antibody targeting VEGF, has been tested in a Phase II multinational trial when added to standard chemoradiation treatment in 46 NPC patients with locally advanced disease. This study proved the protocol to be safe, as only grade 1–2 hemorrhagic events occurred in nine patients. Clinical responses included 90.8% of 2-year distant metastasis-free interval, 74.7% of 2-year progression-free survival, and 90.9% of 2-year overall survival.169 A tyrosine kinase inhibitor targeting VEGF receptor, sunitinib, has also been tested in a Phase II study in 13 metastatic NPC patients. However, this study prematurely stopped because of severe hemorrhagic events affecting nine (69%) patients, even though five patients showed tumor shrinkage, indicating a good clinical response.176 Another small molecule targeting VEGF, sorafenib, has reached Phase II clinical trial, showing its modest efficacy in recurrent or metastatic NPC cases.177 Everolimus, a drug affecting mammalian target of rapamycin, has been tested on NPC cell lines, such as HK1, HONE-1, CNE-1, CNE-2, and C666-1, and was observed to have potential therapeutic effect for NPC.170 Moreover, drugs targeting tumor hypoxia have been developed and tested in a clinical trial showing its capability as a promising strategy for NPC treatment.171
EBV targeting therapies
The presence of EBV in nearly all NPC cells emphasizes its potential to be an effective target in treatment of NPC. Although the natural role of EBV in NPC pathogenesis is not yet fully understood, this association serves as a target of exploitation in a therapeutic capacity.178 Besides the options of targeted therapy mentioned previously, EBV viral antigen expression has attracted many studies for NPC treatment development. These include immunotherapy targeting EBV (EBV-specific T-cell infusions, EBV-based therapeutic vaccinations), EBV-targeted antibody-based therapies, epigenetic approaches, and viral lytic induction treatment.178–180
Immunotherapy targeting EBV
It has been observed that EBV-carrying NPC cells are capable of immunologic processing of internal antigens for CTL recognition and stimulating CTL CD8 elimination.181 Functional CTLs are considered to be competent to attack tumor cells leading to tumor shrinkage.182 However, it requires the tumor cells to express MHC class I and be low in apoptosis resistance function that might counteract the granzyme-B attack by CTLs. Previous studies have indicated significant MHC class I heterogeneity among NPC cases as well as expression of Bcl-2 and XIAP, which correlated with poor prognosis.122–124,183–185 In addition, the immunosuppressive microenvironment of NPC cells in vivo may silence infiltrating activated T-cells, thereby diminishing the intended therapeutic effect.121,122 Despite these observations, several immunotherapy techniques have been developed for EBV-associated malignancies. Two different approaches have been tested to treat NPC, namely adoptive immunotherapy, in which immune cells are passively transferred to patients, and active immunotherapy, in which an immunogen is administered to stimulate a response from the patient’s immune system.179,186
The majority of adoptive immunotherapy studies in NPC patients have applied autologous CTL treatment. The infusion of autologous EBV CTLs expanded ex vivo by repeated stimulation with lymphoblastoid cell lines was first carried out in patients with advanced disease, leading to increased CTL levels and a reduced plasma EBV DNA level. Even though it failed to prove the existence of a clinical benefit, this trial has demonstrated the feasibility of CTL transfer in NPC patients.187 A further trial enrolled ten endstage NPC patients who experienced progression after conventional chemoradiation therapy for intravenous autologus EBV-specific CTLs. All cases showed generation of EBV-specific CTLs that were able to specifically kill in vitro autologous EBV-infected cells. Clinically, this study protocol resulted in disease control in six patients and a progression in four patients.188 Another similar clinical trial included ten NPC patients and demonstrated significant antitumor results. Four patients were in complete remission, whereas the other six had a recurrent or metastatic disease. A mild swelling at the tumor site was the only toxicity effect caused by the study protocol.189 Overall, these early trials showed promising results, including safety and tolerability of the use of EBV-specific CTLs in patients with advanced NPC cases.
In order to optimize the cell therapy approach, a study increased the dose of EBV-specific CTLs and administered it after nonmyeloablative, lymphodepleting chemotherapy. All eleven advanced NPC cases recruited in this trial tolerated the protocol well. Six patients demonstrated a stable disease that lasted for more than 4 months. Although this study confirmed its previous series on the safety of CTL treatment in advanced NPC,188 administration of lymphodepleting chemotherapy did not add any improved tumor control to the previous results.190
Improvement of CTL generation that is more antigen-specific is very crucial to increase treatment efficacy. The above mentioned studies have been applied using lymphoblastoid cell lines to effectively generate CTLs in posttransplant lymphoproliferative disorder (PTLD) cases.191 This modality evokes CTL responses targeting the immunodominant EBV antigens, EBNA3–6. Unlike PTLD, NPC cells express latency type II that includes LMP1, LMP2, and EBNA1, which have poor immunogenicity. To enhance the specificity of CTLs and antitumor response, the immunotherapeutic approach in NPC should only target latency type II antigens. For this reason, an adenovirus-based adoptive immunotherapy has been developed that encodes EBNA1 fused to multiple CD8 T-cell epitopes from LMP1 and LMP2. Clinically, it has been assessed in a Phase I study involving NPC cases with recurrent and metastatic disease. Out of 24 cases, T-cells were successfully expanded from 16 patients. Fourteen patients experienced mild toxicities such as grade 1 flu-like symptoms and malaise. Disease control was achieved with a mean time to progression of 136 days. The study protocol successfully demonstrated an increased overall survival from 220 to 523 days when compared with a patient cohort that did not receive any T-cell therapy. This adoptive immunotherapy indicates that CTL infusions with a polyepitope approach has the potential to prevent recurrent or metastatic disease after primary treatment.180,192 More recently, the safety and tolerability of LMP-specific autologous CTLs were further proven by another study in the case of a recurrent NPC patient with multiple pulmonary metastases. This strategy demonstrated a remarkable effect on metastatic sites, where the majority of pulmonary lesions disappeared although the tumor at the primary site did not decrease.193
A Phase II clinical trial for the first time evaluated CTL infusion therapy as a first-line treatment in locally recurrent or metastatic NPC. The trial recruited only Asian patients to focus on the most prevalent population with the malignancy. The study protocol included up to six sequential infusions containing LMP2-specific T-cell following four cycles of chemotherapy. Of 35 patients receiving EBV-CTL, the study provided a 71.4% response rate with a 2- and 3-year overall survival of 62.9% and 37.1%, respectively. In addition to demonstrating improved survival outcome in advanced NPC patients, this study has set the groundwork for a future Phase III clinical trial of standard chemotherapy with and without EBV-CTL therapy.194
In active immunotherapy, an EBV-specific vaccine was developed, aiming to enhance the immune response in patients with EBV-related malignancy. For this type of EBV-targeted treatment, two strategies have been established: dendritic cell (DC) vaccination and peptide vaccination. DCs are professional antigen-presenting cells that function to activate naïve CD4 and CD8 T-cells. This approach has been developed by culturing autologous monocyte-derived DCs from patients with advanced NPC and pulsed with LMP2-peptide. A clinical trial has applied this vaccination to induce epitope-specific CD8 T-cell responses in 16 local recurrent and metastatic NPC patients. After vaccination, all patients elicited substantial immune response, generated as epitope-specific CTLs in their peripheral blood although the study only showed a few clinical responses (two patients showed partial tumor reduction and 14 patients developed disease progression). Moreover, this study protocol was well tolerated and caused no significant side effects.195 Recently, a Phase II study reported clinical and immunologic effects of a DC vaccine transduced by an adenovirus truncated LMP1 and full length LMP2 in 16 cases of metastatic NPC. Although showing safety in administration, the current vaccine induced only modest efficacy, with only three subjects demonstrating clinical response. Immunologically, delayed-type hypersensitivity responses were shown in nine patients but with no increase in the frequency of peripheral LMP1/2 specific T-cells. Further modifications to the DC and combination with other cellular immunotherapies may be needed to improve the vaccine’s effectiveness.196 In another trial, an autologous DC vaccination was assessed as an adjunct strategy after radiotherapy in 38 patients with stage II/III NPC. The autologous DCs were pulsed with HLA-A2 restricted LMP2 peptides. Following treatment, delayed-type hypersensitivity responses were elicited in nine patients who also showed significant decrease of serum EBV DNA level. Serum levels of IL-2 and interferon gamma as well as the percentage of NK and CD4 T-cells significantly enhanced. Patients also tolerated this regimen well, without any significant toxicity.197
