Back to Journals » Medical Devices: Evidence and Research » Volume 8
The VariLift® Interbody Fusion System: expandable, standalone interbody fusion
Authors Emstad E, Cardenas del Monaco D, Fielding L, Block J
Received 17 March 2015
Accepted for publication 10 April 2015
Published 26 May 2015 Volume 2015:8 Pages 219—230
DOI https://doi.org/10.2147/MDER.S84715
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Erik Emstad,1 Diana Cardenas del Monaco,1 Louis C Fielding,2 Jon E Block2
1Wenzel Spine, Inc., Austin, TX, 2The Jon Block Group, San Francisco, CA, USA
Abstract: Intervertebral fusion cages have been in clinical use since the 1990s. Cages offer the benefits of bone graft containment, restored intervertebral and foraminal height, and a more repeatable, stable procedure compared to interbody fusion with graft material alone. Due to concerns regarding postoperative stability, loss of lordosis, and subsidence or migration of the implant, interbody cages are commonly used with supplemental fixation such as pedicle screw systems or anterior plates. While providing additional stability, supplemental fixation techniques increase operative time, exposure, cost, and morbidity. The VariLift® Interbody Fusion System (VariLift® system) has been developed as a standalone solution to provide the benefits of intervertebral fusion cages without the requirement of supplemental fixation. The VariLift® system, FDA-cleared for standalone use in both the cervical and lumbar spine, is implanted in a minimal profile and then expanded in situ to provide segmental stability, restored lordosis, and a large graft chamber. Preclinical testing and analyses have found that the VariLift® System is durable, and reduces stresses that may contribute to subsidence and migration of other standalone interbody cages. Fifteen years of clinical development with the VariLift® system have demonstrated positive clinical outcomes, continued patient maintenance of segmental stability and lordosis, and no evidence of implant migration. The purpose of this report is to describe the VariLift® system, including implant characteristics, principles of operation, indications for use, patient selection criteria, surgical technique, postoperative care, preclinical testing, and clinical experience. The VariLift® System represents an improved surgical option for a stable interbody fusion without requiring supplemental fixation.
Keywords: TLIF, PLIF, ACDF, standalone cage
Introduction
Fusion of the vertebral bodies in both the lumbar and cervical spine was described by Cloward in the 1950s1,2 and remains important in the treatment of many spinal pathologies. The intervertebral fusion cage was pioneered in the 1970s and 1980s by Bagby to treat cervical nerve root compression in horses.3 The Bagby Basket, as it was called, was later adapted for humans as the Bagby and Kuslich (BAK) intervertebral fusion cage as the first device of this category approved by the United States Food and Drug Administration (FDA).4 Clinical trials of these devices revealed good fusion rates and clinical outcomes, as well as a more cost-effective procedure compared to anteroposterior fusion.5–7 Based on the successful clinical history of these devices, in 2007 the US FDA reclassified intervertebral body fusion devices as Class II devices.8
Over the past decade, cage designs have proliferated and become more widely adopted in both lumbar and cervical spine surgery. The recent trend has been to supplement cages with transpedicular instrumentation in transforaminal and posterior lumbar interbody fusion (TLIF, PLIF) for added biomechanical stability.9 While such supplemental fixation provides a biomechanically stable construct in benchtop testing, pedicle screw placement is associated with significant morbidities, including increased operative time, blood loss, reoperation rate, and significant risk of nerve root injury.10 Violation of the facet joint at the cephalad segment is also common during pedicle screw placement,11–13 and may be associated with symptomatic adjacent segment degeneration (ASD), a common sequela of instrumented fusion.14 In the cervical spine, anterior cervical discectomy and fusion (ACDF) utilizing autologous iliac crest bone graft (ICBG) remains a common procedure for interbody fusion due to the high fusion rate achieved with this technique; the addition of an interbody cage to ACDF has demonstrated lower complication rates compared to ACDF with ICBG.15,16 Anterior cervical plates are another common device used in cervical fusion surgery; however, these have been shown to yield a higher rate of dysphagia compared to interbody cages.17
Despite the benefits and demonstrated clinical success of interbody fusion cages, challenges remain that are inherently associated with many of the commercially available designs. Due to the endplate decortication and impaction required during the implantation procedure, segmental subsidence and implant migration are common occurrences.18–20 Biomechanical studies continue to show higher segmental stiffness when interbody cages are augmented with supplemental instrumentation,21 with the result that additional instrumentation is commonly implanted despite the costs and potential complications. Interbody cages have also been reported to obscure the view of the fusion.20,22
The VariLift® Interbody Fusion System (Wenzel Spine, Austin, TX, USA), a standalone, expandable fusion cage (SAEFC) has been developed to leverage the benefits, safety, and clinical success that interbody cages have demonstrated over the preceding decades, while providing improved stability, preservation of lordosis, and resistance to subsidence and migration that has been seen with earlier interbody cage designs. The purpose of this report is to describe the SAEFC, including implant characteristics, principles of operation, indications for use, patient selection criteria, surgical technique, postoperative care, preclinical testing, and clinical experience. The SAEFC represents a proven surgical option for stable, standalone, minimally invasive interbody fusion.
