Back to Journals » Pharmacogenomics and Personalized Medicine » Volume 10
The HLA-A*31:01 allele: influence on carbamazepine treatment
Authors Yip VLM, Pirmohamed M
Received 3 November 2016
Accepted for publication 14 December 2016
Published 31 January 2017 Volume 2017:10 Pages 29—38
DOI https://doi.org/10.2147/PGPM.S108598
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin Bluth
Vincent Lai Ming Yip,1,2 Munir Pirmohamed1,2
1MRC Centre for Drug Safety Science, Institute of Translational Medicine, Department of Molecular and Clinical Pharmacology, University of Liverpool, 2Department of Clinical Pharmacology,The Royal Liverpool and Broadgreen University Hospital NHS Trust, Liverpool, UK
Abstract: Carbamazepine (CBZ) is an effective anticonvulsant that can sometimes cause hypersensitivity reactions that vary in frequency and severity. Strong associations have been reported between specific human leukocyte antigen (HLA) alleles and susceptibility to CBZ hypersensitivity reactions. Screening for HLA-B*15:02 is mandated in patients from South East Asia because of a strong association with Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). HLA-A*31:01 predisposes to multiple phenotypes of CBZ hypersensitivity including maculopapular exanthema, hypersensitivity syndrome, and SJS/TEN in a range of populations including Europeans, Japanese, South Koreans and Han Chinese, although the effect size varies between the different phenotypes and populations. Between 47 Caucasians and 67 Japanese patients would need to be tested for HLA-A*31:01 in order to avoid a single case of CBZ hypersensitivity. A cost-effectiveness study has demonstrated that HLA-A*31:01 screening would be cost-effective. Patient preference assessment has also revealed that patients prefer pharmacogenetic screening and prescription of alternative anticonvulsants compared to current standard of practice without pharmacogenetic testing. For patients who test positive for HLA-A*31:01, alternative treatments are available. When alternatives have failed or are unavailable, HLA-A*31:01 testing can alert clinicians to 1) patients who are at increased risk of CBZ hypersensitivity who can then be targeted for more intensive monitoring and 2) increase diagnostic certainty in cases where hypersensitivity has already occurred, so patients can be advised to avoid structurally related drugs in the future. On the basis of the current evidence, we would favor screening all patients for HLA-A*31:01 and HLA-B*15:02 prior to starting CBZ therapy.
Keywords: carbamazepine, oxcarbazepine, hypersensitivity, adverse drug reaction, pharmacogenetics, HLA
Introduction
Carbamazepine (CBZ) is an effective anticonvulsant that is also used the in the treatment of psychiatric disorders.1,2 However, it is associated with hypersensitivity reactions in up to 10% of patients.1 These reactions include severe conditions, such as toxic epidermal necrolysis (TEN), Stevens–Johnson syndrome (SJS), hypersensitivity syndrome (HSS) and milder reactions, such as maculopapular exanthema (MPE).3 The mortality rate of TEN at 1 year is 34%,4 and in pediatric patients who survived acute TEN, all patients suffered with long-term complications, which included scarring, visual loss and chronic kidney disease.5 Strong associations have been reported between specific human leukocyte antigen (HLA) alleles and susceptibility to CBZ hypersensitivity reactions.6–8
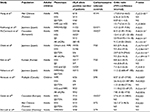
HLA alleles are encoded by the major histocompatibility complex (MHC), are found in all vertebrates and are responsible for presentation of protein-derived peptides to T cells as part of the adaptive immune response.9 There are two main classes of MHC molecules: class I (MHC-I) and class II (MHC-II). MHC-I molecules are encoded by three genes: HLA-A, HLA-B and HLA-C. Similarly, there are three MHC-II molecules called HLA-DR, HLA-DQ and HLA-DP.10 HLA genes constitute the most polymorphic gene cluster in the human genome with most allelic diversity concentrated in peptide binding sites of the HLA molecules enabling different alleles to bind a range of peptides.10 Specific polymorphisms in HLA molecules have been associated with increased susceptibility to a number of hypersensitivity reactions affecting different organs and caused by a wide variety of therapeutically and structurally distinct drugs (Table 1).