Viral vector loading with EBV peptides has been under experiment. A vaccine approach was developed to incorporate scrambled DNA sequences of EBNA1, LMP1, and LMP2 and insert them into an adenoviral vector.198 The construct of this EBV antigen-based NPC vaccine has been described, and the formulation potentially stimulates CD4 and CD8 T-cells against EBNA1 and LMPs. However, clinical trials on this technology are not yet available.199 A different vaccine approach was developed by another group with a modified vaccinia Ankara (MVA) recombination vector expressing NPC-associated viral antigens. The vaccine virus, MVA-EL, was constructed using sequences cloned from a typical Chinese EBV strain and encodes functionally inactive fusion protein containing the C-terminal half of EBNA1 and full length LMP2.200 Further, a Phase I clinical trial tested this strategy in patients who were in remission after standard therapy of NPC, aiming to determine its tolerability and its capacity to induce an EBNA1 and/or LMP2 CTL response. Three intradermal MVA-EL vaccinations were administered every 3 weeks using five escalating dose levels. Of 18 patients recruited, 15 cases showed increasing T-cell responses to one or both vaccine antigens. This trial proved that the MVA-EL vaccine is well tolerated. It also determined the highest and most consistently immunogenic dose to be chosen for further Phase II trial to determine its clinical efficacy.201
Antibody targeting options
Despite some clinical efficacy shown by antibody-based targeted therapy as mentioned above, the utilization of such a management option in developing countries was hampered by high cost. Alternatively, antibodies targeting the LMP1 and LMP2 outer membrane loops may serve as therapeutic targets, as they can mediate cell killing complement activation.202 Such antibodies may not be limited by local immunosupression in the NPC tumor environment and may mediate tumor killing by drug conjugation. Furthermore, they can be generated by antibody phage libraries (Middeldorp patent China CN1526072; US7811581). A previous study successfully developed a novel human antibody fragment, antigen-binding against the LMP1 extracellular domain, which was subsequently conjugated with mitomycin C, thus forming an immunoconjugate. This biotherapy showed an effect on proliferation and apoptosis in NPC cell lines HNE2/LMP1 and the inhibition of growth rate of NPC xenografts in nude mice, proving its potential as a therapeutic agent in the treatment of LMP1-expressing NPC.203 The resulting antibodies may be more specific than those targeting EGFR. Another study showed that immunization against short external loops of viral LMPs could be another low-cost option for antibody-based therapy development.202,204 These extracellular loops are normally barely immunogenic but can be linked to approved human immunogens, such as tetanus toxoid or keyhole limpet hemocyanin, to improve immunogenicity and provide an economically affordable alternative to prior therapeutic vaccine approaches.126 However, this option requires more research, and clinical trials must be conducted to demonstrate its clinical efficacy.
Drugs targeting epigenetic pathway
CpG methylation can be reversed with pharmacological demethylation using epigenetic agents,205,206 providing the opportunity to explore epigenetic treatment as a novel therapeutic approach or as a combinational intervention with other modalities. Reactivating methylated and silenced TSGs would be expected to restore normal cell growth control, promote apoptosis in tumor cells, or evoke immune response. Demethylation would also reactivate the expression of EBV early and lytic genes in latently-infected NPC cells, so that highly immunogenic EBV antigens would be recognized by the immune system, leading to tumor killing. Drugs targeting epigenetic mechanisms include DNA methyltransferase inhibitors (nucleoside analogues such as 5-aza-2′-deoxycytidine/decitabine/DAC, 5-azacytidine, and zebularine)207 and various histone deacetylase (HDAC) inhibitors.205,206 These agents have been tested before in various type of cancers such as colon, head, neck, renal, and lung cancers, which resulted in only partial response in some patients.208,209 A clinical trial of azacitidine was carried out in patients with NPC and EBV-positive AIDS-associated Burkitt’s lymphoma. Comparison on pre- and post-treatment tumor biopsies showed significant demethylation of the latent and early lytic EBV promoters (Cp, Wp, LMP1p [ED-L1], Zp, Rp), with the reactivation of viral antigen expression (Zta),210 signifying the potential of epigenetic therapy for NPC. Moreover, demethylating agents are currently used in combination with drugs inducing EBV lytic phase and nucleoside analogues.
Drugs targeting the viral lytic phase
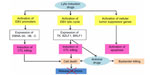
The presence of EBV in the NPC cells may facilitate therapeutic killing of virus-carrying tumor cells. While in latency, DNA methylation of viral promoters protects EBV and suppresses its immunogenic proteins, replicative cycle may induce strong immune response for many viral antigens will be explored to immune system despite viral capability in intervening with it. In lytic replication, EBV-positive tumor cells commonly have intact antigen-presenting capacity to present viral epitopes in the context of MHC class I and/or MHC class II, giving rise to immune recognition and subsequent CTL killing.41 Most NPC patients are also observed to generate functional CTLs with specificity against EBV proteins.181,211 Substances that effectively activate the lytic cycle of EBV include chemotherapeutic agents affecting DNA synthesis and drugs affecting host DNA methylation and histone deacetylation.212–216 EBV can be eliminated during lytic replication in vitro by nucleoside analogues such as acyclovir and ganciclovir.217,218 However, these drugs must first be phosphorylated before incorporation by viral or cellular DNA polymerase into DNA. Cells containing latent EBV infection cannot efficiently phosphorylate either acyclovir or ganciclovir. In contrast, cells infected with the lytic form of viral infection express two virally encoded kinases (EBV thymidine kinase and the BGLF4 gene product, protein kinase), and thus allow phosphorylation or activation of both antiviral drugs in these cells. Simultaneous to viral lytic replication induced by stimulating agents, expression of EBV kinases increases susceptibility of the EBV-infected cells to antiviral treatment. Therefore, the combination of agents inducing viral replication and antiviral nucleoside analogues merits further evaluation as an alternative strategy to selectively eliminate EBV-carrying cells.219,220 Moreover, lytic induction will also reexpress host TSGs, leading to the promotion of apoptosis in the tumor cells. The schematic concept of EBV lytic induction therapy is displayed in Figure 1.
The treatment concept of EBV lytic induction was first applied to a patient suffering from EBV-positive lymphoma using a HDAC inhibitor, arginine butyrate, in combination with antiviral.221 A subsequent study used valproic acid instead of arginine butyrate for activating viral promoters.222 A combination of chemotherapy, 5-fluorouracil, with a HDAC inhibitor was then used to increase the effectiveness of lytic induction. The combination was administered to an endstage NPC patient, while simultaneously adding valganciclovir. This study protocol revealed an increase of viral DNA in the circulation, indicating shedding of apoptotic fragments from the tumor which did not occur before therapy.87 More recently, a novel combination therapy was developed and validated in a naturally EBV-infected NPC and in EBV-positive gastric cancer cell lines, showing strong synergistic effect. The drugs used consisted of chemotherapy gemcitabine, valproic acid, and valganciclovir. Application of this combination was carried out as a new treatment option in three NPC cases for which no curable treatment modalities were available. All patients showed increased levels of viral DNA in the blood. Regarding clinical parameters, patients were in stable condition, developed only transient and moderate side effects, and experienced improvement in quality of life during and after treatment.223 Based on the results in this small population, a clinical trial with a larger sample size is currently underway in our center in collaboration with our Dutch colleagues.
