Back to Journals » Patient Preference and Adherence » Volume 13
The Optimizing-Risk-Communication (OptRisk) randomized trial – impact of decision-aid-based consultation on adherence and perception of cardiovascular risk
Authors Adarkwah CC , Jegan N, Heinzel-Gutenbrunner M, Kühne F, Siebert U , Popert U, Donner-Banzhoff N , Kürwitz S
Received 9 December 2018
Accepted for publication 26 January 2019
Published 27 March 2019 Volume 2019:13 Pages 441—452
DOI https://doi.org/10.2147/PPA.S197545
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Charles Christian Adarkwah,1–3 Nikita Jegan,1 Monika Heinzel-Gutenbrunner,4 Felicitas Kühne,5,6 Uwe Siebert,5–7 Uwe Popert,8 Norbert Donner-Banzhoff,1 Sarah Kürwitz1,9
1Department of General Practice and Family Medicine, University of Marburg, Marburg, Germany; 2Department of Health Services Research and General Practice, Faculty of Life Sciences, University of Siegen, Siegen, Germany; 3Department of Health Services Research, Maastricht University, Maastricht, The Netherlands; 4MH Statistik Beratung, Marburg, Germany; 5Institute of Public Health, Medical Decision Making and Health Technology Assessment, Department of Public Health and Health Technology Assessment, University for Health Sciences, Medical Informatics and Technology, Innsbruck, Austria; 6Division of Public Health Decision Modelling, Health Technology Assessment and Health Economics, ONCOTYROL – Center for Personalized Cancer Medicine, Innsbruck, Austria; 7Center for Health Decision Science, Department of Health Policy and Management, Harvard School of Public Health, Boston, MA, USA; 8Department of General Practice, University of Göttingen, Göttingen, Germany; 9Department of Public Health, University of Bielefeld, Germany
Background: Shared decision-making is a well-established approach to increasing patient participation in medical decisions. Increasingly, using lifetime-risk or time-to-event (TTE) formats has been suggested, as these might have advantages in comparison with a 10-year risk prognosis, particularly for younger patients, whose lifetime risk for some events may be considerably greater than their 10-year risk. In this study, a randomized trial, the most popular 10-year risk illustration in the decision-aid software Arriba (emoticons), is compared with a newly developed TTE illustration, which is based on a Markov model. The study compares the effect of these two methods of presenting cardiovascular risk to patients on their subsequent adherence to intervention.
Methods: A total of 294 patients were interviewed 3 months after they had had a consultation with their GP on cardiovascular risk prevention. Adherence to behavioral change or medication intervention was measured as the primary outcome. The latter was expressed as a generated score. Furthermore, different secondary outcomes were measured, ie, patient perception of risk and self-rated importance of avoiding a cardiovascular event, as well as patient numeracy, which was used as a proxy for patient health literacy.
Results: Overall, no significant difference in patient adherence was found depending on risk representation. In the emoticon group, the number of interventions had a significant impact on the adherence score (P=0.025). Perception of risk was significantly higher in patients counseled with the TTE risk display, whereas the importance of avoiding a cardiovascular event was rated equally highly in both groups and actually increased over time.
Conclusion: The TTE format is an appropriate means for counseling patients. Adherence is a very complex construct, which cannot be fully explained by our findings. The study results support our call for considering TTE illustrations as a valuable alternative to current decision-support tools covering cardiovascular prevention. Nevertheless, further research is needed to shed light on patient motivation and adherence with regard to cardiovascular risk prevention.
Trial registration: The study was registered at the German Clinical Trials Register and at the WHO International Clinical Trials Register Platform (ICTRP, ID DRKS00004933); registered February 2, 2016 (retrospectively registered).