VariLift® Interbody Fusion System
Prosthesis characteristics
To draw upon both the benefits and clinical success that interbody cages have demonstrated and further address some of the shortcomings of previous designs, the following design objectives were established for the SAEFC:
- Support biomechanical loads without supplemental fixation
- Provide immediate stability
- Resist subsidence and migration
- Restore anatomic alignment
- Minimize exposure and nerve retraction
- Preserve native anatomy
- Contain substantial graft volume
- Provide view of fusion
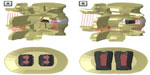
The VariLift® Interbody Fusion System (Figure 1) was designed to fulfill the design objectives for successful standalone interbody fusion. The system is fabricated from titanium alloy (Ti6Al4V), which has an extensive history of use in spinal implants. The device is initially cylindrical in shape, with ridges that allow for advancement into the intervertebral space without impaction, preserving the integrity of the vertebral endplates. A sliding expansion plate expands the device in situ, locking the device in place while preserving the cortical vertebral endplates. In addition to endplate preservation, this technique provides immediate postoperative stability23 and a solid foundation for device fixation and maintenance of segmental lordosis due to the wedge shape of the deployed implant. Once deployed, a large hollow inner chamber and wide fenestrations allow placement of local bone graft and ultimate growth of the intervertebral fusion throughout the implant and endplates. The device’s fenestrations allow for postoperative fusion assessment.
  | Figure 1 VariLift®-L (A) and VariLift®-C (B). |
Principles of operation
The SAEFC achieves fusion of the vertebral bodies through the distraction-compression method described by Bagby in the 1980s.3 The zero-insertion profile of the device allows for insertion without disruption of the cortical endplates. Once deployed, the SAEFC provides immediate stability and preservation of segmental lordosis. The large central graft chamber and fenestrations allow for incorporation of local bone graft into a biomechanically sound interbody arthrodesis.
Indications for use
Two models of SAEFC, VariLift® Lumbar Interbody Fusion System (VariLift®-L) and VariLift® Cervical Interbody Fusion System (VariLift®-C), are indicated for use in the lumbar and cervical spines, respectively. Both devices are intended for use with degenerative disk disease (DDD) and up to grade I spondylolisthesis. The lumbar product VariLift®-L may be implanted bilaterally via a PLIF approach or transversely via a TLIF approach at one or two contiguous levels. Importantly, both the VariLift®-L and VariLift®-C devices may be implanted with or without supplemental fixation. The indications for use of each device, as cleared by the US FDA, are provided in the following sub-sections.
VariLift®-L indications for use
VariLift®-L is indicated for intervertebral body fusion of the lumbar spine, from L2 to S1, in skeletally mature patients who have had 6 months of nonoperative treatment. The device is intended for use at either one level or two contiguous levels for the treatment of DDD with up to grade I spondylolisthesis. DDD is defined as back pain of discogenic origin with degeneration of the disk confirmed by history and radiographic studies.
VariLift®-L is designed to be implanted bilaterally via a posterior (PLIF) approach or as a single device via a transverse (TLIF) approach. VariLift®-L may be implanted with or without supplemental fixation and are intended for use with autograft to facilitate fusion.
VariLift®-C indications for use
VariLift®-C is indicated for use in skeletally mature patients with DDD of the cervical spine with accompanying radicular symptoms at one disk level. DDD is defined as discogenic pain with degeneration of the disk confirmed by patient history and radiographic studies. These DDD patients may have up to grade I spondylolisthesis or retrolisthesis at the involved level.
The Wenzel Spine VariLift®-C is used to facilitate intervertebral body fusion in the cervical spine and is placed in a unilateral or a bilateral fashion via an anterior approach at the C3 to C7 disk levels using autograft bone. The Wenzel Spine VariLift®-C may be used with or without supplemental fixation. Patients should have at least 6 weeks of nonoperative treatment prior to treatment with an intervertebral fusion device.