Conclusion
NPC is highly prevalent in certain regions including southern China and Southeast Asia. EBV infection is associated with the vast majority of cases shown by the presence of viral transcripts and protein antigens in tumor cells. Given the premise of this tight relationship, EBV serves as a target for therapeutic implications. The fact that patients may relapse after primary treatment using radiotherapy, a combination of chemoradiation, or systemic therapy urges the development of personalized medicine that provides better disease control and prevents recurrence or metastases. Novel therapies targeting EBV have currently become a center of interest in research and development of NPC treatment. Currently, immune-based strategies represent options with the most clinical benefit. Such treatment offers promising application and success in patients with EBV-associated NPC and may augment clinical response to achieve disease control and reduce risk of recurrence, especially in cases with limited response after conventional therapy. Further Phase III trials are needed to assess the clinical efficacy of these strategies. Beside immune-based treatment, viral lytic induction therapy also shows potential as a treatment strategy in patients with NPC and currently has reached early phase clinical trials.
Disclosure
The authors report no conflicts of interest in this work.
References
Sham JS, Wei WI, Zong YS, et al. Detection of subclinical nasopharyngeal carcinoma by fibreoptic endoscopy and multiple biopsy. Lancet. 1990;335(8686):371–374. | |
Shanmugaratnam K, Sobin LH. The World Health Organization histological classification of tumours of the upper respiratory tract and ear. A commentary on the second edition. Cancer. 1993;71(8):2689–2697. | |
Reddy SP, Raslan WF, Gooneratne S, Kathuria S, Marks JE. Prognostic significance of keratinization in nasopharyngeal carcinoma. Am J Otolaryngol. 1995;16(2):103–108. | |
Parkin DM, Whelan SL, Ferlay J, Raymond L, Young J, editors. Cancer Incidence in Five Continents. Vol VII. IARC Scientific Publications No 143. Lyon; 1997. | |
Yu MC, Yuan JM. Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12(6):421–429. | |
Chen CJ, You SL, Lin LH, Hsu WL, Yang YW. Cancer epidemiology and control in Taiwan: a brief review. Jpn J Clin Oncol. 2002;32 Suppl:S66–S81. | |
Devi BC, Pisani P, Tang TS, Parkin DM. High incidence of nasopharyngeal carcinoma in native people of Sarawak, Borneo Island. Cancer Epidemiol Biomarkers Prev. 2004;13(3):482–486. | |
Wee JT, Ha TC, Loong SL, Qian CN. Is nasopharyngeal cancer really a “Cantonese cancer”? Chin J Cancer. 2010;29(5):517–526. | |
Trejaut J, Lee CL, Yen JC, Loo JH, Lin M. Ancient migration routes of Austronesian-speaking populations in oceanic Southeast Asia and Melanesia might mimic the spread of nasopharyngeal carcinoma. Chin J Cancer. 2011;30(2):96–105. | |
Levine PH, Pocinki AG, Madigan P, Bale S. Familial nasopharyngeal carcinoma in patients who are not Chinese. Cancer. 1992;70(5):1024–1029. | |
Friborg J, Wohlfahrt J, Melbye M. Familial risk and clustering of nasopharyngeal carcinoma in Guangdong, China. Cancer. 2005;103(1):211. | |
Ung A, Chen CJ, Levine PH, et al. Familial and sporadic cases of nasopharyngeal carcinoma in Taiwan. Anticancer Res. 1999;19(1B):661–665. | |
Ng WT, Choi CW, Lee MC, Chan SH, Yau TK, Lee AW. Familial nasopharyngeal carcinoma in Hong Kong: epidemiology and implication in screening. Fam Cancer. 2009;8(2):103–108. | |
Feng BJ, Huang W, Shugart YY, et al. Genome-wide scan for familial nasopharyngeal carcinoma reveals evidence of linkage to chromosome 4. Nat Genet. 2002;31(4):395–399. | |
Xiong W, Zeng ZY, Xia JH, et al. A susceptibility locus at chromosome 3p21 linked to familial nasopharyngeal carcinoma. Cancer Res. 2004;64(6):1972–1974. | |
Hu LF, Qiu QH, Fu SM, et al. A genome-wide scan suggests a susceptibility locus on 5p 13 for nasopharyngeal carcinoma. Eur J Hum Genet. 2008;16(3):343–349. | |
Hildesheim A, Wang CP. Genetic predisposition factors and nasopharyngeal carcinoma risk: a review of epidemiological association studies, 2000–2011: Rosetta Stone for NPC: genetics, viral infection, and other environmental factors. Semin Cancer Biol. 2012;22(2):107–116. | |
Hsu WL, Tse KP, Liang S, et al. Evaluation of human leukocyte antigen-A (HLA-A), other non-HLA markers on chromosome 6p21 and risk of nasopharyngeal carcinoma. PLoS One. 2012;7(8):e42767. | |
Yu MC, Huang TB, Henderson BE. Diet and nasopharyngeal carcinoma: a case-control study in Guangzhou, China. Int J Cancer. 1989;43(6):1077–1082. | |
Guo X, Johnson RC, Deng H, et al. Evaluation of nonviral risk factors for nasopharyngeal carcinoma in a high-risk population of Southern China. Int J Cancer. 2009;124(12):2942–2947. | |
Jia WH, Luo XY, Feng BJ, et al. Traditional Cantonese diet and nasopharyngeal carcinoma risk: a large-scale case-control study in Guangdong, China. BMC Cancer. 2010;10:446. | |
Farrow DC, Vaughan TL, Berwick M, Lynch CF, Swanson GM, Lyon JL. Diet and nasopharyngeal cancer in a low-risk population. Int J Cancer. 1998;78(6):675–679. | |
Jeannel D, Hubert A, de Vathaire F, et al. Diet, living conditions and nasopharyngeal carcinoma in Tunisia – a case-control study. Int J Cancer. 1990;46(3):421–425. | |
Xue WQ, Qin HD, Ruan HL, Shugart YY, Jia WH. Quantitative association of tobacco smoking with the risk of nasopharyngeal carcinoma: a comprehensive meta-analysis of studies conducted between 1979 and 2011. Am J Epidemiol. 2013;178(3):325–338. | |
Fang CY, Huang SY, Wu CC, et al. The synergistic effect of chemical carcinogens enhances Epstein-Barr virus reactivation and tumor progression of nasopharyngeal carcinoma cells. PLoS One. 2012;7(9):e44810. | |
Hildesheim A, Levine PH. Etiology of nasopharyngeal carcinoma: a review. Epidemiol Rev. 1993;15(2):466–485. | |
Middeldorp JM, Brink AA, van den Brule AJ, Meijer CJ. Pathogenic roles for Epstein-Barr virus (EBV) gene products in EBV-associated proliferative disorders. Crit Rev Oncol Hematol. 2003;45(1):1–36. | |
Roizman B. Herpesviridae: general description, taxonomy and classification. In: Roizman B, editor. The Herpesviruses. London: Plenum Press, 1996:1_/23.)/Rickinson, A.B.; Kieff, E. Epstein-Barr Virus, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; pp 2575–2627. | |
Kieff E. Epstein-Barr virus and its replication. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. Philadelphia, PA: Lippincott-Raven; 1996:2343–2396. | |
Crawford DH, Macsween KF, Higgins CD, et al. A cohort study among university students: identification of risk factors for Epstein-Barr virus seroconversion and infectious mononucleosis. Clin Infect Dis. 2006;43(3):276–282. | |
Kutok JL, Wang F. Spectrum of Epstein-Barr virus-associated diseases. Annu Rev Pathol. 2006;1:375–404. | |
Okano M, Gross TG. Acute or chronic life-threatening diseases associated with Epstein-Barr virus infection. Am J Med Sci. 2012;343(6):483–489. | |
