Back to Journals » Clinical Interventions in Aging » Volume 14
The LipiDiDiet trial: what does it add to the current evidence for Fortasyn Connect in early Alzheimer’s disease?
Authors Rasmussen J
Received 10 April 2019
Accepted for publication 14 June 2019
Published 15 August 2019 Volume 2019:14 Pages 1481—1492
DOI https://doi.org/10.2147/CIA.S211739
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Jill Rasmussen1,2
1Primary Care Specialist Mental Health in Dementia and Learning Disability, Surrey, UK; 2Royal College of General Practitioners Representative for Dementia, London, UK
Abstract: Nutritional factors can influence the risk of developing Alzheimer’s disease (AD) and its rate of progression, and there is, therefore, increasing interest in nutrition as a modifiable risk factor for the disease. Synaptic loss is an important feature of early AD, and the formation of new synapses is dependent on key nutritional elements that are known to be deficient in patients with AD. The daily medical food, Souvenaid, contains Fortasyn Connect, a multinutrient combination developed to specifically address these deficiencies, comprising docosahexaenoic acid, eicosapentaenoic acid, uridine monophosphate, choline, phospholipids, selenium, folic acid, and vitamins B12, B6, C, and E. Although yielding heterogeneous findings, clinical studies of Fortasyn Connect provide preliminary evidence of clinically relevant benefits on cognitive outcomes in prodromal and early AD. The LipiDiDiet trial investigated the effects of Fortasyn Connect on cognition and related measures in prodromal AD, and is the first randomized, controlled, double-blind, multicenter trial study of a non-pharmacological intervention in this setting. The primary efficacy endpoint was change over 24 months in a composite score of cognitive performance using a neuropsychological test battery. Fortasyn Connect had no significant effect on this endpoint, but demonstrated a significant benefit on secondary endpoints, including domains of cognition affected by AD (attention, memory, executive function) and hippocampal atrophy, suggesting a potential benefit on disease progression. Other studies have demonstrated benefits for Fortasyn Connect on nutritional markers and levels of plasma homocysteine. Taken together, current evidence indicates that Fortasyn Connect may show benefit on domains of cognition affected by AD and nutritional measures that influence risk factors for its progression; that it has greater potential for benefit earlier rather than later in the disease; and that it is safe and well tolerated, alone or in combination with AD medications. Further research into its potential role in AD management is therefore warranted.
Keywords: nutrition, medical food, cognition, modifiable risk factors
In the absence of a disease-modifying therapy for people at risk of, or with, Alzheimer’s disease (AD), there is increased interest in modifying risk factors for AD and evaluating the potential of non-pharmacological approaches to management, including cognitive stimulation therapy and nutrition. Accumulating evidence from epidemiological studies indicates that nutritional factors can influence both the risk of developing AD and the rate of disease progression. Deficiencies of key nutritional elements that are critical for synapse formation are of specific interest. This article reviews one of the major trials in this area – LipiDiDiet – and looks at some of the key findings relating to early signals of AD, including cognition and brain volume changes. The data are presented within the context of findings from other studies, and their implications for health care professionals, with respect to the advice and support they might offer people at risk of AD, with mild cognitive impairment, or in the very early stages of disease, are discussed.