Patient selection criteria
Patients selected to receive SAEFC should conform to the indications for use stated above. As described further below, good clinical results have been obtained in patients with DDD, spondylolisthesis (grade I), failed back syndrome and disk herniation, with preoperative symptoms including chronic low back pain and/or radiculopathy refractory to nonoperative care.
A patient may have multiple pain generators due to advanced degeneration of the spine (eg, intervertebral disk, facets, or bony stenosis). These conditions may be present at the index level or adjacent levels. Careful review of the clinical record, including radiographic studies and applicable diagnostic tests, should be performed to make the appropriate diagnosis. Concomitant conditions may reduce the effectiveness of the surgery and this should be discussed with the patient.
Surgical technique
The surgical technique for SAEFC implantation is a simple, minimally invasive procedure, with only minor differences in technique between the PLIF, TLIF, and ACDF procedures. The key steps common to all the three procedures are:
- Decompression performed and disk space accessed via surgeon preferred, standard approach
- Disk removed in a standard fashion
- Endplates prepared for direct graft-to-bone contact
- Sizing drill or trial used to select device size and prepare the opening of the disk space, with careful attention to preserving the integrity of the endplates
- VariLift® device(s) rotated into place and then expanded
- Device(s) packed with morselized locally acquired bone graft
- End cap(s) threaded into place, securing the bone graft (VariLift®-L only).
Highlights of the three procedures are illustrated in Figures 2–4.
In all cases, preoperative radiography should be used to identify the level(s) to be fused, decompression techniques, and to provide an initial estimate of implant size. The patient is positioned appropriately for the specific procedure: prone or kneeling for PLIF, in flexion (local kyphosis) for TLIF, or supine with the neck slightly extended for the cervical procedure.
The implantation site is then accessed, decompression performed as required, and complete discectomy performed with removal of the cartilaginous tissue from the endplates using surgeon preferred tools such as rongeurs, curettes, and shaving spatulas. The PLIF approach typically involves bilateral laminotomies and medial facetectomies to expose the entire width between the pedicles, as well as foraminotomies along the nerve root trajectories. In the TLIF approach, the ipsilateral facet is removed to prepare the disk space through an annulotomy that preserves the annulus, and may be performed through either an open operative exposure or a minimally invasive approach using an operating microscope. A spatula spacer can be used to open the disk space, which is then maintained with the TLIF spacer provided with the TLIF instrument set.
A sizing tool is used to both select the appropriate device size and prepare the opening of the disk space, without damaging the cortical endplates. A sizing tool and Dura protector are used for the PLIF and TLIF procedures. With intraoperative fluoroscopy, sizing and disk space opening are easily performed while the nerve roots are protected. For the cervical model, sequentially sized taps are used for this step.
The selected device size is then loaded onto an insertion instrument. By rotating the insertion instrument clockwise, the SAEFC advances into the disk space without impaction. Lateral fluoroscopy is used to attain the desired implant depth and orientation. The implant is then expanded and packed with local autograft tissue obtained during surgical access. In lumbar procedures, an end cap is then installed to contain the packed graft material. If needed, the lumbar implants may be repositioned or removed by removing the end cap and graft, reducing (unexpanding) the implant, and repositioning or removing the implant using the insertion tools. Cervical implants cannot be reduced, but can be removed using a simple removal procedure.
Postoperative care
Postoperative care after SAEFC implantation includes normal precautions for lumbar and cervical fusion. Accepted surgical practices should be followed for postoperative care. The patient should be cautioned to govern his/her activities accordingly as the risk of implant failure increases with weight and activity levels of the patient. The surgeon should advise the patient to be careful not to place significant loads on the spine for the first 3 months after surgery. The surgeon may advise the patient to limit their activity or wear a brace. Careful management of the load will enable the fusion mass to heal and reduce the likelihood of nonunion. Radiographic confirmation of a mature fusion mass may be used as a guide in the lifting of these restrictions.
Preclinical testing
Mechanical performance testing
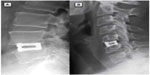
The VariLift® Interbody Fusion System has been subjected to an extensive battery of preclinical mechanical tests (Table 1). Testing conformed to standard specifications governed by ASTM International and recognized by the US FDA. Static tests included compression, compression/shear, expulsion, and subsidence testing. In all cases, the SAEFC withstood super-physiologic loading without failure. Dynamic fatigue tests included five-million cycles of compressive and torsional loading, all at super-physiologic loads. The test setups for static and dynamic axial compression testing and compressive shear testing are illustrated in Figure 5. All tests represented the worst-case implant configuration, ie, the smallest implant size. This battery of testing demonstrated that the VariLift® Interbody Fusion System can withstand the in vivo loading environment with minimal risk of in situ failure.