Lossius A, Johansen JN, Torkildsen Ø, Vartdal F, Holmøy T. Epstein-Barr virus in systemic lupus erythematosus, rheumatoid arthritis and multiple sclerosis – association and causation. Viruses. 2012;4(12):3701–3730. | |
Niedobitek G. The Epstein-Barr virus: a group 1 carcinogen? Virchows Arch. 1999;435(2):79–86. | |
Babcock GJ, Decker LL, Volk M, Thorley-Lawson DA. EBV persistence in memory B cells in vivo. Immunity. 1998;9(3):395–404. | |
Pegtel DM, Middeldorp J, Thorley-Lawson DA. Epstein-Barr virus infection in ex vivo tonsil epithelial cell cultures of asymptomatic carriers. J Virol. 2004;78(22):12613–12624. | |
Tao Q, Young LS, Woodman CB, Murray PG. Epstein-Barr virus (EBV) and its associated human cancers – genetics, epigenetics, pathobiology and novel therapeutics. Front Biosci. 2006;11:2672–2713. | |
Thorley-Lawson DA, Hawkins JB, Tracy SI, Shapiro M. The pathogenesis of Epstein-Barr virus persistent infection. Curr Opin Virol. 2013;3(3):227–232. | |
Menon MP, Pittaluga S, Jaffe ES. The histological and biological spectrum of diffuse large B-cell lymphoma in the World Health Organization classification. Cancer J. 2012;18(5):411–420. | |
Hadinoto V, Shapiro M, Sun CC, Thorley-Lawson DA. The dynamics of EBV shedding implicate a central role for epithelial cells in amplifying viral output. PLoS Pathog. 2009;5(7):e1000496. | |
Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu Rev Immunol. 2007;25:587–617. | |
Hochberg D, Middeldorp JM, Catalina M, Sullivan JL, Luzuriaga K, Thorley-Lawson DA. Demonstration of the Burkitt’s lymphoma Epstein-Barr virus phenotype in dividing latently infected memory cells in vivo. Proc Natl Acad Sci U S A. 2004;101(1):239–244. | |
Laichalk LL, Thorley-Lawson DA. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J Virol. 2005;79(2):1296–1307. | |
Laichalk LL, Hochberg D, Babcock GJ, Freeman RB, Thorley-Lawson DA. The dispersal of mucosal memory B cells: evidence from persistent EBV infection. Immunity. 2002;16(5):745–754. | |
Roughan JE, Torgbor C, Thorley-Lawson DA. Germinal center B cells latently infected with Epstein-Barr virus proliferate extensively but do not increase in number. J Virol. 2010;84(2):1158–1168. | |
Kis LL, Salamon D, Persson EK, et al. IL-21 imposes a type II EBV gene expression on type III and type I B cells by the repression of C- and activation of LMP-1-promoter. Proc Natl Acad Sci U S A. 2010;107(2):872–877. | |
Raab-Traub N. EBV-induced oncogenesis. In: Arvin A, Campadelli-Fiume G, Mocarski E, et al; editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press; 2007. Chapter 55. | |
Pathmanathan R, Prasad U, Chandrika G, Sadler R, Flynn K, Raab-Traub N. Undifferentiated, nonkeratinizing, and squamous cell carcinoma of the nasopharynx. Variants of Epstein-Barr virus-infected neoplasia. Am J Pathol. 1995;146(6):1355–1367. | |
Pathmanathan R, Prasad U, Sadler R, Flynn K, Raab-Traub N. Clonal proliferations of cells infected with Epstein-Barr virus in preinvasive lesions related to nasopharyngeal carcinoma. N Engl J Med. 1995; 333(11):693–698. | |
Raab-Traub N, Flynn K. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell. 1986;47(6):883–889. | |
Tao Q, Srivastava G, Chan AC, Chung LP, Loke SL, Ho FC. Evidence for lytic infection by Epstein-Barr virus in mucosal lymphocytes instead of nasopharyngeal epithelial cells in normal individuals. J Med Virol. 1995;45(1):71–77. | |
Sam CK, Brooks LA, Niedobitek G, Young LS, Prasad U, Rickinson AB. Analysis of Epstein-Barr virus infection in nasopharyngeal biopsies from a group at high risk of nasopharyngeal carcinoma. Int J Cancer. 1993;53(6):957–962. | |
Frappier L. Contributions of Epstein-Barr nuclear antigen 1 (EBNA1) to cell immortalization and survival. Viruses. 2012;4(9):1537–1547. | |
Frappier L. EBNA1 and host factors in Epstein-Barr virus latent DNA replication. Curr Opin Virol. 2012;2(6):733–739. | |
Dawson CW, Port RJ, Young LS. The role of the EBV-encoded latent membrane proteins LMP1 and LMP2 in the pathogenesis of nasopharyngeal carcinoma (NPC). Semin Cancer Biol. 2012;22(2):144–153. | |
Seto E, Yang L, Middeldorp J, et al. Epstein-Barr virus (EBV)-encoded BARF1 gene is expressed in nasopharyngeal carcinoma and EBV-associated gastric carcinoma tissues in the absence of lytic gene expression. J Med Virol. 2005;76(1):82–88. | |
Hoebe EK, Le Large TY, Greijer AE, Middeldorp JM. BamHI-A rightward frame 1, an Epstein-Barr virus-encoded oncogene and immune modulator. Rev Med Virol. 2013;23(6):367–383. | |
Takada K. Role of EBER and BARF1 in nasopharyngeal carcinoma (NPC) tumorigenesis. Semin Cancer Biol. 2012;22(2):162–165. | |
Marquitz AR, Raab-Traub N. The role of miRNAs and EBV BARTs in NPC. Semin Cancer Biol. 2012;22(2):166–172. | |
Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4(10):757–768. | |
Yoshizaki T, Kondo S, Wakisaka N, et al. Pathogenic role of Epstein-Barr virus latent membrane protein-1 in the development of nasopharyngeal carcinoma. Cancer Lett. 2013;337(1):1–7. | |
Kieff E, Rickinson A. Epstein-Barr virus and its replication. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:2603–2654. | |
Yoshizaki T, Sato H, Furukawa M, Pagano JS. The expression of matrix metalloproteinase 9 is enhanced by Epstein-Barr virus latent membrane protein 1. Proc Natl Acad Sci U S A. 1998;95(7):3621–3626. | |
Endo K, Kondo S, Shackleford J, et al. Phosphorylated ezrin is associated with EBV latent membrane protein 1 in nasopharyngeal carcinoma and induces cell migration. Oncogene. 2009;28(14):1725–1735. | |
Shair KH, Schnegg CI, Raab-Traub N. Epstein-Barr virus latent membrane protein-1 effects on junctional plakoglobin and induction of a cadherin switch. Cancer Res. 2009;69(14):5734–5742. | |
Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic. 2008;9(6):871–881. | |
Simons M, Raposo G. Exosomes – vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21(4):575–581. | |
Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010;107(14):6328–6333. | |
Dukers DF, Meij P, Vervoort MB, et al. Direct immunosuppressive effects of EBV-encoded latent membrane protein 1. J Immunol. 2000;165(2):663–670. | |
Flanagan J, Middeldorp J, Sculley T. Localization of the Epstein-Barr virus protein LMP 1 to exosomes. J Gen Virol. 2003;84(Pt 7):1871–1879. | |
Middeldorp JM, Pegtel DM. Multiple roles of LMP1 in Epstein-Barr virus induced immune escape. Semin Cancer Biol. 2008;18(6):388–396. | |
Verweij FJ, van Eijndhoven MA, Hopmans ES, et al. LMP1 association with CD63 in endosomes and secretion via exosomes limits constitutive NF-κB activation. EMBO J. 2011;30(11):2115–2129. | |
Miller WE, Earp HS, Raab-Traub N. The Epstein-Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor. J Virol. 1995;69(7):4390–4398. | |
Hu LF, Minarovits J, Cao SL, et al. Variable expression of latent membrane protein in nasopharyngeal carcinoma can be related to methylation status of the Epstein-Barr virus BNLF-1 5′-flanking region. J Virol. 1991;65(3):1558–1567. | |