In addition to the hallmark pathologies of AD – amyloid plaques and tau tangles – one of the important features that occur early in the disease is a pronounced synaptic loss, which is itself linked to memory loss. The formation of new synapses depends on the availability of certain nutrients that are required to synthesize phospholipids that make up neuronal membranes (see Figure 1).1 These include the omega-3 polyunsaturated fatty acid, docosahexaenoic acid (DHA), uridine, choline, folate, vitamin B12, vitamin B6, vitamin E, vitamin C, and selenium.2,3
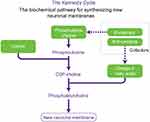 |
Figure 1 Kennedy Cycle: the biochemical pathway for synthesizing new neuronal membranes. Developed from Kennedy et al, 19561 and adapted with permission of Annual Reviews, from Use of phosphatide precursors to promote synaptogenesis, Wurtman RJ, Cansev M, Sakamoto T, Ulus IH, 29, 2009; permission conveyed through Copyright Clearance Center, Inc.2 |
Despite eating a normal diet, people with AD have been shown to have deficiencies in elements that are critical to the Kennedy Cycle, including:
- Lower brain levels of DHA4
- Reduced plasma levels of folate, and vitamins B12, C, and E5,6
- Reduced uridine monophosphate synthesis.7
There is also decreased uptake of choline in the aging brain.8
LipiDiDiet trial
The LipiDiDiet trial9 is the first randomized, controlled, double-blind, multicenter study of a non-pharmacological intervention in prodromal AD. It was performed in accordance with the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice guidelines, and is registered with the Dutch Trial Register (number NTR1705).9 The study protocol and consent forms were approved by the local ethical committees of all participating sites, and all participants provided written informed consent prior to participation.9 To address the rate-limiting supply of compounds for brain phospholipid synthesis, the active treatment group in LipiDiDiet received a daily medical food, Souvenaid (Nutricia Advanced Medical Nutrition), the active component of which is Fortasyn Connect. Fortasyn Connect is a multinutrient combination containing DHA; eicosapentaenoic acid (EPA); uridine monophosphate; choline; vitamins B12, B6, C, E, and folic acid; phospholipids; and selenium.3 Results from animal studies showed that this multinutrient combination improved neuronal membrane composition; increased the formation of synapses, cholinergic neurotransmission, and cerebral blood flow and perfusion; preserved neuronal integrity; restored hippocampal neurogenesis; reduced β-amyloid pathology; and improved cognition.10–16
In LipiDiDiet, a total of 311 patients with prodromal AD, defined according to International Working Group-1 criteria,17 were recruited from 11 sites in Finland, Germany, the Netherlands, and Sweden, and randomized to a 24-month treatment period with an optional 12-month double-blind extension. Patients taking putative non-prescription/prescription cognitive enhancers (eg, ginkgo) and statins could be included if the dosage had been stable for at least 3 months prior to randomization, and doses were to be kept stable during the study, if possible. Patients taking omega-3 preparations, or folic acid, vitamins B6, B12, C, and/or E at >200% the recommended daily intake, were excluded. The primary outcome variable was the change over 24 months in the neuropsychological test battery (NTB) composite z-score, based on Consortium to Establish a Registry for AD (CERAD) 10-word list learning immediate recall, CERAD 10-word delayed recall, CERAD 10-word recognition, category fluency, and the Letter Digit Substitution Test. Secondary outcomes included:
- NTB total (16-item), and memory and executive function domains
- Clinical Dementia Rating-Sum of Boxes (CDR-SB)
- Magnetic resonance imaging (MRI) measurement of hippocampal, ventricular, and whole brain volumes
- Serum concentrations of high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and plasma fatty acids (DHA and EPA)
- Cerebrospinal fluid concentration of DHA
- Safety parameters, comprising adverse events, concomitant medications, consumption of nutritional supplements, study product compliance, vital signs, and clinical safety laboratory tests.
Statistical considerations
The modified intention-to-treat (mITT) population included all randomized patients but excluded data after the start of rescue medication. The per-protocol (PP) population was defined as all participants from the mITT population excluding those with major protocol deviations. The most common reason for exclusion from the PP analysis was substantial irregular study product intake. Adherence was confirmed by significant biochemical changes in plasma DHA and EPA. All randomized participants who had taken at least one dose of study medication were included in the safety analyses.
The two treatment groups had comparable demographic characteristics except for Mini-Mental State Examination (MMSE), which was found to be a significant predictor of outcome parameters and a potential prognostic factor. The MMSE was therefore included as a covariate in all statistical models, except MMSE subgroup analyses.
Key findings
Of the 311 patients who were randomized, 245 (79%) completed the 24-month trial. During the trial period, 59 (37%) participants in the control group and 62 (41%) in the active group were diagnosed with dementia (p=0.642). A summary of the results for the primary and secondary outcome assessments is presented in Table 1.
 |
Table 1 Overview of primary and main secondary endpoints at 24 months in the LipiDiDiet trial. Adapted from Soininen H, Solomon A, Visser PJ, et al. LipiDiDiet clinical study group. 24-month intervention with a specific multinutrient in people with prodromal Alzheimer’s disease (LipiDiDiet): a randomised, double blind, controlled trial. Lancet Neurol. 2017;16(12):965–975. Creative commons license and disclaimer from: http://creativecommons.org/licenses/by/4.0/legalcode9 |
There was no statistically significant difference between groups for either the NTB primary endpoint or the secondary NTB composite. However, several secondary outcomes showed significantly less worsening in the active group compared with the control group:
- Less CDR-SB deterioration (45%, p=0.005).As baseline MMSE was an effect modifier for CDR-SB, an exploratory analysis was undertaken of CDR-SB performance across the range of baseline MMSE scores (≥24 to ≥29). The results suggested that the treatment effect was more pronounced with higher baseline MMSE scores
- Less reduction in hippocampal volume (26%, p=0.005)
- Less increase in ventricular volume (16%, p=0.046).