  | Table 1 Mechanical performance and reliability testing |
  | Figure 5 Mechanical test setups for (A) static and dynamic axial compression testing and (B) compressive shear testing. |
Finite element analysis
A finite element analysis (FEA) of the SAEFC device was conducted by a team at the University of Toledo (Toledo, OH, USA), led by Dr Vijay K Goel, distinguished university professor and expert in the field.23 The study utilized a validated model of the L4-L5 spinal segment and finite element models of the VariLift®-L and BAK cage for comparison to a traditional rigid cylindrical cage (Figure 6). The FEA study modeled a 400 N compressive follower preload and an 8 Nm bending moment to represent physiological loadings simulating flexion, extension, lateral bending, and axial rotation of the spinal segment. The simulation was run for these loading configurations with the VariLift®-L or the BAK implanted bilaterally through annulotomies in a PLIF application. Segmental rotation and forces on the vertebral endplates were analyzed for each of the simulated motions.
Compared to the BAK cages, the FEA simulation with the VariLift®-L showed greater reduction of motion in flexion, extension and axial rotation, and equivalent reduction in lateral bending. The total normal force on the vertebral endplate was equivalent for the two devices; as the VariLift®-L has a 62% larger area in contact with the endplate compared to the BAK, this translates to significantly lower tissue stresses that could lead to subsidence. The University of Toledo authors state that compared to other interbody cages, the VariLift-L device is better able to adjust to the lordotic curvature, improves load-sharing, and more effectively resists posterior migration toward the spinal canal, and ultimately conclude:
Biomechanically, the VariLift®-L interbody fusion device is a superior alternative compared to the traditional ALIF interbody fixation devices for fusion surgery of the lumbar spine segment.23
Clinical experience
Clinical follow-up data have been obtained for hundreds of patients treated with the VariLift® Interbody Fusion System. Here, we summarize three large retrospective studies of the lumbar and cervical VariLift® devices.
Retrospective series of 157 patients implanted with lumbar SAEFC
In 2002, Attia reported results for 157 consecutive patients treated with PLIF and VariLift®-L implantation.24,25 Of the 157 patients, 131 (83.4%) received standalone VariLift® devices, and 26 (16.6%) had supplementary posterior instrumentation due to spondylolisthesis, multi-level fusion, or concerns about stability due to wide laminectomy. Follow-up ranged from 12 months to 60 months. There were 140 patients (89.2%) with satisfactory outcomes, ten (6.4%) with fair outcomes, and seven (4.4%) patients who had poor outcomes. Fusion was deemed solid in 150 patients (95.5%). No patient’s symptoms worsened. Major complications noted were three cases of foot drop, one of which partially resolved and the other two completely resolved, as well as one case of unilateral thrombosis of the central retinal artery. There was no incidence of implant failure or migration. Lordosis of the fused segment was maintained at a mean 6.9°.
Retrospective study of 470 patients treated with lumbar SAEFC
From August 2003 to October 2009, 470 patients (209 male, 261 female) with a mean age of 57.6 years (range 19–86 years) were surgically treated for symptomatic disk herniation with instability, and/or DDD using the VariLift®-L device at one or two levels. The VariLift®-L device was implanted bilaterally in all patients at one level (298 patients, 63.4%) or two contiguous levels (172 patients, 36.6%). A total of 642 levels were treated, with the L4-L5 vertebral level accounting for nearly half of all implanted levels. The VariLift®-L was used standalone in 469 cases (99.8%). Additional supplemental fixation with pedicle screws was used in one single-level, L4-L5 case.
Patients were followed according to the surgeons’ typical clinical follow-up period. Patients were assessed preoperatively and postoperatively at approximately 2–4 weeks (n=455, 96.8%), 2–6 months (n=419, 89.7%), and 9–12 months or last follow-up (n=352, 74.9%). Average pain scores improved from 8.5±1.5 preoperatively to 0.8±1.5 postoperatively at the last follow-up. Additionally, of the 352 patients who had pain assessments 9 months or more following surgery, 334 (93.6%) had a clinically significant decrease from preoperative baseline of 3.8 points or more at their last recorded follow-up visit. Eighteen patients (3.8%) required reoperation following the original fusion procedure with 16 (88.9%) of these patients presenting with ASD. The mean time between surgery for those patients presenting with ASD was 2.7 years (range 0.9–5.3 years). Only two patients (<1%) of the 352 patients with 9- to 12-month follow-up required reoperation at the index level. One patient fell and started having additional back pain and the other patient required explantation of the VariLift®-L due to a deep disk space infection.