Khabir A, Karray H, Rodriguez S, et al. EBV latent membrane protein 1 abundance correlates with patient age but not with metastatic behavior in north African nasopharyngeal carcinomas. Virol J. 2005;2:39. | |
Tao Q, Ho FC, Loke SL, Srivastava G. Epstein-Barr virus is localized in the tumour cells of nasal lymphomas of NK, T or B cell type. Int J Cancer. 1995;60(3):315–320. | |
Chiang AK, Tao Q, Srivastava G, Ho FC. Nasal NK- and T-cell lymphomas share the same type of Epstein-Barr virus latency as nasopharyngeal carcinoma and Hodgkin’s disease. Int J Cancer. 1996;68(3):285–290. | |
Decaussin G, Sbih-Lammali F, de Turenne-Tessier M, Bouguermouh A, Ooka T. Expression of BARF1 gene encoded by Epstein-Barr virus in nasopharyngeal carcinoma biopsies. Cancer Res. 2000;60(19):5584–5588. | |
Stevens SJ, Verkuijlen SA, Hariwiyanto B, et al. Noninvasive diagnosis of nasopharyngeal carcinoma: nasopharyngeal brushings reveal high Epstein-Barr virus DNA load and carcinoma-specific viral BARF1 mRNA. Int J Cancer. 2006;119(3):608–614. | |
Cosmopoulos K, Pegtel M, Hawkins J, et al. Comprehensive profiling of Epstein-Barr virus microRNAs in nasopharyngeal carcinoma. J Virol. 2009;83(5):2357–2367. | |
Barth S, Pfuhl T, Mamiani A, et al. Epstein-Barr virus-encoded microRNA miR-BART2 down-regulates the viral DNA polymerase BALF5. Nucleic Acids Res. 2008;36(2):666–675. | |
Lo AK, To KF, Lo KW, et al. Modulation of LMP1 protein expression by EBV-encoded microRNAs. Proc Natl Acad Sci U S A. 2007;104(41):16164–16169. | |
Choy EY, Siu KL, Kok KH, et al. An Epstein-Barr virus-encoded microRNA targets PUMA to promote host cell survival. J Exp Med. 2008;205(11):2551–2560. | |
Barth S, Meister G, Grässer FA. EBV-encoded miRNAs. Biochim Biophys Acta. 2011;1809(11–12):631–640. | |
Israel BF, Kenney SC. EBV lytic infection. In: Robertson ES, editor. Epstein-Barr Virus. Philadelphia, PA: Caister Academic Press; 2011:571–611. | |
Kenney SC, Mertz JE. Regulation of the latent-lytic switch in Epstein-Barr virus. Semin Cancer Biol. Epub January 20, 2014. | |
Stevens SJ, Zwaan CM, Verkuijlen SA, Middeldorp JM. Epstein-Barr virus (EBV) serology for predicting distant metastases in a white juvenile patient with nasopharyngeal carcinoma and no clinical response to EBV lytic induction therapy. Head Neck. 2006;28(11):1040–1045. | |
Fachiroh J, Schouten T, Hariwiyanto B, et al. Molecular diversity of Epstein-Barr virus IgG and IgA antibody responses in nasopharyngeal carcinoma: a comparison of Indonesian, Chinese, and European subjects. J Infect Dis. 2004;190(1):53–62. | |
Ji MF, Wang DK, Yu YL, et al. Sustained elevation of Epstein-Barr virus antibody levels preceding clinical onset of nasopharyngeal carcinoma. Br J Cancer. 2007;96(4):623–630. | |
Cao SM, Liu Z, Jia WH, et al. Fluctuations of Epstein-Barr virus serological antibodies and risk for nasopharyngeal carcinoma: a prospective screening study with a 20-year follow-up. PLoS One. 2011;6(4):e19100. | |
Gan YJ, Chodosh J, Morgan A, Sixbey JW. Epithelial cell polarization is a determinant in the infectious outcome of immunoglobulin A-mediated entry by Epstein-Barr virus. J Virol. 1997;71(1):519–526. | |
Bose S, Yap LF, Fung M, et al. The ATM tumour suppressor gene is down-regulated in EBV-associated nasopharyngeal carcinoma. J Pathol. 2009;217(3):345–352. | |
Sivachandran N, Cao JY, Frappier L. Epstein-Barr virus nuclear antigen 1 hijacks the host kinase CK2 to disrupt PML nuclear bodies. J Virol. 2010;84(21):11113–11123. | |
Huang SY, Fang CY, Tsai CH, et al. N-methyl-N′-nitro-N-nitrosoguanidine induces and cooperates with 12-O-tetradecanoylphorbol-1,3-acetate/sodium butyrate to enhance Epstein-Barr virus reactivation and genome instability in nasopharyngeal carcinoma cells. Chem Biol Interact. 2010;188(3):623–634. | |
de Sanjosé S, Bosch R, Schouten T, et al. Epstein-Barr virus infection and risk of lymphoma: immunoblot analysis of antibody responses against EBV-related proteins in a large series of lymphoma subjects and matched controls. Int J Cancer. 2007;121(8):1806–1812. | |
Ressing ME, Horst D, Griffin BD, et al. Epstein-Barr virus evasion of CD8(+) and CD4(+) T cell immunity via concerted actions of multiple gene products. Semin Cancer Biol. 2008;18(6):397–408. | |
Merlo A, Turrini R, Dolcetti R, et al. The interplay between Epstein-Barr virus and the immune system: a rationale for adoptive cell therapy of EBV-related disorders. Haematologica. 2010;95(10):1769–1777. | |
Middeldorp JM, Herbrink P. Epstein-Barr virus specific marker molecules for early diagnosis of infectious mononucleosis. J Virol Methods. 1988;21(1–4):133–146. | |
Callan MF, Steven N, Krausa P, et al. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nat Med. 1996; 2(8):906–911. | |
Callan MF, Tan L, Annels N, et al. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus In vivo. J Exp Med. 1998;187(9):1395–1402. | |
Yates JL, Warren N, Sugden B. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature. 1985;313(6005):812–815. | |
Tellam J, Connolly G, Green KJ, et al. Endogenous presentation of CD8+ T cell epitopes from Epstein-Barr virus-encoded nuclear antigen 1. J Exp Med. 2004;199(10):1421–1431. | |
Voo KS, Fu T, Wang HY, et al. Evidence for the presentation of major histocompatibility complex class I-restricted Epstein-Barr virus nuclear antigen 1 peptides to CD8+ T lymphocytes. J Exp Med. 2004;199(4):459–470. | |
Levitskaya J, Sharipo A, Leonchiks A, Ciechanover A, Masucci MG. Inhibition of ubiquitin/proteasome-dependent protein degradation by the Gly-Ala repeat domain of the Epstein-Barr virus nuclear antigen 1. Proc Natl Acad Sci U S A. 1997;94(23):12616–12621. | |
Klibi J, Niki T, Riedel A, et al. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood. 2009;113(9):1957–1966. | |
Keryer-Bibens C, Pioche-Durieu C, Villemant C, et al. Exosomes released by EBV-infected nasopharyngeal carcinoma cells convey the viral latent membrane protein 1 and the immunomodulatory protein galectin 9. BMC Cancer. 2006;6:283. | |
Cohen JI, Lekstrom K. Epstein-Barr virus BARF1 protein is dispensable for B-cell transformation and inhibits alpha interferon secretion from mononuclear cells. J Virol. 1999;73(9):7627–7632. | |
Huang YT, Sheen TS, Chen CL, et al. Profile of cytokine expression in nasopharyngeal carcinomas: a distinct expression of interleukin 1 in tumor and CD4+ T cells. Cancer Res. 1999;59(7):1599–1605. | |
Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1(1):11–22. | |
Chan KH, Gu YL, Ng F, et al. EBV specific antibody-based and DNA-based assays in serologic diagnosis of nasopharyngeal carcinoma. Int J Cancer. 2003;105(5):706–709. | |
Fachiroh J, Paramita DK, Hariwiyanto B, et al. Single-assay combination of Epstein-Barr Virus (EBV) EBNA1- and viral capsid antigen-p18-derived synthetic peptides for measuring anti-EBV immunoglobulin G (IgG) and IgA antibody levels in sera from nasopharyngeal carcinoma patients: options for field screening. J Clin Microbiol. 2006;44(4):1459–1467. | |