Keywords: randomized trial, Arriba, decision aid, adherence, risk perception, shared decision-making 10-year prognosis, risk assessment, lifetime risk, time to event, cardiovascular disease
Introduction
Cardiovascular diseases are a burden for all health care systems, especially in the Western world. As such, guidelines strongly recommend predicting cardiovascular risk and tailoring preventive efforts accordingly.1,2 Shared decision-making between patients and health professionals is increasingly becoming the norm for health-related decisions. Studies have shown that increased participation in the decision-making process leads to greater satisfaction on the part of both doctors and patients and to better adherence and clinical outcomes.3,4 Chronic conditions, such as cardiovascular disease, require information for lifestyle changes leading to early prevention, and when needed lifelong treatment. In developed countries, adherence to long-term therapies is only around 50%. Nonadherence can lead to increased morbidity, premature mortality and increasing costs for health care.5 Therefore improving adherence to treatment or lifestyle changes is an important goal for health care providers. The World Health Organization describes adherence as “… the extent to which a person’s behavior, [ie], taking medication, following a diet, and/or executing lifestyle changes, corresponds with agreed recommendations from a health care provider”.5
Decision aids are designed to help patients make informed choices by delivering evidence-based information on options and outcomes, eg, regarding cardiovascular events.6 They typically cover diagnostic, therapeutic, and preventive decisions, and are also able to inform the counseling process, as they can be delivered in different formats before, during, or after the consultation.7 Furthermore, they have been found to be appropriate tools for increasing adherence by improving risk communication and shared decision-making.8 Decision aids usually provide individualized absolute risk estimates for a limited time, such as 10 years. This format has been criticized, even by guidelines,2 for underestimating high-risk constellations in younger patients.9–11 Although this patient population may have unfavorable risk profiles, since their absolute risk is still low, this does not become obvious and an opportunity for early intervention may be missed.9 To overcome this problem, using lifetime-risk and time-to-event (TTE) formats have been suggested.1,9–13 These formats were developed and assessed within the OptRisk trial14,15 as part of Arriba, a computerized decision-aid software.16 Arriba is well-established in Germany,17 and is being introduced in other European countries. Within Arriba, the calculation of absolute cardiovascular risks is based on the Framingham risk algorithm.16 The TTE displays evaluated in this study are based on a Markov model, which was constructed for the purpose of our study (unpublished data). It has been tested extensively and found to be a valuable tool in primary-care practice.18–21 Further details regarding Arriba, the new risk-representation format, and the data set behind their use have been published elsewhere.14,18–21 At the end of the OptRisk trial, the highest-rated TTE representation (Figure S1) was compared with the most popular 10-year display (“emoticons”, Figure S2) within a randomized trial.14 Within this trial, patients were consecutively recruited by their GPs. Immediately after giving their informed consent, patients were randomized to consultation with the emoticons or the TTE illustration. GPs entered a study ID into the decision-support software, which automatically allocated each patient into one of the two conditions according to an a priori randomized sequence. GPs learned about each patient’s allocation by the illustration displayed by the software. They then started a discussion with their patients on the basis of the allocated display, ie, either emoticons or TTE. After the consultation, patients were asked to fill in a questionnaire covering immediate-outcome assessments. GPs recorded the decision made, such as specific medications, dose adjustments, behavioral measures, or no change at all.14
It was demonstrated that the new TTE illustration is not inferior in comparison with the well-established emoticons regarding the primary outcome “participation in the shared decision-making process”. Furthermore, the noninferiority of the innovative TTE could be confirmed for all secondary outcome variables, eg, decisional conflict and perception of risk. The explorative analysis even indicated advantages in younger patients (<46 years of age), especially with regard to perception of risk and decisional conflict.15 The patients’ willingness to take preventive actions, ie, weight loss, smoking cessation, antihypertensive drugs, and diet, is one thing; sticking to these healthier behaviors is another. In this paper, we present follow-up data gathered 3 months after the initial consultation, in order to assess the adherence of behavioral change activities patients had agreed upon, as well as to explore changes in perception over time, depending on the risk representation applied.
Methods
Design and setting
This prospective, randomized trial was performed in general practices in the greater area of Marburg, Germany, between October 2012 and January 2013. The study was performed in accordance with the Declaration of Helsinki and approved by the research ethics committee of the University of Marburg. The study was registered on the German Clinical Trials Register (DRKS-ID: DRKS00004933).