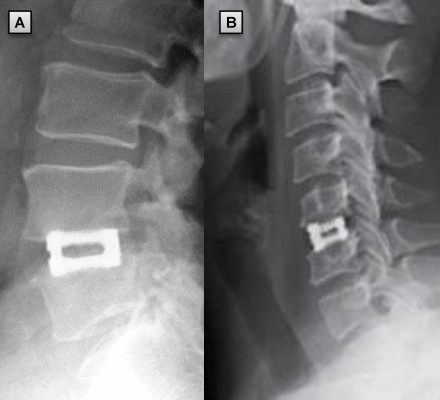
Postoperative radiographs were available for 281 patients (59.0%) at the 9-month follow-up or more (Figure 7). Fusion was achieved for 263 of these patients (93.6%). Radiographs at 6 months were available to measure fusion progression for 68 additional patients. It was determined that 62 (91.0%) of these patients showed early signs of fusion success. Radiographs were not available to assess fusion or the early signs of fusion for the remainder of the patients (127 patients, 26.7%).
Radiographs at two postoperative time points were available for 326 patients (69.0%) and 445 levels (69.0%). Subsidence and migration values were calculated for these patients. Subsidence of greater than 3 mm was measured for ten (2.2%) of the analyzed levels. Two of these patients went on to receive further treatment using the VariLift®-L device at the adjacent level below the original fusion; however, the original operative level was asymptomatic for all patients that were determined to have subsidence. There was no migration greater than 3 mm measured for any of the analyzed levels.
Retrospective study of 86 patients treated with cervical SAEFC
From October 1999 to September 2011, 86 patients (43 male, 40 female, sex not reported for three patients) with a mean age of 47.5 years (range 24–89 years) were surgically treated for symptomatic disk herniation with instability and/or DDD using the VariLift®-C expandable, standalone interbody fusion device at one level. Patients with greater than grade I spondylolisthesis were not included. All patients had preoperative neck and/or arm pain. Surgical treatment decisions were based on physical and neurological examinations, and radiographic imaging assessments using plain radiographs, MRI scans, and/or cervical myelogram/CT scans. Surgeries were performed by three surgeons at three institutions. One site was located in the US and two sites were located in Europe. Only patients with a minimum of 1 year follow-up were included in this review. Exclusion criteria included: 1) surgical treatment at more than one level; 2) treatment for ASD; or 3) greater than grade I spondylolisthesis.
All patients received VariLift®-C implantation at a single cervical level. Twenty-three patients (26.7%) received a single device, and 63 patients (73.3%) received two VariLift®-C devices implanted bilaterally in the disk space. Implantations performed at C5-C6 and C6-C7 accounted for over 80% of all procedures. The VariLift®-C was used standalone in 82 cases (95.3%). Additional supplemental fixation with anterior plating was used in four cases. The surgeon chose additional fixation for two of these patients because they were heavy smokers.
Three procedure-related complications were noted, two of which required an additional operation to resolve. One patient required surgery to repair a cerebrospinal fluid leak and the other patient suffered from dysphagia, which was resolved through placement of a temporary G tube. The third patient required evacuation of an epidural hematoma.
Patients were followed according to the surgeons’ typical clinical follow-up period. Patients were assessed preoperatively and postoperatively at approximately 2–8 weeks (n=70, 81.4%), 2–6 months (n=63, 72.1%), and 6–12 months or last follow-up (n=66, 76.7%). All patients were followed at least one time during the first 6 months and 81 patients (94.2%) had more than one follow-up over their course of treatment. Patients were evaluated using a 4-point (0–4) pain scale, with preoperative and postoperative pain scores compared using two-tailed Student’s t-tests and a significance level of P≤0.05.
Average pain scores improved from 2.9±0.3 preoperatively to 0.4±0.9 postoperatively at the last follow-up. Additionally, 21 (24.4%) patients returned for a follow-up visit at greater than 5 years postoperatively (range 5.2–10.9 years). Pain scores for this group of patients improved to 0.5±0.8 from a preoperative score of 2.9±0.3. The improvements in pain for preoperative vs postoperative time points represent statistically significant improvements (P<0.001).
Postoperative radiographs were available for 68 patients (79.1%) at the 6-month follow-up or more (Figure 5). Fusion was achieved for 65 of these patients (95.6%). Encouraging progression of fusion was observed for the remaining three patients; however, the surgeon was unable to make a definitive determination. All patients had a radiographic assessment before the 6-month postoperative follow-up. Early signs of fusion were present in 85 of these cases (98.8%).