There were no statistically significant differences between groups for changes in whole brain volume.
Differences between the active and control groups in changes from baseline for cognition-related scores and MRI were more pronounced in the predefined subgroup analyses for the PP population than for the mITT population:
- PP: Significant difference from placebo in favor of Fortasyn Connect on NTB composite 10-item z score, NTB memory domain, CDR-SB, MRI hippocampal volume, and ventricular volume
- mITT: Significant difference from placebo in favor of Fortasyn Connect on CDR-SB and hippocampal volume.
Although HDL cholesterol values increased significantly in the active group, compared with the control group, the absolute changes were small (<5%) and not considered clinically relevant. There were no differences between groups for changes in LDL cholesterol.
The incidences of adverse events and serious adverse events were not significantly different between the overall treatment groups, or the subgroups of participants who dropped out (66 [21%] overall; 33 [21%] in the control group and 33 [22%] in the active group). None of the serious adverse events were regarded as related to the study product. The most common reasons for discontinuation from the study were withdrawal of informed consent (10 [6%] in the control group; 8 [5%] in the active group) and adverse events (6 [4%] in the control group; 9 [6%] in the active group). In the active group, adverse events contributing to discontinuation that were considered related to study product comprised eczema (2), upper abdominal pain (1), regurgitation (1), and lactose intolerance (1); none of these adverse events was serious.
Other clinical studies of Fortasyn Connect
In addition to the LipiDiDiet trial, data are available from five other clinical studies of Fortasyn Connect (Table 2). Four involved patients with AD (Souvenir I,18 Souvenir II,19 the open-label continuation study of Souvenir II,20 and S-Connect21) and a fifth was a proof of concept study in patients with the behavioral variant of frontotemporal dementia (bvFTD).22
 |
 |
 |
Table 2 Overview of clinical trial design and results for Fortasyn Connect |
The Souvenir I and II studies were conducted in drug-naïve patients with mild AD, while the S-Connect study recruited patients with mild-to-moderate AD (MMSE 14–24) taking stable doses of approved symptomatic AD treatments (cholinesterase inhibitors and/or memantine). In the 24-week, double-blind S-Connect study, where Fortasyn Connect or control product was added to current medication, Fortasyn Connect did not result in additional benefit on cognitive (Alzheimer’s Disease Assessment Scale-cognitive subscale [ADAS-cog]), functional or global outcomes. When discussing these results, the authors commented that a nutritional intervention targeting synaptogenesis would be more likely to show benefit in earlier disease.
Although two of the studies (Souvenir I and LipiDiDiet) failed to show significance on the primary outcome (see discussion below for factors that may have contributed to this), the overall impression from the three trials in prodromal and early AD is that Fortasyn Connect shows benefits on domains of cognition that are affected by AD (attention, memory, and executive function) and on nutritional measures that influence risk factors for its progression, and that it may have greater potential for benefit earlier rather than later in the disease. Current evidence also indicates that Fortasyn Connect is a safe and well-tolerated product with good patient acceptability.
Assessment of the whole brain, hippocampal, and ventricular volumes was only assessed in LipiDiDiet. The benefits on hippocampal volume (26% less reduction) and ventricular volume (16% less increase) in the active group, compared with the control group, need to be confirmed in future studies but may suggest an interaction of active treatment with the disease process. In a secondary analysis of the Souvenir II study (in which drug-naïve patients with mild AD were randomized to receive Fortasyn Connect or an iso-caloric control product once daily for 24 weeks), electroencephalography data were used to construct brain networks, and graph theory was then employed to quantify complex brain structure.23 Results showed that there was significantly greater preservation of the networks in the Fortasyn Connect group compared with the control group, indicating a potential benefit on synaptic integrity and function.23
The improvements seen in nutritional measures, which are important for the functioning of the Kennedy Cycle (ie, formation of neuronal membranes and synapses), suggest that deficiencies of key nutrients seen in people at risk of, or with, mild dementia can be corrected.