Patient recruitment
As previously described,14 patients were eligible for the study if they were aged 30–80 years and their GP felt a need to discuss behavioral change regarding cardiovascular risk. This age range was chosen because data on cardiovascular morbidity and mortality were available for this population. The need to discuss behavioral change could have been, for example, the biannual health check, “CheckUp 35+”, offered to adults >35 years every 2 years, a disease management–program consultation, done every 3–6 months, or a discussion of medication after specialist consultation or hospital discharge. Patients were also eligible if they addressed cardiovascular risk and possible prevention themselves. This could include interventions like exercise, cessation of smoking, taking medications (statins, low-dose aspirin, antihypertensive drugs, or dose adjustments), or dietary changes. Patients were excluded from the study if they were (according to the judgment of the GP) significantly mentally retarded and thus not able to follow a shared decision making–based consultation on their cardiovascular risk, had no interest in taking an active part in the decision process, or had insufficient knowledge of the German language. Patients who fulfilled the inclusion criteria at baseline were invited to participate, and if they agreed, enrolled in the study. After the baseline visit, patients were informed that a member of the study team would get in touch for a telephone interview after 3 months.
Interventions
Patients were contacted for a telephone interview 3 months after the initial recruitment by a well-instructed study nurse. They were asked if they were able to remember the appointment at their GP’s practice within the frame of the study. They were then asked for further details regarding the content of the counseling talk with Arriba, ie, which preventive actions both doctor and patient agreed upon. Initially, patients were randomized to consultation with the TTE illustration (Figure S1) or the emoticons (Figure S2). GPs entered a study ID into the decision-support software, which automatically allocated each patient into one of the two decision-aid formats according to an a priori randomized sequence. GPs learned about each patient’s allocation by the illustration displayed by the software. They then started a discussion with their patients on the basis of the allocated display, ie, emoticons or TTE.
Primary outcome
The main objective of the study was to evaluate the performance of the new TTE illustration in comparison with the emoticons regarding their impact on patient adherence to the behavioral change interventions that the patient had agreed upon in the consultation 3 months prior to the interview. To measure adherence, a score was developed by the study group, as no validated instrument was available for the purpose of our study. For each item, a distinction was made according to whether a patient had been completely adherent, partially adherent (which could mean that the patient started with adherence, but did not adhere to all of the criteria completely), or nonadherent (the patient did not adhere to the behavioral change or intervention at all). We labeled the grade of adherence as 2= fully adherent, 1= partially adherent, and 0= non-adherent.
In the end, total values were added and divided by the number of interventions to make a score, which might range between 0 and 2. The higher the score, the more adherent the patient. We finally classified the scores as <1= nonadherent, 1= partly adherent, >1–<2= predominantly adherent, and 2= fully adherent.
Secondary outcomes
In the wider context of adherence, we also evaluated to what extent patients remembered the behavioral change interventions they had agreed upon, as this is a precondition to being adherent. In addition, we evaluated several secondary end points. Patients were shown two visual analogue scales: first to rate the importance of avoiding a cardiovascular event, and second to estimate their own cardiovascular risk (perception of risk). Both scales ranged from 0 (no risk/not important) to 10 (maximum risk/maximum importance). These results were compared with patient estimates immediately after the consultation at baseline (t0), ie, 3 months prior. Furthermore, numeracy was assessed using two statistical questions in the structured telephone-interview guideline. Thereby, we investigated the ability of participants to understand health information to improve the evaluation of our results. This is to estimate to what extent information given about health is well-perceived and understood. We chose two questions suitable for a structured telephone interview from Lipkus et al22 and slightly adjusted these to our research topic on risk communication of cardiovascular diseases (Question 1 – Which of the following numbers represents the biggest risk of getting a disease? a) 1 in 10, b) 1 in 100, c) 1 in 1,000; Question 2 – Which of the following represents the biggest risk of getting a disease? a) 1%, b) 5%, c) 10%).
Statistical analyses
All statistical calculations were performed with IBM SPSS.23 Patients’ characteristics are demonstrated by means ± SD for metric variables and frequencies and percentages for categorical variables. Student’s t-test was used for comparisons between patient groups regarding primary and secondary outcomes. In order to examine the importance of the differences between the two graphic renditions, effect sizes (Cohen’s δ) were calculated.24
We compared the two illustrations with respect to one primary outcome variable, adherence, and additionally to several secondary outcome variables (eg, numeracy as a proxy for health literacy, risk perception, and the importance of avoiding a cardiovascular event). Results for risk perception and importance of avoiding a cardiovascular event were compared with results at baseline by means of paired t-tests. SPSS Crosstabs with χ2 or Fisher’s exact test were used to compare the categorical (binary) variables between treatment groups. In line with the exploratory nature of the tests concerning the secondary outcome variables, we applied no α-correction for multiple testing.