Discussion
Interbody fusion remains an important surgical technique in both the lumbar and cervical spine. The advantages of the PLIF and TLIF procedures include: 1) decompression of the spinal canal, thecal sac, and nerve roots; 2) interbody fusion promoting axial load bearing by the anterior column; 3) immediate restoration of disk height and foraminal patency; 4) compressive loading of the interbody bone graft; and 5) avoidance of the comorbidities and potential complications of an anterior approach. These advantages are well documented.20,26–28 However, complications including subsidence, migration, and difficulty assessing bony fusion have also been reported, particularly when using cylindrical interbody fusion devices that do not preserve the cortical vertebral endplates.29 These drawbacks have led to the recent trend of adding supplemental fixation such as pedicle screw systems to interbody fusion, despite the well-known risks of pedicle screw placement.10–13
Subsidence of an interbody fusion can lead to narrowing of the neural foramina, segmental spinal instability, and loss of disk height and lordosis.18 Clinical experience with VariLift® has demonstrated a very low subsidence rate that is further supported by benchtop subsidence testing per ASTM standards30 and FEA studies comparing the VariLift® device to cylindrical cages.23 The preservation of the cortical endplates has been shown to be important in preventing subsidence of interbody fusion devices.19 Oxland et al determined that the removal of the endplate reduced the strength and stiffness of the lower lumbar vertebral bodies that could lead to subsidence.31 Additionally, Zdeblick and Phillips discussed the effect of facet resection on the destabilizing effect on standalone interbody fusion devices.20 Both of these issues have been addressed in the design and surgical technique for the lumbar SAEFC device. The VariLift®-L surgical technique describes both the need to preserve the bony endplates and the importance of minimal facet resection prior to implantation. The zero-insertion profile of the devices further enables nonimpacted implantation with minimal endplate disruption. Therefore, low subsidence rates with the VariLift®-L device are likely related to these factors, in addition to the strong immediate fixation provided upon expanding the device in situ in the disk space. Furthermore, because pedicle screws are avoided, this procedure offers the benefits of a minimally invasive technique, including a smaller incision and preservation of much of the native posterior anatomy.
In the cervical spine, ACDF has been used to treat disk herniation and DDD since the late 1950s.2 Since its inception, there have been many studies conducted and implants designed to improve the efficiency of the operation and the associated patient outcomes. However, there are still comorbidities associated with the operation that include dysphagia and speech dysfunction in the short term,32,33 and potential ASD in the long term.34–38
Dysphagia is a comorbidity associated with the ACDF procedure that occurs in approximately 16% of patients.39 This complication has been associated with a number of causes including anterior plate prominence33,40 and incision lengths that can disrupt the underlying soft tissues such as the trachea and esophagus.32 Zero-profile interbody fusion devices have been designed in an effort to decrease the likelihood of this complication by creating a device that can be inserted through a smaller incision and will not extend beyond the disk space. Scholz et al showed that these types of standalone devices can decrease the risk of dysphagia in a small population with 6 months follow-up.41
ASD is a long-term complication associated with both lumbar and cervical fusion. While the causes of ASD continue to be unclear, the results of the retrospective studies described above suggest encouraging long-term follow-up results in patients that have been implanted with the SAEFC device. In the study of 470 patients who received VariLift®-L, revision surgery rates due to ASD were determined to be 3.4% with an average of 2.7 years between operations. Only two patients (2.3%) in the cervical study returned for additional surgery at an adjacent level, less than the annualized incidence rate of 2.9% described by Hilibrand and Robbins.37 These data are encouraging; additional long-term data (>5 years) would be helpful to more accurately demonstrate the ASD rate in patients receiving the VariLift® device.
Conclusion
SAEFC devices such as the VariLift® Interbody Fusion System have been designed to take advantage of the safety and successful clinical history of interbody fusion devices, while addressing some of the shortcomings of previous interbody implants. Laboratory testing and simulations have demonstrated that these objectives have been achieved, and that the VariLift® devices are robust and reliable. More than 15 years of clinical experience have demonstrated positive clinical outcomes with high fusion rates and continuously reported low rates of subsidence, reoperation or ASD. Possible advantages of this less invasive option for spinal fusion surgery include a shorter procedure and a quicker return to the activities of daily living, while retaining the benefits of an open exposure (ie, a bilateral visualization of the disk space after decompression allowing for easy device insertion). The VariLift system represents an improved surgical option for a stable interbody fusion without requiring supplemental fixation.
Disclosure
Erik Emstad is an employee of Wenzel Spine, Inc., Austin, TX, USA (salary, employee stock options). Diana Cardenas del Monaco is a consultant to Wenzel Spine. Louis C Fielding and Jon E Block (Jon Block Group authors) received financial support from Wenzel Spine. The authors report no other conflicts of interest in this work.