The results of the proof-of-concept study in bvFTD suggest a benefit not only on cognition but also on behavioral symptoms associated with dementia.
Considerations in the interpretation of clinical trial results
Several methodological issues may have influenced the ability of the Fortasyn Connect studies to demonstrate a difference between active intervention and control on the primary and secondary outcome variables, including choice of outcomes, compliance, patient population, performance of the control group, and study duration.
Decline in cognition in AD is not linear, with more mildly affected patients showing a slower rate of decline. The ADAS-Cog has been the standard measure of cognition in AD clinical trials. However, in recent trials in early stages of the disease, ADAS-Cog has shown limited sensitivity because it does not include assessment of the cognitive domains most affected in these populations, such as attention and executive function.24 A similar problem appears to be emerging when all domains of the NTB are evaluated together as a composite, rather than by evaluating the domains most likely to be affected. This is illustrated by results of the Fortasyn Connect trials, which were more likely to demonstrate a significant benefit on cognitive outcomes assessing attention, memory, and executive function.
There are similar considerations when choosing functional outcomes in more mildly affected populations. The Disability Assessment for Dementia functional scale, used in the Souvenir II study and its open-label extension, showed a large percentage of patients (26%) with maximum score (ie, no disability) at baseline. The CDR-SB may be a more appropriate scale to use in people with early AD, as it demonstrates negligible floor and ceiling effects and uses real-life activities.25
The inability to demonstrate significance on a primary endpoint can be affected by decline in the control group. The LipiDiDiet trial was performed soon after the first criteria for prodromal AD had been published. Experience since then has shown that changes in cognitive performance with currently used tests are not very pronounced in early AD. In LipiDiDiet, the estimates of decline and the trial’s power calculation were based on a previous 12-month trial in AD dementia. As the decline on the NTB primary endpoint in the control group was only one-quarter of that predicted over 24 months, the study lacked sufficient power to demonstrate significance.
Another way of exploring the clinical relevance of results is to consider effect sizes. For the two studies in patients with mild AD (Souvenir I and II), the effect sizes were 0.20 (95% confidence intervals: 0.10, 0.34) for one of the co-primary outcomes (delayed verbal recall task of the Wechsler Memory Scale revised) and 0.21 (95% confidence intervals: −0.06, 0.49) for the primary outcome (NTB memory z-score), respectively.26 This compares favorably with the effect sizes previously reported for cholinesterase inhibitors: 0.15, 0.23, and 0.28 for low, medium, and high doses, respectively, based on the primary endpoint of ADAS-Cog.27
Data have also shown that B-vitamin supplementation markedly slows cognitive decline and grey matter atrophy in areas of the brain related to AD.28 MRI studies have demonstrated that the hippocampus is the brain region of highest and earliest atrophy in AD, and that rate of hippocampal atrophy is a reliable measure of AD progression.29 Synaptic loss is a hallmark feature of early AD, and the constituents of Fortasyn Connect have been specifically designed to enhance synapse formation. The findings from Souvenir II on brain activity-based networks, taken together with the MRI findings from LipiDiDiet, suggest a potential benefit for Fortasyn Connect on underlying pathology processes associated with early AD. The benefit of Fortasyn Connect on nutritional measures, the implications of these for the Kennedy Cycle (neuronal membrane and synapse formation), and the link to effects on hippocampal and ventricular volumes, suggest that further evaluation of the effects of Fortasyn Connect on disease progression is warranted.
The potential for improving synaptic function is also relevant in bvFTD, where synaptic loss is considered one of the key elements in the neurodegenerative process leading to cognitive and behavioral symptoms. The results of the proof-of-concept study in bvFTD also deserve further investigation and indicate the potential value of Fortasyn Connect in other dementia subtypes.