CBZ-induced SJS/TEN has been strongly associated with carriage of HLA-B*15:02 in patients from South East Asian countries.6,11–15 A prospective cohort study in Taiwan demonstrated the clinical utility of pharmacogenetic screening for HLA-B*15:02 in preventing CBZ-induced SJS/TEN.16 Regulatory agencies, such as the US Food and Drug Administration and the European Medicines Agency, have included warnings in the drug label and summary of product characteristic (SmPC), respectively, advising pharmacogenetic screening in patients from particular populations in South East Asia.17 The strong association of HLA-B*15:02 with CBZ-induced SJS/TEN in South East Asian countries, but not in other countries, reflects the higher prevalence of this allele in those countries (Figure 1). Despite the fact that HLA-B*15:02 is rare in Caucasians (prevalence <0.01%), if a Northern European patient is positive for this allele, it would be important not to challenge the patient with CBZ,18 although there is no specific evidence of the association in Caucasians.
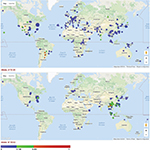  | Figure 1 Allele frequency distribution for HLA-A*31:01 and HLA-B*15:02. Notes: HLA-A*31:01 is widely distributed in comparison with HLA-B*15:02, which is predominantly concentrated in South East Asia. Adapted from Gonzalez-Galarza FF, Takeshita LY, Santos EJ, et al. New features for HLA epitopes, KIR and disease and HLA adverse drug reaction associations. Nucleic Acid Research. 2015;28:D784–788.64 |
HLA-A*31:01 has also been associated with CBZ-induced hypersensitivity reactions in multiple populations including European and Japanese patients but pharmacogenetic screening is not currently mandated before initiation of CBZ therapy.7,8 It is included in a number of drug labels worldwide but the association with HLA-A*31:01 is mentioned for information only. Unlike HLA-B*15:02, which is largely restricted to South East Asia, HLA-A*31:01 is present in many different populations worldwide (Figure 1). The allele frequency of HLA-A*31:01 in European populations ranges between 2.1% and 3.6%. The frequency of HLA-A*31:01 varies across Asian populations: Han Chinese (2.8%–3.6%), Korean (5.6%) and Japanese (7.1%–12%). The highest frequencies have been reported in South American countries, such as Argentina (25%–38.6%) and Brazil (2.6%–18.5%; www.allelefrequencies.net).
This review aims to summarize the association between HLA-A*31:01 and CBZ hypersensitivity, evaluate the association studies that have been performed to date, discuss the proposed interaction between CBZ and HLA-A*31:01, identify the challenges in applying pharmacogenetic screening for HLA-A*31:01 and proposals for overcoming these barriers.
HLA-A*31:01 and CBZ hypersensitivity
Retrospective case–control studies in multiple patient populations have reported associations between HLA-A*31:01 and CBZ hypersensitivity (Table 2). The first association between HLA-A*31:01 and CBZ hypersensitivity was reported in Han Chinese patients from Taiwan.19 In this study, a significant association was reported between HLA-A*31:01 and CBZ-induced MPE but not for SJS-TEN.
Subsequently, two independent genome-wide association studies (GWAS) in European and Japanese patients demonstrated that carriage of HLA-A*31:01 was significantly associated with all phenotypes of CBZ hypersensitivity.7,8 In the European study, HLA-A*31:01 was significantly associated with HSS (P=3.5×10−8) in a GWAS. Subsequent analyses of replication cohorts showed HLA-A*31:01 to be associated with all phenotypes of CBZ hypersensitivity: CBZ-induced SJS/TEN (n=12 cases, odds ratio [OR]=25.93 [95% confidence interval [CI]: 4.93–116.18], P=8×10−5), HSS (n=27 cases, OR=12.41 [95% CI: 1.27–121.03], P=0.03) and MPE (n=106 cases, OR=8.33 [95% CI: 3.59–19.36], P=8.0×10−7).7 Similar results were reported in the Japanese GWAS with HLA-A*31:01 significantly associated with all phenotypes of CBZ hypersensitivity both individually and in pooled analysis (n=77 cases, OR=9.5 [95% CI: 5.6–16.3], Pc=1.09×10−16).8 The association between HLA-A*31:01 and all clinical presentations of CBZ hypersensitivity in Japanese patients has been replicated in two further case–control studies.20,21
Subsequent studies have also investigated the association between HLA-A*31:01 and CBZ hypersensitivity in different populations. In Han Chinese, one study detected a significant association between HLA-A*31:01 and CBZ-induced MPE (n=18 cases, OR=17.5 [95% CI: 4.6–66.5], Pc=2.2×10−3) but not HSS; however, only 13 patients with HSS were investigated.19 Another study in Han Chinese detected a strong association between HLA-A*31:01 and CBZ-induced HSS (n=10 cases; OR=26.3 [95% CI: 7.2–96.5], P<0.001) but no patients with MPE were included in this study.22 However, neither study showed an association between HLA-A*31:01 and CBZ-induced SJS/TEN in Han Chinese patients,19,22 where there is already a very strong association between HLA-B*15:02 and SJS/TEN. The molecular mechanisms by which HLA-B*15:02 predisposes to SJS/TEN only, whereas HLA-A*31:01 predisposes to several different phenotypes is not known, but may be a reflection of differences in the affinity and mechanisms of binding and antigen presentation in the two HLA alleles. Thus, if a patient is positive for both HLA-B*15:02 and HLA-A*31:01, binding to the former allele could be greater than to the latter, leading to the development of SJS/TEN rather than another CBZ hypersensitivity phenotype. Clearly, this is a hypothesis which needs further investigation.