References
Cloward RB. The treatment of ruptured lumbar intervertebral discs by vertebral body fusion. I. Indications, operative technique, after care. J Neurosurg. 1953;10(2):154–168. | |
Cloward RB. The treatment of ruptured lumbar intervertebral discs by vertebral body fusion. I. Indications, operative technique, after care. J Neurosurg. 1953;10(2):154–168. | |
Cloward RB. The anterior approach for removal of ruptured cervical disks. J Neurosurg. 1958;15(6):602–617. | |
Bagby GW. Arthrodesis by the distraction-compression method using a stainless steel implant. Orthopedics. 1988;11(6):931–934. | |
FDA. Premarket Approval of BAK™ Interbody Fusion System with Instrumentation. Silver Spring: United States Food and Drug Administration; 1996:950002. | |
Kuslich SD, Ulstrom CL, Griffith SL, Ahern JW, Dowdle JD. The Bagby and Kuslich method of lumbar interbody fusion. History, techniques, and 2-year follow-up results of a United States prospective, multicenter trial. Spine (Phila Pa 1976). 1998;23(11):1267–1278. [discussion 1279]. | |
Kuslich SD, Danielson G, Dowdle JD, et al. Four-year follow-up results of lumbar spine arthrodesis using the Bagby and Kuslich lumbar fusion cage. Spine (Phila Pa 1976). 2000;25(20):2656–2662. | |
Hacker RJ. Comparison of interbody fusion approaches for disabling low back pain. Spine (Phila Pa 1976). 1997;22(6):660–665. [discussion 665–666]. | |
FDA. Class II Special Controls Guidance Document: Intervertebral Body Fusion Device. Guidance for Industry and FDA Staff. Silver Spring: United States Food and Drug Administration; 2007. | |
Cole CD, McCall TD, Schmidt MH, Dailey AT. Comparison of low back fusion techniques: transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr Rev Musculoskelet Med. 2009;2(2):118–126. | |
Thomsen K, Christensen FB, Eiskjaer SP, Hansen ES, Fruensgaard S, Bunger CE. 1997 Volvo Award winner in clinical studies. The effect of pedicle screw instrumentation on functional outcome and fusion rates in posterolateral lumbar spinal fusion: a prospective, randomized clinical study. Spine (Phila Pa 1976). 1997;22(24):2813–2822. | |
Kosmopoulos V, Schizas C. Pedicle screw placement accuracy: a meta-analysis. Spine (Phila Pa 1976). 2007;32(3):E111–E120. | |
Patel RD, Graziano GP, Vanderhave KL, Patel AA, Gerling MC. Facet violation with the placement of percutaneous pedicle screws. Spine (Phila Pa 1976). 2011;36(26):E1749–E1752. | |
Babu R, Park JG, Mehta AI, et al. Comparison of superior-level facet joint violations during open and percutaneous pedicle screw placement. Neurosurgery. 2012;71(5):962–970. | |
Lee CS, Hwang CJ, Lee SW, et al. Risk factors for adjacent segment disease after lumbar fusion. Eur Spine J. 2009;18(11):1637–1643. | |
Jacobs W, Willems PC, Kruyt M, et al. Systematic review of anterior interbody fusion techniques for single- and double-level cervical degenerative disc disease. Spine (Phila Pa 1976). 2011;36(14):E950–E960. | |
Hacker RJ, Cauthen JC, Gilbert TJ, Griffith SL. A prospective randomized multicenter clinical evaluation of an anterior cervical fusion cage. Spine (Phila Pa 1976). 2000;25(20):2646–2654. [discussion 2655]. | |
Shin JS, Oh SH, Cho PG. Surgical outcome of a zero-profile device comparing with stand-alone cage and anterior cervical plate with iliac bone graft in the anterior cervical discectomy and fusion. Korean J Spine. 2014;11(3):169–177. | |
Chen L, Yang H, Tang T. Cage migration in spondylolisthesis treated with posterior lumbar interbody fusion using BAK cages. Spine (Phila Pa 1976). 2005;30(19):2171–2175. | |
Choi JY, Sung KH. Subsidence after anterior lumbar interbody fusion using paired stand-alone rectangular cages. Eur Spine J. 2006;15(1):16–22. | |
Zdeblick TA, Phillips FM. Interbody cage devices. Spine (Phila Pa 1976). 2003;28(15 Suppl):S2–S7. | |
Ames CP, Acosta FL Jr, Chi J, et al. Biomechanical comparison of posterior lumbar interbody fusion and transforaminal lumbar interbody fusion performed at 1 and 2 levels. Spine (Phila Pa 1976). 2005;30(19):E562–E566. | |