Results
A sample of 304 study participants (Figure 1) was recruited by 32 GPs in 28 practices. An average of 9.5 patients (range three to 15) per GP were enrolled. Of the 304 study participants who were initially enrolled, 294 took part in the follow-up survey (Figure 1). Six were not available by phone (change of phone number), and four declined to participate. A total of 146 patients were initially shown emoticons, and 148 received their risk information on the basis of the TTE illustration. The characteristics of the two groups (emoticons and TTE) are shown in Table 1. Both study arms were well balanced regarding sociodemographic and clinical variables.
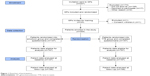  | Figure 1 Flowchart of participation. |
Primary outcome
A total of 294 patients agreed upon zero to six possible interventions. These interventions include exercise, smoking, taking medications (statins, low-dose aspirin, antihypertensive drugs, or dose adjustments), or dietary changes, and did not differ between the study arms (Figure 2). Fourteen patients were excluded from the adherence analysis, as no behavioral change intervention was agreed upon during the consultation. For instance, a total of 280 patients were analyzed, with a mean of 1.0417±0.412 interventions agreed upon and a median of 1. Only half the population were able to remember fully the agreement that was made with the GP, while 35% were able to recap parts and about 15% did not remember any intervention having been agreed upon. With regard to risk representations, no significant difference was demonstrated, with a mean for emoticons of 1.04±0.44 and that for TTE representation of 1.05±0.39. In addition, an exploratory analysis showed that in the emoticon group, the number of intervention had a significant impact on the adherence score (P=0.025), whereas there was no systematic pattern notable (eg, the fewer interventions the higher the score). In the TTE group, the number of interventions discussed had no significant impact on adherence score (Table 2). The frequency distribution for the adherence score showed that the majority of patients were partly adherent (Figure 3). Full adherence was rare. Overall, the risk representation had no significant impact on adherence in our study.
  | Figure 2 Frequency distribution of number of interventions discussed and agreed upon per patient per study arm. |
  | Table 2 Mean adherence in relation to number of interventions discussed |
  | Figure 3 Frequency distribution of adherence score per study arm. |
Secondary outcomes
The perception of risk was significantly higher in the TTE group than the emoticons group (P=0.032) 3 months after the initial consultation. In addition, the effect size was δ=0.25, which represented a small effect. Assuming that higher risk perception is a precondition for adherence and behavioral change, the TTE representation was superior in comparison with the emoticons. In comparison with the baseline results, obtained immediately after the consultation, there was no significant change at 3 months in the emoticon group, whereas the perception of risk had decreased significantly at 3 months in the TTE group (P=0.02). Despite this decrease, perception of risk in the TTE group at the follow-up was significantly higher than that in the emoticon group.
Next, the importance of avoiding a cardiovascular event was rated highly in both groups (Table 3). Furthermore, this importance increased significantly over time (P<0.0005) in both groups. Finally, patient numeracy was assessed as a proxy for health literacy. Spearman’s correlation between numeracy and adherence score was ρ=–0.086 (P=0.178). Altogether, 273 of 294 patients agreed to answer the questions regarding numeracy (93%) at the end of the interview: 38.8% answered both questions regarding numeracy correctly, 32.2% gave at least one correct answer, and 28.9% gave two false answers.