Hutajulu SH, Ng N, Jati BR, et al. Seroreactivity against Epstein-Barr virus (EBV) among first-degree relatives of sporadic EBV-associated nasopharyngeal carcinoma in Indonesia. J Med Virol. 2012;84(5):768–776. | |
Liu Z, Ji MF, Huang QH, et al. Two Epstein-Barr virus-related serologic antibody tests in nasopharyngeal carcinoma screening: results from the initial phase of a cluster randomized controlled trial in Southern China. Am J Epidemiol. 2013;177(3):242–250. | |
Meij P, Vervoort MB, Bloemena E, et al. Antibody responses to Epstein-Barr virus-encoded latent membrane protein-1 (LMP1) and expression of LMP1 in juvenile Hodgkin’s disease. J Med Virol. 2002;68(3):370–377. | |
Lennette ET, Winberg G, Yadav M, Enblad G, Klein G. Antibodies to LMP2A/2B in EBV-carrying malignancies. Eur J Cancer. 1995; 31A(11):1875–1878. | |
Tanner JE, Wei MX, Alfieri C, et al. Antibody and antibody-dependent cellular cytotoxicity responses against the BamHI A rightward open-reading frame-1 protein of Epstein-Barr virus (EBV) in EBV-associated disorders. J Infect Dis. 1997;175(1):38–46. | |
Hoebe EK, Hutajulu SH, van Beek J, et al. Purified hexameric Epstein-Barr virus-encoded BARF1 protein for measuring anti-BARF1 antibody responses in nasopharyngeal carcinoma patients. Clin Vaccine Immunol. 2011;18(2):298–304. | |
Croft NP, Shannon-Lowe C, Bell AI, et al. Stage-specific inhibition of MHC class I presentation by the Epstein-Barr virus BNLF2a protein during virus lytic cycle. PLoS Pathog. 2009;5(6):e1000490. | |
Rowe M, Glaunsinger B, van Leeuwen D, et al. Host shutoff during productive Epstein-Barr virus infection is mediated by BGLF5 and may contribute to immune evasion. Proc Natl Acad Sci U S A. 2007;104(9):3366–3371. | |
Zuo J, Quinn LL, Tamblyn J, et al. The Epstein-Barr virus-encoded BILF1 protein modulates immune recognition of endogenously processed antigen by targeting major histocompatibility complex class I molecules trafficking on both the exocytic and endocytic pathways. J Virol. 2011;85(4):1604–1614. | |
Vicari AP, Trinchieri G. Interleukin-10 in viral diseases and cancer: exiting the labyrinth? Immunol Rev. 2004;202:223–236. | |
Li J, Zeng XH, Mo HY, et al. Functional inactivation of EBV-specific T-lymphocytes in nasopharyngeal carcinoma: implications for tumor immunotherapy. PLoS One. 2007;2(11):e1122. | |
Oudejans JJ, Harijadi H, Kummer JA, et al. High numbers of granzyme B/CD8-positive tumour-infiltrating lymphocytes in nasopharyngeal carcinoma biopsies predict rapid fatal outcome in patients treated with curative intent. J Pathol. 2002;198(4):468–475. | |
Oudejans JJ, Harijadi A, Cillessen SA, et al. Absence of caspase 3 activation in neoplastic cells of nasopharyngeal carcinoma biopsies predicts rapid fatal outcome. Mod Pathol. 2005;18(7):877–885. | |
Ogino T, Moriai S, Ishida Y, et al. Association of immunoescape mechanisms with Epstein-Barr virus infection in nasopharyngeal carcinoma. Int J Cancer. 2007;120(11):2401–2410. | |
Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res. 1998;72:141–196. | |
Takacs M, Banati F, Koroknai A, et al. Epigenetic regulation of latent Epstein-Barr virus promoters. Biochim Biophys Acta. 2010;1799(3–4):228–235. | |
Tao Q, Chan AT. Nasopharyngeal carcinoma: molecular pathogenesis and therapeutic developments. Expert Rev Mol Med. 2007;9(12):1–24. | |
Woisetschlaeger M, Yandava CN, Furmanski LA, Strominger JL, Speck SH. Promoter switching in Epstein-Barr virus during the initial stages of infection of B lymphocytes. Proc Natl Acad Sci U S A. 1990;87(5):1725–1729. | |
Ruf IK, Moghaddam A, Wang F, Sample J. Mechanisms that regulate Epstein-Barr virus EBNA-1 gene transcription during restricted latency are conserved among lymphocryptoviruses of Old World primates. J Virol. 1999;73(3):1980–1989. | |
Tao Q, Robertson KD. Stealth technology: how Epstein-Barr virus utilizes DNA methylation to cloak itself from immune detection. Clin Immunol. 2003;109(1):53–63. | |
Spruck CH, Tsai YC, Huang DP, et al. Absence of p53 gene mutations in primary nasopharyngeal carcinomas. Cancer Res. 1992;52(17):4787–4790. | |
Sun Y, Hegamyer G, Colburn NH. Nasopharyngeal carcinoma shows no detectable retinoblastoma susceptibility gene alterations. Oncogene. 1993;8(3):791–795. | |
Fries KL, Miller WE, Raab-Traub N. Epstein-Barr virus latent membrane protein 1 blocks p53-mediated apoptosis through the induction of the A20 gene. J Virol. 1996;70(12):8653–8659. | |
Lo KW, Huang DP, Lau KM. p16 gene alterations in nasopharyngeal carcinoma. Cancer Res. 1995;55(10):2039–2043. | |
Lo KW, Huang DP. Genetic and epigenetic changes in nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12(6):451–462. | |
Li LL, Shu XS, Wang ZH, Cao Y, Tao Q. Epigenetic disruption of cell signaling in nasopharyngeal carcinoma. Chin J Cancer. 2011;30(4):231–239. | |
Niller HH, Wolf H, Minarovits J. Epigenetic dysregulation of the host cell genome in Epstein-Barr virus-associated neoplasia. Semin Cancer Biol. 2009;19(3):158–164. | |
Kwong J, Lo KW, To KF, Teo PM, Johnson PJ, Huang DP. Promoter hypermethylation of multiple genes in nasopharyngeal carcinoma. Clin Cancer Res. 2002;8(1):131–137. | |
Zhou L, Jiang W, Ren C, et al. Frequent hypermethylation of RASSF1A and TSLC1, and high viral load of Epstein-Barr Virus DNA in nasopharyngeal carcinoma and matched tumor-adjacent tissues. Neoplasia. 2005;7(9):809–815. | |
Hutajulu SH, Indrasari SR, Indrawati LP, et al. Epigenetic markers for early detection of nasopharyngeal carcinoma in a high risk population. Mol Cancer. 2011;10:48. | |
Li HP, Leu YW, Chang YS. Epigenetic changes in virus-associated human cancers. Cell Res. 2005;15(4):262–271. | |
Tsai CL, Li HP, Lu YJ, et al. Activation of DNA methyltransferase 1 by EBV LMP1 Involves c-Jun NH(2)-terminal kinase signaling. Cancer Res. 2006;66(24):11668–11676. | |
Seo SY, Kim EO, Jang KL. Epstein-Barr virus latent membrane protein 1 suppresses the growth-inhibitory effect of retinoic acid by inhibiting retinoic acid receptor-beta2 expression via DNA methylation. Cancer Lett. 2008;270(1):66–76. | |
Lee H, Seo SY, Tiwari I, Jang KL. Epstein-Barr Virus latent membrane protein 1 overcomes all-trans retinoic acid-induced apoptosis by inhibiting retinoic acid receptor-β2 expression. Biochem Biophys Res Commun. 2012;423(2):313–318. | |
Mohammad HP, Baylin SB. Linking cell signaling and the epigenetic machinery. Nat Biotechnol. 2010;28(10):1033–1038. | |
Song LB, Li J, Liao WT, et al. The polycomb group protein Bmi-1 represses the tumor suppressor PTEN and induces epithelial-mesenchymal transition in human nasopharyngeal epithelial cells. J Clin Invest. 2009;119(12):3626–3636. | |
Rottey S, Madani I, Deron P, Van Belle S. Modern treatment for nasopharyngeal carcinoma: current status and prospects. Curr Opin Oncol. 2011;23(3):254–258. | |
Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16(4):1310–1317. | |
Wee J, Tan EH, Tai BC, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol. 2005;23(27):6730–6738. | |