In addition to age, cardiovascular disease, stroke, and elevated homocysteine are recognized risk factors for dementia and AD. Levels of homocysteine are increased by inadequate availability of vitamins B2, B6, B12, and folate, which are known to be deficient in people with AD despite a normal diet. Authors of the Framingham Study also concluded that homocysteine was a strong risk factor for dementia and AD, since there was almost double the rate of dementia in people with the highest quartile of plasma homocysteine.30 The potential for lowering homocysteine levels should, therefore, be regarded as an indicator of benefit in people at risk, or with, mild AD.
One would anticipate that an intervention such as a special food for medical purposes would take time to have an effect, would require good compliance and patient acceptability, and would benefit people earlier in their disease course. This is suggested by the findings of LipiDiDiet and other trials, which indicate that significance was more likely to be observed in the PP populations and in the longer duration studies. It will also, therefore, be particularly interesting to see if the benefits of Fortasyn Connect become more apparent in the long-term trial data still to be released.
Implications for clinical practice and future research
Although the role of nutrition in established dementia is unclear, there is accumulating evidence to support the value of healthy lifestyle and diet in helping to reduce the risk for dementia. Recent studies, such as the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER)31 and the Multidomain Alzheimer Preventive Trial (MAPT),32 have evaluated combinations of interventions that affect risk factors for AD progression. For example, in FINGER, participants were randomized to receive a 2-year multi-domain intervention (nutritional guidance; exercise; cognitive training and social activity; and management of metabolic and vascular risk factors) or regular health advice (control).31 Recent results from the study demonstrated that adherence to a healthy diet at baseline predicted improvement in global cognition, regardless of which intervention participants received (p=0.003), and that dietary improvement was associated with beneficial changes in executive function, particularly in the multi-domain intervention group (p=0.008; p=0.051 for the groups combined).33 Additional analysis demonstrated that the observed beneficial intervention effects on the primary cognitive outcome were unaffected by the presence of cardiovascular comorbidity (history of stroke, myocardial infarction, or diabetes); indeed, none of the participants’ characteristics were found to influence the intervention effects on executive functioning, processing speed, or memory, with the exception of diastolic blood pressure, which appeared to modify the intervention effects on processing speed, the effects being significantly more pronounced in those with lower vs higher diastolic blood pressure.34 The potential impact of comorbidities and/or the presence of modifiable cardiovascular and cerebrovascular risk factors on the observed findings in LipiDiDiet have not been reported. Similarly, patients’ medications and baseline supplemented nutrients were not reported in LipiDiDiet and may also potentially have had a confounding influence on the trial’s outcomes (although the use of omega-3 preparations, and the intake of folic acid and vitamins B6, B12, C, and/or E at >200% the recommended daily intake, were exclusion criteria). The influence of such factors requires further research, in order to clarify whether there may be certain subgroups of patients most likely to benefit from targeted intervention.
In current clinical practice, more attention is being paid to nutrition and lifestyle in people with long-term conditions, such as cardiovascular disease and diabetes, which are risk factors for dementia, but little attention is paid to nutrition in other populations, especially the elderly. Therefore, there needs to be increased awareness, education, and training about the importance of nutrition for health and social care professionals, people with dementia and their families and carers. Moreover, nutritional assessment and dietetic support should be commissioned as part of the dementia pathway.
It is now recognized that there is a long asymptomatic phase of AD before the onset of symptoms that can be tracked by monitoring various biomarkers, such as amyloid plaques, tau tangles, and inflammation. Future studies should target populations not only with risk factors but also with biomarkers of disease.
Fortasyn Connect contains a unique combination of nutrients at levels difficult to achieve from diet alone. The data demonstrate the safety and potential benefit of Fortasyn Connect in the dietary management of early AD and suggest further study is warranted into its effects when used alone and in combination with other preventative strategies.
Acknowledgments
Editorial support was provided by mXm Medical Communications. Editorial support was funded by Nutricia Advanced Medical Nutrition.
Disclosure
Current roles: Royal College of General Practitioners clinical representative for dementia, independent consultant for PsiNapse and advisor for Kent Surrey Sussex Academic Health Science Network. Past roles: clinical lead, Dementia South East Clinical Network, general practitioner in Surrey. The author reports no other conflicts of interest in this work.
References
1. Kennedy EP, Weiss SB. The function of cytidine coenzymes in the biosynthesis of phospholipids. J Biol Chem. 1956;222(1):193–214.