  | Table 3 Risk perception and self-rated importance of avoiding a cardiovascular event over time, depending on risk representation |
Discussion
Regarding our primary outcome, adherence, there was no significant difference between the emoticon group and the TTE group. It was obvious that a majority of patients were adherent in parts. We can only speculate why this is the case. After all, almost all patients remembered the consultation at their GP’s office about cardiovascular risk 3 months before (290 of 294 patients). However, only half the population were able to remember fully the agreement that had been made with the GP, while 35% recapped parts and about 15% did not remember any intervention having been agreed upon. Logically, it is not possible to be adherent to something patients do not even think about. This problem could be solved, eg, by making a written note for the GP and patient, which the patient takes home in order to recapitulate and remember the agreement over time. Whether this would contribute to better adherence results needs to be examined in a further study. The exploratory finding that the number of interventions had a significant impact on adherence score (P=0.025) in the emoticon group remains unexplained. No clear trend was notable with this observation (Table 2). Further studies with larger patient groups are necessary to investigate this. Significantly higher scores were achieved in the TTE group with regard to the perception of risk, while scores in both groups decreased over time. Higher scores in perception of risk mostly arose in younger patients, who seem to react more to the TTE representation of risk, as shown in a previous study,15 which explains this observation. The slight decrease over time could also have been because patients did not consider the content or consequences of the GP counseling in their daily lives over time. Interestingly, the importance of avoiding a cardiovascular event, already high at baseline, had significantly increased at the 3-month follow-up. As such, the SD for importance decreased from baseline to follow-up, most likely due to a decrease in heterogeneity. Why exactly this is the case remains unclear.
Nevertheless, our study had some limitations. First, the behavioral change interventions that were discussed with the patients need to be translated into actions over the long term. A follow-up period of 3 months might be too short to capture the total impact of the counseling process. Studies are needed that take a longer time horizon into account, ie, 12 months and more. However, 15% of study participants did not remember the agreement with their GPs at all after 3 months, as already mentioned. We can only speculate that a longer follow-up would lead to an increase in this proportion of patients. Second, no validated instrument was available to capture adherence in this scenario, which is why the authors developed a tool by themselves. Third, we did not generally distinguish between adherence regarding medication intake and adherence with respect to lifestyle changes. It should be considered that there might be a difference in efforts whether patients have to take medication on a regular basis or if they are trying to implement a lifestyle change.
Despite these limitations, we think that our study provides valid estimates regarding the outcome measure. Our study is the first to investigate real patients in the setting of a GP consultation with regard to cardiovascular decisions concerning themselves looking at adherence and various other secondary outcomes mentioned and discussed in detail herein. In fact, issues regarding their own cardiovascular risks and preventive options were discussed and real decisions were taken. Current guidelines criticize the 10-year period for younger individuals.2 They point out that up to half the adult population have a low 10-year risk (<10%), but a high risk of future events (>39%) over their lifetime.2,25 It is pointed out as a “key change” in the new Joint British Societies 3 guidelines that a lifetime-risk approach be adopted in addition to 10-year absolute-risk estimates. This is a crucial and fundamental change that strengthens our study design and line of action. To our knowledge, there is no study available that examines the effect of a TTE risk display on patient adherence to medication or lifestyle changes. There are a few studies available that investigated the effect of decision aids on adherence in general.26 Three studies found that patients who were exposed to decision aids had higher adherence to treatment than those who had received usual counseling.27–29 In another study, patients exposed to decision aids during consultation had lower adherence,30 while six studies showed no difference in adherence between patients who were exposed to decision aids and patients who received usual care.31–36 Other studies have distinguished between different types of adherence. Trenaman et al37 suggested distinguishing between intentional and unintentional adherence. Moreover, the authors recommend making a difference between adherence to choice (measures the proportion of individuals who choose a treatment at follow-up) and adherence to treatment (measures the degree to which a treatment is being used at follow-up). These aspects should be considered for further validation in future studies to assess the appropriateness of the instrument. According to Trenaman et al,37 it is difficult to draw conclusions regarding the effect of decision aids on adherence, due to the heterogeneity of how adherence is measured. For instance, in contrast to Trenaman et al and various other studies mentioned, we did not compare the use of decision aids to no decision aid, but rather compared two different ways of risk counseling using the same decision-aid software using different representations of risk: emoticons in one group and TTE in the other group.