Lee AW, Lin JC, Ng WT. Current management of nasopharyngeal cancer. Semin Radiat Oncol. 2012;22(3):233–244. | |
Lee AW, Sze WM, Au JS, et al. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2005;61(4):1107–1116. | |
Bakst RL, Lee N, Pfister DG, et al. Hypofractionated dose-painting intensity modulated radiation therapy with chemotherapy for nasopharyngeal carcinoma: a prospective trial. Int J Radiat Oncol Biol Phys. 2011;80(1):148–153. | |
Tham IW, Hee SW, Yeo RM, et al. Treatment of nasopharyngeal carcinoma using intensity-modulated radiotherapy-the national cancer centre singapore experience. Int J Radiat Oncol Biol Phys. 2009;75(5):1481–1486. | |
Spratt DE, Lee N. Current and emerging treatment options for nasopharyngeal carcinoma. Onco Targets Ther. 2012;5:297–308. | |
Leung TW, Tung SY, Sze WK, Sze WM, Wong VY, O SK. Salvage brachytherapy for patients with locally persistent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2000;47(2):405–412. | |
Wildeman MA, Nyst HJ, Karakullukcu B, Tan BI. Photodynamic therapy in the therapy for recurrent/persistent nasopharyngeal cancer. Head Neck Oncol. 2009;1:40. | |
Leung TW, Wong VY, Tung SY. Stereotactic radiotherapy for locally recurrent nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2009;75(3):734–741. | |
Wei WI, Chan JY, Ng RW, Ho WK. Surgical salvage of persistent or recurrent nasopharyngeal carcinoma with maxillary swing approach – Critical appraisal after 2 decades. Head Neck. 2011;33(7):969–975. | |
Zhang L, Zhu YX, Wang Y, Huang CP, Wu Y, Ji QH. Salvage surgery for neck residue or recurrence of nasopharyngeal carcinoma: a 10-year experience. Ann Surg Oncol. 2011;18(1):233–238. | |
Zhang L, Chen QY, Liu H, Tang LQ, Mai HQ. Emerging treatment options for nasopharyngeal carcinoma. Drug Des Devel Ther. 2013;7:37–52. | |
Xu T, Tang J, Gu M, Liu L, Wei W, Yang H. Recurrent nasopharyngeal carcinoma: a clinical dilemma and challenge. Curr Oncol. 2013;20(5):e406–e419. | |
Ali H, al-Sarraf M. Chemotherapy in advanced nasopharyngeal cancer. Oncology (Williston Park). 2000;14(8):1223–1230; discussion 1232–1227, 1239–1242. | |
Chen MY, Jiang R, Guo L, et al. Locoregional radiotherapy in patients with distant metastases of nasopharyngeal carcinoma at diagnosis. Chin J Cancer. 2013;32(11):604–613. | |
Bensouda Y, Kaikani W, Ahbeddou N, et al. Treatment for metastatic nasopharyngeal carcinoma. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128(2):79–85. | |
Leong SS, Wee J, Tay MH, et al. Paclitaxel, carboplatin, and gemcitabine in metastatic nasopharyngeal carcinoma: a Phase II trial using a triplet combination. Cancer. 2005;103(3):569–575. | |
Chua DT, Wei WI, Wong MP, Sham JS, Nicholls J, Au GK. Phase II study of gefitinib for the treatment of recurrent and metastatic nasopharyngeal carcinoma. Head Neck. 2008;30(7):863–867. | |
Ma B, Hui EP, King A, et al. A phase II study of patients with metastatic or locoregionally recurrent nasopharyngeal carcinoma and evaluation of plasma Epstein-Barr virus DNA as a biomarker of efficacy. Cancer Chemother Pharmacol. 2008;62(1):59–64. | |
Lee NY, Zhang Q, Pfister DG, et al. Addition of bevacizumab to standard chemoradiation for locoregionally advanced nasopharyngeal carcinoma (RTOG 0615): a phase 2 multi-institutional trial. Lancet Oncol. 2012;13(2):172–180. | |
Ma BB, Lui VW, Hui EP, et al. The activity of mTOR inhibitor RAD001 (everolimus) in nasopharyngeal carcinoma and cisplatin-resistant cell lines. Invest New Drugs. 2010;28(4):413–420. | |
Hong B, Lui VW, Hashiguchi M, Hui EP, Chan AT. Targeting tumor hypoxia in nasopharyngeal carcinoma. Head Neck. 2013;35(1):133–145. | |
Chua DT, Nicholls JM, Sham JS, Au GK. Prognostic value of epidermal growth factor receptor expression in patients with advanced stage nasopharyngeal carcinoma treated with induction chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59(1):11–20. | |
Chan AT, Hsu MM, Goh BC, et al. Multicenter, phase II study of cetuximab in combination with carboplatin in patients with recurrent or metastatic nasopharyngeal carcinoma. J Clin Oncol. 2005;23(15):3568–3576. | |
Chan SL, Ma BB. Novel systemic therapeutic for nasopharyngeal carcinoma. Expert Opin Ther Targets. 2012;16(Suppl 1):S63–S68. | |
Guang-Wu H, Sunagawa M, Jie-En L, et al. The relationship between microvessel density, the expression of vascular endothelial growth factor (VEGF), and the extension of nasopharyngeal carcinoma. Laryngoscope. 2000;110(12):2066–2069. | |
Hui EP, Ma BB, King AD, et al. Hemorrhagic complications in a phase II study of sunitinib in patients of nasopharyngeal carcinoma who has previously received high-dose radiation. Ann Oncol. 2011;22(6):1280–1287. | |
Elser C, Siu LL, Winquist E, et al. Phase II trial of sorafenib in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or nasopharyngeal carcinoma. J Clin Oncol. 2007;25(24):3766–3773. | |
Shah KM, Young LS. Epstein-Barr virus and carcinogenesis: beyond Burkitt’s lymphoma. Clin Microbiol Infect. 2009;15(11):982–988. | |
Caponigro F, Longo F, Ionna F, Perri F. Treatment approaches to nasopharyngeal carcinoma: a review. Anticancer Drugs. 2010;21(5):471–477. | |
Tsang J, Lee VH, Kwong DL. Novel therapy for nasopharyngeal carcinoma – Where are we. Oral Oncol. Epub January 22, 2014. | |
Khanna R, Busson P, Burrows SR, et al. Molecular characterization of antigen-processing function in nasopharyngeal carcinoma (NPC): evidence for efficient presentation of Epstein-Barr virus cytotoxic T-cell epitopes by NPC cells. Cancer Res. 1998;58(2):310–314. | |
Khanna R, Moss D, Gandhi M. Technology insight: Applications of emerging immunotherapeutic strategies for Epstein-Barr virus-associated malignancies. Nat Clin Pract Oncol. 2005;2(3):138–149. | |
Yao Y, Minter HA, Chen X, Reynolds GM, Bromley M, Arrand JR. Heterogeneity of HLA and EBER expression in Epstein-Barr virus-associated nasopharyngeal carcinoma. Int J Cancer. 2000;88(6):949–955. | |
Zhen Y, Liu Z, Yang H, et al. Tumor suppressor PDCD4 modulates miR-184-mediated direct suppression of C-MYC and BCL2 blocking cell growth and survival in nasopharyngeal carcinoma. Cell Death Dis. 2013;4:e872. | |
Kontos CK, Fendri A, Khabir A, Mokdad-Gargouri R, Scorilas A. Quantitative expression analysis and prognostic significance of the BCL2-associated X gene in nasopharyngeal carcinoma: a retrospective cohort study. BMC Cancer. 2013;13:293. | |
De Paoli P. Novel virally targeted therapies of EBV-associated tumors. Curr Cancer Drug Targets. 2008;8(7):591–596. | |
Chua D, Huang J, Zheng B, et al. Adoptive transfer of autologous Epstein-Barr virus-specific cytotoxic T cells for nasopharyngeal carcinoma. Int J Cancer. 2001;94(1):73–80. | |
Comoli P, Pedrazzoli P, Maccario R, et al. Cell therapy of stage IV nasopharyngeal carcinoma with autologous Epstein-Barr virus-targeted cytotoxic T lymphocytes. J Clin Oncol. 2005;23(35):8942–8949. | |