2. Wurtman RJ, Cansev M, Sakamoto T, Ulus IH. Use of phosphatide precursors to promote synaptogenesis. Annu Rev Nutr. 2009;29:59–87. doi:10.1146/annurev-nutr-080508-141059
3. van Wijk N, Broersen LM, de Wilde MC, et al. Targeting synaptic dysfunction in Alzheimer’s disease by administering a specific nutrient combination. J Alzheimer’s Dis. 2014;38(3):459–479. doi:10.3233/JAD-130998
4. Jicha GA, Markesbery WR. Omega-3 fatty acids: potential role in the management of early Alzheimer’s disease. Clin Interv Aging. 2010;5:45–61. doi:10.2147/CIA.S5231
5. Smach MA, Jacob N, Golmard J-L, et al. Folate and homocysteine in the cerebrospinal fluid of patients with Alzheimer’s disease or dementia: a case control study. Eur Neurol. 2011;65(5):270–278. doi:10.1159/000326301
6. Glasø M, Nordbø G, Diep L, Bøhmer T. Reduced concentrations of several vitamins in normal weight patients with late-onset dementia of the Alzheimer type without vascular disease. J Nutr Health Aging. 2004;8(5):407–413.
7. Olde Rikkert MG, Verhey FR, Sijben JW, et al. Differences in nutritional status between very mild Alzheimer’s disease patients and healthy controls. J Alzheimers Dis. 2014;(41):261–271. doi:10.3233/JAD-131892
8. Cohen BM, Renshaw PF, Stoll AL, Wurtman RJ, Yurgelun-Todd D, Babb SM. Decreased brain choline uptake in older adults. An in vivo proton magnetic resonance spectroscopy study. JAMA. 1995;274(11):902–907.doi:10.1001/jama.1995.03530110064037
9. Soininen H, Solomon A, Visser PJ, et al. LipiDiDiet clinical study group. 24-month intervention with a specific multinutrient in people with prodromal Alzheimer’s disease (LipiDiDiet): a randomised, double-blind, controlled trial. Lancet Neurol. 2017;16(12):965–975. doi:10.1016/S1474-4422(17)30332-0
10. Broersen LM, Kuipers AA, Balvers M, et al. A specific multi-nutrient diet reduces alzheimer-like pathology in young adult AbetaPPswe/PS1dE9 mice. J Alzheimers Dis. 2013;33(1):177–190. doi:10.3233/JAD-2012-112039
11. Koivisto H, Grimm MO, Rothhaar TL, et al. Special lipid-based diets alleviate cognitive deficits in the APPswe/PS1dE9 transgenic mouse model of Alzheimer’s disease independent of brain amyloid deposition. J Nutr Biochem. 2014;25(2):157–169. doi:10.1016/j.jnutbio.2013.09.015
12. Zerbi V, Jansen D, Wiesmann M, et al. Multinutrient diets improve cerebral perfusion and neuroprotection in a murine model of Alzheimer’s disease. Neurobiol Aging. 2014;35(3):600–613. doi:10.1016/j.neurobiolaging.2013.09.038
13. Holguin S, Martinez J, Chow C, Wurtman R. Dietary uridine enhances the improvement in learning and memory produced by administering DHA to gerbils. FASEB J. 2008;22(11):3938–3946. doi:10.1096/fj.08-112425
14. Jansen D, Zerbi V, Arnoldussen IA, et al. Effects of specific multi-nutrient enriched diets on cerebral metabolism, cognition and neuropathology in AbetaPPswe-PS1dE9 mice. PLoS One. 2013;8(9):e75393. doi:10.1371/journal.pone.0075393
15. Cansev M, van Wijk N, Turkyilmaz M, Orhan F, Sijben JW, Broersen LM. Specific multi-nutrient enriched diet enhances hippocampal cholinergic transmission in aged rats. Neurobiol Aging. 2015;36(1):344–351. doi:10.1016/j.neurobiolaging.2014.07.021
16. Janickova H, Rudajev V, Dolejsi E, et al. Lipid-based diets improve muscarinic neurotransmission in the hippocampus of transgenic APPswe/PS1dE9 Mice. Curr Alzheimer Res. 2015;12(10):923–931. doi:10.2174/1567205012666151027130350
17. Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6(8):734–746. doi:10.1016/S1474-4422(07)70178-3
18. Scheltens P, Kamphuis PJ, Verhey FR, et al. Efficacy of a medical food in mild Alzheimer’s disease: a randomized, controlled trial. Alzheimers Dement. 2010;6(1):1–10.e1. doi:10.1016/j.jalz.2009.10.003