McAfee PC. Interbody fusion cages in reconstructive operations on the spine. J Bone Joint Surg Am. 1999;81(6):859–880. | |
Kiapour A, Kiapour MA, Kodigudla M, Hill GM, Mishra S, Goel VK. A biomechanical finite element study of subsidence and migration tendencies in stand-alone fusion procedures – comparison of an in situ expandable device with a rigid device. J Spine. 2012;1:4. | |
Attia D. PLIF using the VariLift expandable cages: an experience reaching 5 years about stand alone cages. J Bone Joint Surg Br. 2002; 84-B(Suppl III):298. | |
Attia D. PLIF using VariLift expandable cages. More than 5 years of experience of standalone cages. Paper presented at: Swiss Spine Institute 3rd International Symposium: Progress in Spinal Fixation; June 22, 2002; Bern, Switzerland. | |
Ray CD. Threaded titanium cages for lumbar interbody fusions. Spine (Phila Pa 1976). 1997;22(6):667–679. [discussion 679–680]. | |
Zhao J, Wang X, Hou T, He S. One versus two BAK fusion cages in posterior lumbar interbody fusion to L4-L5 degenerative spondylolisthesis: a randomized, controlled prospective study in 25 patients with minimum two-year follow-up. Spine (Phila Pa 1976). 2002;27(24):2753–2757. | |
Kasis AG, Marshman LA, Krishna M, Bhatia CK. Significantly improved outcomes with a less invasive posterior lumbar interbody fusion incorporating total facetectomy. Spine (Phila Pa 1976). 2009; 34(6):572–577. | |
Polikeit A, Ferguson SJ, Nolte LP, Orr TE. The importance of the endplate for interbody cages in the lumbar spine. Eur Spine J. 2003;12(6):556–561. | |
ASTM. Standard Test Method for Measuring Load Induced Subsidence of Intervertebral Body Fusion Device under Static Axial Compression. Pennsylvania: ASTM International; 2004. [ASTM F2267-04]. | |
Oxland TR, Grant JP, Dvorak MF, Fisher CG. Effects of endplate removal on the structural properties of the lower lumbar vertebral bodies. Spine (Phila Pa 1976). 2003;28(8):771–777. | |
Frempong-Boadu A, Houten JK, Osborn B, et al. Swallowing and speech dysfunction in patients undergoing anterior cervical discectomy and fusion: a prospective, objective preoperative and postoperative assessment. J Spinal Disord Tech. 2002;15(5):362–368. | |
Lee MJ, Bazaz R, Furey CG, Yoo J. Influence of anterior cervical plate design on dysphagia: a 2-year prospective longitudinal follow-up study. J Spinal Disord Tech. 2005;18(5):406–409. | |
Bydon M, Xu R, Macki M, et al. Adjacent segment disease after anterior cervical discectomy and fusion in a large series. Neurosurgery. 2014;74(2):139–146. [discussion 146]. | |
Cho SK, Riew KD. Adjacent segment disease following cervical spine surgery. J Am Acad Orthop Surg. 2013;21(1):3–11. | |
Helgeson MD, Bevevino AJ, Hilibrand AS. Update on the evidence for adjacent segment degeneration and disease. Spine J. 2013;13(3):342–351. | |
Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J. 2004;4 (6 Suppl):190S–194S. | |
Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81(4):519–528. | |
Martin RE, Neary MA, Diamant NE. Dysphagia following anterior cervical spine surgery. Dysphagia. 1997;12(1):2–8. [discussion 9–10]. | |
Stein MI, Nayak AN, Gaskins RB 3rd, Cabezas AF, Santoni BG, Castellvi AE. Biomechanics of an integrated interbody device versus ACDF anterior locking plate in a single-level cervical spine fusion construct. Spine J. 2014;14(1):128–136. | |
Scholz M, Schnake KJ, Pingel A, Hoffmann R, Kandziora F. A new zero-profile implant for stand-alone anterior cervical interbody fusion. Clin Orthop Relat Res. 2011;469(3):666–673. | |
ASTM International. Test Methods for Intervertebral Body Fusion Devices. Pennsylvania: ASTM International; 2003. [ASTM F2077-03]. | |
ASTM International. Static Push-Out Test Method for Intervertebral Body Fusion Devices. Pennsylvania: ASTM International; 2000. [ASTM Draft Standard F-04.25.02.02]. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.