In Korean patients, a significant association for HLA-A*31:01 was detected for CBZ-induced HSS (n=17 cases, OR=8.8 [95% CI: 2.5–30.7], Pc=0.011), but not SJS/TEN; however, only 7 SJS/TEN patients were included, 3 of whom were positive for HLA-A*31:01.23 In Caucasian adults with CBZ-induced SJS/TEN only 3/20 subjects possessed HLA-A*31:01 compared with 10/257 tolerant controls.22 The same study reported a strong association of HLA-A*31:01 with HSS (n=10 cases, OR=57.6 [95% CI: 11.0–340], P<0.001). A study in children with multiple ethnic backgrounds from Canada reported significant associations between HLA-A*31:01 and CBZ-induced MPE (n=26 cases, OR=8.57 [95% CI: 1.67–57.50], P=0.0037) and HSS (n=6 cases, OR=26.36 [95% CI: 2.53–307.89], P=0.0025).24 None of the 9 children presenting with SJS/TEN were positive for HLA-A*31:01.
Recently, the presence of the HLA-A*31:01 allele was confirmed in a familial case of CBZ-induced HSS.25 The index case, a 23-year-old Caucasian male, developed HSS after 2 weeks of CBZ therapy for epilepsy. Three months later, his mother also presented with symptoms compatible with HSS after 9 weeks of therapy with CBZ for trigeminal neuralgia. Carriage of HLA-A*31:01 was confirmed in both subjects with the authors advising other family members to avoid CBZ in the future.
The association between HLA-A*31:01 and CBZ hypersensitivity was not detected in a population from Norway.26 There were 48 cases of CBZ hypersensitivity in this study, but nearly all patients (43/48 [89.6%]) were diagnosed with MPE according to the phenotype standardization for immune-mediated drug-induced skin injury guidance.27 A major issue with MPE is that causality determination is more difficult as many other factors including concomitant viral infections can cause mild cutaneous eruptions.