Furthermore, the health literacy of patients was assessed using numeracy as a proxy measure. Health literacy is considered a prerequisite for competence in understanding, assessing, and applying health-related information.38 This is essential for managing health risks and diseases in daily life and being able to make well-informed and pertinent decisions.39 In comparison with usual care, decision aids increased participants’ knowledge, as well as the accuracy of their perception of risk, and decreased decisional conflict related to feeling uninformed.26 Literature on the effect of lifetime-risk predictions with respect to cardiovascular events or disease on patient decision-making is scarce, and has been discussed extensively elsewhere.14 For the most part, previous studies were not performed in the setting of a GP consultation, and no real decisions were taken.
Previous research has shown some evidence concerning the link between health literacy and adherence. Health-literacy interventions increased health literacy and adherence outcomes.40 Patients with inadequate health literacy had lower adherence to cardiovascular drugs than those with adequate health literacy.41 The results show no significant link between numeracy and adherence in our study. However, the study was not designed to examine this issue.
Conclusion
The results of our study show that the illustration of event-free survival (TTE) is appropriate for cardiovascular risk information and with regard to adherence. According to our results, there was no significant association between the type of risk display and adherence to the previously discussed health interventions. Adherence is a complex construct that is subject to many different influencing factors, such as health literacy, communication skills, the side effects of medication, and many more.42 Therefore, we recommend offering a more holistic and continuous approach regarding risk communication, ie, regular risk consultations, to show the effect of the chosen treatment options as a motivating factor and to provide patient coaching. Future studies should examine the construct of adherence in further detail, also involving qualitative methods to obtain deeper insights into patient motivation and understanding.
Abbreviations
DMP, disease-management program; GP, general practitioner; OptRisk, Optimizing Risk Communication; SDM, shared decision-making; TTE, time to event.
Ethics statement
The study was performed in accordance with the Declaration of Helsinki and approved by the research ethics committee of the University of Marburg (AZ 167/12, 10/10/2012). We obtained written informed consent from each study participant.
Availability of data and materials
All study data and materials are available upon request. This includes the study version of Arriba and specifications regarding the Markov model.
Acknowledgments
We thank all the GPs and their teams who participated in this study for a very smooth recruitment process. Furthermore, we would like to thank our study nurses – Dr Elisabeth Szabo, Muazzez Ilhan, and Marion Herz-Schuchardt – for their excellent work in data collection, data entry, and office support. This study was funded by the German Federal Ministry of Education and Research (grant 016X1045).
Disclosure
NDB is cochairman of Gesellschaft für Patientenzentrierte Kommunikation (GPZK), a nonprofit organization distributing decision-support software. He receives no salary or other regular payment from GPZK; only such expenses as travel costs are covered. The other authors report no conflicts of interest in this work.
References
Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. J Am Coll Cardiol. 2014;63(25):2935–2959. doi:10.1016/j.jacc.2013.11.005 | ||
JBS3 Board. Joint British Societies’ consensus recommendations for the prevention of cardiovascular disease (JBS3). Heart. 2014;100(Suppl 2):ii1–ii67. doi:10.1136/heartjnl-2014-305693 | ||
Härter M, Loh A, Spies C. Gemeinsam entscheiden – erfolgreich behandeln. Köln: Deutscher Ärzteverlag; 2005. | ||