Straathof KC, Bollard CM, Popat U, et al. Treatment of nasopharyngeal carcinoma with Epstein-Barr virus – specific T lymphocytes. Blood. 2005;105(5):1898–1904. | |
Secondino S, Zecca M, Licitra L, et al. T-cell therapy for EBV-associated nasopharyngeal carcinoma: preparative lymphodepleting chemotherapy does not improve clinical results. Ann Oncol. 2012;23(2):435–441. | |
Merlo A, Turrini R, Dolcetti R, Zanovello P, Rosato A. Immunotherapy for EBV-associated malignancies. Int J Hematol. 2011;93(3):281–293. | |
Smith C, Tsang J, Beagley L, et al. Effective treatment of metastatic forms of Epstein-Barr virus-associated nasopharyngeal carcinoma with a novel adenovirus-based adoptive immunotherapy. Cancer Res. 2012;72(5):1116–1125. | |
Lutzky VP, Crooks P, Morrison L, et al. Cytotoxic T cell adoptive immunotherapy as a treatment for nasopharyngeal carcinoma. Clin Vaccine Immunol. 2014;21(2):256–259. | |
Chia WK, Teo M, Wang WW, et al. Adoptive T-cell transfer and chemotherapy in the first-line treatment of metastatic and/or locally recurrent nasopharyngeal carcinoma. Mol Ther. 2014;22(1):132–139. | |
Lin CL, Lo WF, Lee TH, et al. Immunization with Epstein-Barr Virus (EBV) peptide-pulsed dendritic cells induces functional CD8+ T-cell immunity and may lead to tumor regression in patients with EBV-positive nasopharyngeal carcinoma. Cancer Res. 2002;62(23):6952–6958. | |
Chia WK, Wang WW, Teo M, et al. A phase II study evaluating the safety and efficacy of an adenovirus-ΔLMP1-LMP2 transduced dendritic cell vaccine in patients with advanced metastatic nasopharyngeal carcinoma. Ann Oncol. 2012;23(4):997–1005. | |
Li F, Song D, Lu Y, Zhu H, Chen Z, He X. Delayed-type hypersensitivity (DTH) immune response related with EBV-DNA in nasopharyngeal carcinoma treated with autologous dendritic cell vaccination after radiotherapy. J Immunother. 2013;36(3):208–214. | |
Duraiswamy J, Sherritt M, Thomson S, et al. Therapeutic LMP1 polyepitope vaccine for EBV-associated Hodgkin disease and nasopharyngeal carcinoma. Blood. 2003;101(8):3150–3156. | |
Lutzky VP, Corban M, Heslop L, et al. Novel approach to the formulation of an Epstein-Barr virus antigen-based nasopharyngeal carcinoma vaccine. J Virol. 2010;84(1):407–417. | |
Taylor GS, Haigh TA, Gudgeon NH, et al. Dual stimulation of Epstein-Barr Virus (EBV)-specific CD4+- and CD8+-T-cell responses by a chimeric antigen construct: potential therapeutic vaccine for EBV-positive nasopharyngeal carcinoma. J Virol. 2004;78(2):768–778. | |
Hui EP, Taylor GS, Jia H, et al. Phase I trial of recombinant modified vaccinia ankara encoding Epstein-Barr viral tumor antigens in nasopharyngeal carcinoma patients. Cancer Res. 2013;73(6):1676–1688. | |
Paramita DK, Fatmawati C, Juwana H, et al. Humoral immune responses to Epstein-Barr virus encoded tumor associated proteins and their putative extracellular domains in nasopharyngeal carcinoma patients and regional controls. J Med Virol. 2011;83(4):665–678. | |
Chen R, Zhang D, Mao Y, et al. A human Fab-based immunoconjugate specific for the LMP1 extracellular domain inhibits nasopharyngeal carcinoma growth in vitro and in vivo. Mol Cancer Ther. 2012;11(3):594–603. | |
Delbende C, Verwaerde C, Mougel A, Tranchand Bunel D. Induction of therapeutic antibodies by vaccination against external loops of tumor-associated viral latent membrane protein. J Virol. 2009;83(22):11734–11745. | |
Suzuki H, Gabrielson E, Chen W, et al. A genomic screen for genes upregulated by demethylation and histone deacetylase inhibition in human colorectal cancer. Nat Genet. 2002;31(2):141–149. | |
Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res. 2007;5(10):981–989. | |
Cheng JC, Yoo CB, Weisenberger DJ, et al. Preferential response of cancer cells to zebularine. Cancer Cell. 2004;6(2):151–158. | |
Abele R, Clavel M, Dodion P, et al. The EORTC Early Clinical Trials Cooperative Group experience with 5-aza-2′-deoxycytidine (NSC 127716) in patients with colo-rectal, head and neck, renal carcinomas and malignant melanomas. Eur J Cancer Clin Oncol. 1987;23(12):1921–1924. | |
van Groeningen CJ, Leyva A, O’Brien AM, Gall HE, Pinedo HM. Phase I and pharmacokinetic study of 5-aza-2′-deoxycytidine (NSC 127716) in cancer patients. Cancer Res. 1986;46(9):4831–4836. | |
Chan AT, Tao Q, Robertson KD, et al. Azacitidine induces demethylation of the Epstein-Barr virus genome in tumors. J Clin Oncol. 2004;22(8):1373–1381. | |
Lee SP, Chan AT, Cheung ST, et al. CTL control of EBV in nasopharyngeal carcinoma (NPC): EBV-specific CTL responses in the blood and tumors of NPC patients and the antigen-processing function of the tumor cells. J Immunol. 2000;165(1):573–582. | |
Feng WH, Israel B, Raab-Traub N, Busson P, Kenney SC. Chemotherapy induces lytic EBV replication and confers ganciclovir susceptibility to EBV-positive epithelial cell tumors. Cancer Res. 2002;62(6):1920–1926. | |
Feng WH, Hong G, Delecluse HJ, Kenney SC. Lytic induction therapy for Epstein-Barr virus-positive B-cell lymphomas. J Virol. 2004;78(4):1893–1902. | |
Feng WH, Kenney SC. Valproic acid enhances the efficacy of chemotherapy in EBV-positive tumors by increasing lytic viral gene expression. Cancer Res. 2006;66(17):8762–8769. | |
Hui KF, Ho DN, Tsang CM, Middeldorp JM, Tsao GS, Chiang AK. Activation of lytic cycle of Epstein-Barr virus by suberoylanilide hydroxamic acid leads to apoptosis and tumor growth suppression of nasopharyngeal carcinoma. Int J Cancer. 2012;131(8):1930–1940. | |
Ben-Sasson SA, Klein G. Activation of the Epstein-Barr virus genome by 5-aza-cytidine in latently infected human lymphoid lines. Int J Cancer. 1981;28(2):131–135. | |
Datta AK, Colby BM, Shaw JE, Pagano JS. Acyclovir inhibition of Epstein-Barr virus replication. Proc Natl Acad Sci U S A. 1980;77(9):5163–5166. | |
Meerbach A, Holý A, Wutzler P, De Clercq E, Neyts J. Inhibitory effects of novel nucleoside and nucleotide analogues on Epstein-Barr virus replication. Antivir Chem Chemother. 1998;9(3):275–282. | |
Moore SM, Cannon JS, Tanhehco YC, Hamzeh FM, Ambinder RF. Induction of Epstein-Barr virus kinases to sensitize tumor cells to nucleoside analogues. Antimicrob Agents Chemother. 2001;45(7):2082–2091. | |
Kenney S, Theodore E. Woodward Award: development of novel, EBV-targeted therapies for EBV-positive tumors. Trans Am Clin Climatol Assoc. 2006;117:55–73; discussion 73–74. | |
Mentzer SJ, Fingeroth J, Reilly JJ, Perrine SP, Faller DV. Arginine butyrate-induced susceptibility to ganciclovir in an Epstein-Barr-virus-associated lymphoma. Blood Cells Mol Dis. 1998;24(2):114–123. | |
Jones K, Nourse J, Corbett G, Gandhi MK. Sodium valproate in combination with ganciclovir induces lysis of EBV-infected lymphoma cells without impairing EBV-specific T-cell immunity. Int J Lab Hematol. 2010;32(1 Pt 1):e169–e174. | |
Wildeman MA, Novalic Z, Verkuijlen SA, et al. Cytolytic virus activation therapy for Epstein-Barr virus-driven tumors. Clin Cancer Res. 2012;18(18):5061–5070. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.