19. Scheltens P, Twisk JW, Blesa R, et al. Efficacy of Souvenaid in mild Alzheimer’s disease: results from a randomized, controlled trial. J Alzheimers Dis. 2012;31(1):225–236. doi:10.3233/JAD-2012-121189
20. Olde Rikkert MG, Verhey FR, Blesa R, et al. Tolerability and safety of Souvenaid in patients with mild Alzheimer’s disease: results of multi-center, 24-week, open-label extension study. J Alzheimers Dis. 2015;44(2):471–480. doi:10.3233/JAD-141305
21. Shah RC, Kamphuis PJ, Leurgans S, et al. The S-Connect study: results from a randomized, controlled trial of Souvenaid in mild-to-moderate Alzheimer’s disease. Alzheimers Res Ther. 2013;5(6):59. doi:10.1186/alzrt224
22. Pardini M, Serrato C, Guida S, et al. Souvenaid reduces behavioral deficits and improves social cognition skills in frontotemporal dementia: a proof-of-concept study. Neurodegener Dis. 2015;15(1):58–62. doi:10.1159/000369811
23. de Waal H, Stam CJ, Lansbergen MM, et al. The effect of souvenaid on functional brain network organisation in patients with mild Alzheimer’s disease: a randomised controlled study. PLoS One. 2014;9(1):e86558. doi:10.1371/journal.pone.0086558
24. Podhorna J, Krahnke T, Shear M, Harrison JE. Alzheimer’s Disease Neuroimaging Initiative. Alzheimer’s Disease Assessment Scale–cognitive subscale variants in mild cognitive impairment and mild Alzheimer’s disease: change over time and the effect of enrichment strategies. Alzheimer’s Res Ther. 2016;8:8. doi:10.1186/s13195-016-0170-5
25. Samtani MN, Raghavan N, Novak G, Nandy P, Narayan VA. Disease progression model for Clinical Dementia Rating–Sum of Boxes in mild cognitive impairment and Alzheimer’s subjects from the Alzheimer’s Disease Neuroimaging Initiative. Neuropsychiatr Dis Treat. 2014;10:929–952. doi:10.2147/NDT.S62323
26. Cummings J, Scheltens P, McKeith I, et al. Effect size analyses of Souvenaid in patients with Alzheimer’s disease. J Alzheimers Dis. 2017;55(3):1131–1139. doi:10.3233/JAD-160745
27. Rockwood K. Size of the treatment effect on cognition of cholinesterase inhibition in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004;75(5):677–685. doi:10.1136/jnnp.2003.029074
28. Douaud G, Refsum H, de Jager CA, et al. Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc Natl Acad Sci USA. 2013;110(23):9523–9528. doi:10.1073/pnas.1301816110
29. Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13(6):614–629. doi:10.1016/S1474-4422(14)70090-0
30. Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346(7):476–483. doi:10.1056/NEJMoa011613
31. Kivipelto M, Solomon A, Ahtiluoto S, et al. The Finnish Geriatric Intervention Study to prevent cognitive impairment and disability (FINGER): study design and progress. Alzheimers Dement. 2013;9(6):657–665. doi:10.1016/j.jalz.2012.09.012
32. Gillette-Guyonnet S, Andrieu S, Dantoine T, Dartigues JF, Touchon J, Vellas B; MAPT Study Group. Commentary on “A roadmap for the prevention of dementia II. Leon Thal Symposium 2008.” The Multidomain Alzheimer Preventive Trial (MAPT): a new approach to the prevention of Alzheimer’s disease. Alzheimers Dement. 2009;5(2):114–121. doi:10.1016/j.jalz.2009.01.008
33. Lehtisalo J, Levälahti E, Lindström J, et al. Dietary changes and cognition over 2 years within a multidomain intervention trial-The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER). Alzheimers Dement. 2019;15(3):410–417. doi:10.1016/j.jalz.2018.10.001
34. Rosenberg A, Ngandu T, Rusanen M, et al. Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: the FINGER trial. Alzheimers Dement. 2018;14(3):263–270. doi:10.1016/j.jalz.2017.09.006
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