Coulter A, Ellins J. Effectiveness of strategies for informing, educating, and involving patients. BMJ. 2007;335:24–27. doi:10.1136/bmj.39246.581169.80 | ||
World Health Organization. Adherence to Long-Term Therapies: Evidence for Action. [Internet]. Geneva: World Health Organization; 2003. Available from: http://whqlibdoc.who.int/publications/2003/9241545992.pdf. Accessed May 23, 2017. | ||
Hirsch O, Keller H, Krones T, Donner-Banzhoff N. Arriba-lib: evaluation of an electronic library of decision aids in primary care physicians. BMC Med Inform Decis Mak. 2012;12:48. doi:10.1186/1472-6947-12-48 | ||
O’Donnell S, Cranney A, Jacobsen MJ, Graham ID, O’Connor AM, Tugwell P. Understanding and overcoming the barriers of implementing patient decision aids in clinical practice. J Eval Clin Pract. 2006;12(2):174–181. doi:10.1111/j.1365-2753.2006.00613.x | ||
Kambhampati S, Ashvetiya T, Stone NJ, Blumenthal RS, Martin SS. Shared decision-making and patient empowerment in preventive cardiology. Curr Cardiol Rep. 2016;18(5):49. doi:10.1007/s11886-016-0729-6 | ||
Ulrich S. What is the optimal age for starting lipid lowering treatment? A mathematical model. BMJ. 2000;320(7242):1134–1140. | ||
Sniderman AD, Toth PP, Thanassoulis G, Pencina MJ, Furberg CD. Taking a longer term view of cardiovascular risk: the causal exposure paradigm. BMJ. 2014;348:g3047. doi:10.1136/bmj.g3047 | ||
Elward KS, Simpson RJ, Mendys P. Improving cardiovascular risk reduction for primary prevention-utility of lifetime risk assessment. Postgrad Med. 2010;122(4):192–199. doi:10.3810/pgm.2010.07.2186 | ||
Persell SD, Zei C, Cameron KA, Zielinski M, Lloyd-Jones DM. Potential use of 10-year and lifetime coronary risk information for preventive cardiology prescribing decisions: a primary care physician survey. Arch Intern Med. 2010;170(5):470–477. doi:10.1001/archinternmed.2009.525 | ||
Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113(6):791–798. doi:10.1161/CIRCULATIONAHA.105.548206 | ||
Adarkwah CC, Jegan N, Heinzel-Gutenbrunner M, et al. Time-to-event versus ten-year-absolute-risk in cardiovascular risk prevention – does it make a difference? Results from the Optimizing-Risk-Communication (OptRisk) randomized-controlled trial. BMC Med Inform Decis Mak. 2016;16(1):152. | ||
Adarkwah CC, Jegan N, Kürwitz S, Heinzel-Gutenbrunner M, Donner-Banzhoff N. Cardiovascular risk assessment in younger patients: implications of different risk representation on risk perception and decisional conflict. Z Allg Med. 2017;93(9):353–359. | ||
Donner-Banzhoff N, Popert U. Hausärztliche Beratung zur kardiovaskulären Prävention (Version 4.2); 2015. Available from: http://arriba-hausarzt.de/downloads/arriba_broschuere.pdf. Accessed August 10, 2015. | ||
Hirner B, Rehwald U, Geserick R. Newsletter Thema Allgemeinmedizin: gesundheitsforschung: forschung für den Menschen. Berlin; 2007. Available from: http://www.gesundheitsforschung-bmbf.de/_media/13_NL_Allgemeinmedizin.pdf. Accessed August 10, 2015. | ||
Krones T, Keller H, Sönnichsen AC, Sadowski EM, Baum E, Donner-Banzhoff N. Partizipative Entscheidungsfindung in der kardiovaskulären Risikoprävention: ergebnisse der Pilotstudie von ARRIBA-Herz, einer konsultationsbezogenen Entscheidungshilfe für die allgemeinmedizinische Praxis. Z Med Psychol. 2006;15(2):61–70. | ||
Krones T, Keller H, Sonnichsen A, et al. Absolute cardiovascular disease risk and shared decision making in primary care: a randomized controlled trial. Ann Fam Med. 2008;6(3):218–227. doi:10.1370/afm.854 | ||
Krones T, Keller H, Becker A, Sönnichsen A, Baum E, Donner-Banzhoff N. The theory of planned behaviour in a randomized trial of a decision aid on cardiovascular risk prevention. Patient Educ Couns. 2010;78(2):169–176. doi:10.1016/j.pec.2009.06.010 | ||
Sadowski EM, Eimer C, Keller H, et al. Evaluation komplexer Interventionen: implementierung von ARRIBA-Herz ☺, einer Beratungsstrategie für die Herz-Kreislaufprävention. Z Allg Med. 2005;81(10):429–434. doi:10.1055/s-2005-872475 | ||
Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21(1):37–44. doi:10.1177/0272989X0102100105 | ||
IBM SPSS. Statistics for Windows. Armonk, NY: IBM Corp; 2012. | ||
Cohen J. Statistical power analysis. Curr Dir Psychol Sci. 1992;1(3):98–101. doi:10.1111/1467-8721.ep10768783 | ||
Marma AK, Berry JD, Ning H, Persell SD, Lloyd-Jones DM. Distribution of 10-year and lifetime predicted risks for cardiovascular disease in US adults: findings from the National Health and Nutrition Examination Survey 2003 to 2006. Circ Cardiovasc Qual Outcomes. 2010;3:8–14. doi:10.1161/CIRCOUTCOMES.109.869727 | ||
Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;12(4):CD001431. doi:10.1002/14651858.CD001431.pub5 | ||
Montori VM, Shah ND, Pencille LJ, Branda ME, Van Houten HK, Swiglo BA. Use of a decision aid to improve treatment decisions in osteoporosis: the osteoporosis choice randomized trial. Am J Med. 2011;124(6):549–556. doi:10.1016/j.amjmed.2011.01.013 | ||
Sheridan SL, Draeger LB, Pignone MP, et al. A randomized trial of an intervention to improve use and adherence to effective coronary heart disease prevention strategies. BMC Health Serv Res. 2011;11:331. doi:10.1186/1472-6963-11-331 | ||
Weymiller AJ, Montori VM, Jones LA, et al. Helping patients with type 2 diabetes mellitus make treatment decisions: statin choice randomized trial. Arch Intern Med. 2007;167(10):1076–1082. doi:10.1001/archinte.167.10.1076 | ||
Mullan RJ, Montori VM, Shah ND, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med. 2009;169(17):1560–1568. doi:10.1001/archinternmed.2009.293 | ||
Mott JM, Stanley MA, Street RL Jr, Grady RH, Teng EJ. Increasing engagement in evidence-based PTSD treatment through shared decision-making: a pilot study. Mil Med. 2014;179(2):143–149. doi:10.7205/MILMED-D-14-00052 | ||
Loh A, Simon D, Wills CE, Kriston L, Niebling W, Harter M. The effects of a shared decision-making intervention in primary care of depression: a cluster-randomized controlled trial. Patient Educ Couns. 2007;67(3):324–332. doi:10.1016/j.pec.2007.02.003 | ||
Emmett CL, Montgomery AA, Peters TJ, Fahey T. Three-year follow-up of a factorial randomised controlled trial of two decision aids for newly diagnosed hypertensive patients. Br J Gen Pract. 2005;55(516):551–553. | ||
Mann DM, Ponieman D, Montori VM, Arciniega J, McGinn T. The statin choice decision aid in primary care: a randomized trial. Patient Educ Couns. 2010;80(1):138–140. doi:10.1016/j.pec.2009.10.008 | ||
Oakley S, Walley T. A pilot study assessing the effectiveness of a decision aid on patient adherence with oral bisphosphonate medication. Pharm J. 2006;276(7399):536–538. | ||
LeBlanc A, Wang AT, Wyatt K, et al. Encounter decision aid vs. clinical decision support or usual care to support patient-centered treatment decisions in osteoporosis: the osteoporosis choice randomized trial II. PLoS One. 2015;10(5):1–13. doi:10.1371/journal.pone.0128063 | ||
Trenaman L, Selva A, Desroches S, et al. A measurement framework for adherence in patient decision aid trials applied in a systematic review subanalysis. J Clin Epidemiol. 2016;77:15e23. | ||
Sørensen K, Van Den Broucke S, Fullam J, et al. Health literacy and public health: a systematic review and integration of definitions and models. BMC Public Health. 2012;12:80. doi:10.1186/1471-2458-12-80 | ||
Schaeffer D, Pelikan J. Health Literacy. Göttingen: Hogrefe; 2016. | ||
Miller TA. Health literacy and adherence to medical treatment in chronic and acute illness: a meta-analysis. Patient Educ Couns. 2016;99(7):1079–1086. doi:10.1016/j.pec.2016.01.020 | ||
Noureldin M, Plake KS, Morrow DG, Tu W, Wu J, Murray MD. Effect of health literacy on drug adherence in patients with heart failure. Pharmacotherapy. 2012;32(9):819–826. doi:10.1002/j.1875-9114.2012.01109.x | ||
Brown MT, Bussell JK. Medication adherence: WHO cares. Mayo Clin Proc. 2011;86(4):304–314. doi:10.4065/mcp.2010.0575 |
Supplementary materials
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.