These studies confirm an association between carriage of HLA-A*31:01 and increased susceptibility to CBZ-induced hypersensitivity reactions. While the association with HSS seems clear from all the studies, whether HLA-A*31:01 is also associated with MPE and SJS-TEN is more controversial (Table 2). In Han Chinese patients, significant associations have been reported with MPE19 and HSS22 but not SJS.22 In Japanese patients, the GWAS detected significant associations between HLA-A*31:01 and MPE, HSS and SJS/TEN.8 The original GWAS in Caucasian patients reported significant associations between carriage of HLA-A*31:01 and CBZ-induced MPE, HSS and SJS/TEN.7 A subsequent study detected an association with HSS but not SJS,22 whereas the most recent study in a Norwegian population was unable to detect any association between HLA-A*31:01 and CBZ-induced MPE.26 The discrepancies in the studies are most likely to be due to a combination of small sample sizes, incorrect classification/diagnosis of cases and difficulty in determining causality particularly in milder cases. It is important to note that diagnosis of CBZ hypersensitivity reactions is complex because many patients are prescribed multiple medications preceding a reaction with diverse clinical presentations and variable times to onset of hypersensitivity, and the difficulty in excluding other nondrug etiologies.28 Standardized criteria for classification of drug-induced hypersensitivity reactions have been developed and should be used in clinical studies.27
HLA-A*31:01 and oxcarbazepine (OXC) hypersensitivity
OXC is a 10-keto analog of CBZ with an altered pharmacokinetic profile designed to reduce formation of reactive metabolites in comparison with CBZ.29 OXC has equal efficacy to CBZ for seizure control, but may also have a reduced tendency to cause liver toxicity, anemia and agranulocytosis.30 The incidence of cutaneous ADRs to OXC is lower than with CBZ.31 A study of 40 Korean patients with OXC-induced MPE identified 6 carriers of HLA-A*31:01 but the association was not significant when compared with tolerant controls (OR=0.85 [95% CI: 0.29–2.48], P=1.000).32 The study also failed to identify an association between OXC-MPE and HLA-B*15:02. Other studies have included small numbers of patients with OXC, with some patients being positive for HLA-A*31:01.24 Taken together, the numbers of patients tested for HLA-A*31:01 who have had OXC hypersensitivity reactions are small, and no conclusions can be made at this stage as to whether this allele is a predisposing factor.
Pathophysiology of CBZ hypersensitivity reactions
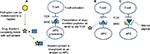
Drug-induced hypersensitivity reactions are characterized by the activation of T cells by drugs or reactive metabolites.33 The hapten hypothesis,34 direct pharmacologic interaction of drugs with immune receptors (PI)35 and the altered peptide repertoire model36 have been proposed as potential mechanisms for activation of T cells by drugs (Figure 2).
In the hapten hypothesis chemically reactive small molecules, such as drugs or reactive metabolites, act as haptens to bind and irreversibly modify self-proteins. These modified proteins are recognized as antigens and presented in association with MHC to T-cell receptors (TCRs), leading to the activation of immune system and hypersensitivity.34 According to the PI model, small molecules, such as drugs, are able to bind directly and noncovalently to either the MHC or TCR to activate the immune system.37 In the altered peptide repertoire model, low molecular weight drugs bind to the antigen binding cleft of the HLA-class I molecules leading to conformational changes and an altered repertoire of peptides that are presented, which may now include self-peptides.36
Much of the research in CBZ hypersensitivity has focused on the interaction between HLA-B*15:02, CBZ and the TCR. There is evidence to suggest that CBZ can activate the immune system via a combination of pathways. Reactive metabolites of CBZ, such as CBZ 10,11-epoxide, are able to modify serum proteins, such as human serum albumin, leading to the generation of chemically modified peptides that have the potential to activate the immune system via a hapten mechanism.38 CBZ is able to bind directly with HLA-B*15:02 independent of intracellular metabolism or processing consistent with a PI mechanism.39 Structural modeling suggests that CBZ is located at the interface between the HLA-B*15:02/peptide and TCR, with direct contact to the antigen peptide and bound within the TCR pocket.40 Further evidence to support direct interaction between CBZ and the TCR is the clonal expansion of specific TCRs observed in SJS/TEN patients compared with tolerant controls.41 These CBZ-specific CD8+ T cells secrete granulysin and interferon-gamma, which mediate keratinocyte apoptosis in a HLA-B*15:02-dependent manner consistent with the known pathogenesis of SJS/TEN.41,42 Finally, preliminary studies suggest that binding of CBZ to HLA-B*15:02 may lead to an alteration of the repertoire of presented self-peptides36 and activation of T cells only in the presence of endogenous peptides, but further work is needed to substantiate this.39
There have been very limited studies investigating the role of HLA-A*31:01 in CBZ hypersensitivity. A case study in a HLA-A*31:01 positive patient presenting with CBZ-induced MPE with eosinophilia and lymphocytosis demonstrated expansion of HLA-A*31:01 restricted CD8+ T-cell clones and DRB1*04:04 restricted CD4+ T-cell clones, indicating that a common HLA haplotype may contribute to the multiclonal T-cell response seen in European patients with CBZ hypersenstivity.43 It is unclear at present why HLA-B*15:02 predisposes to CBZ-induced SJS/TEN only, whereas HLA-A*31:01 is associated with multiple phenotypes of hypersensitivity. Further studies including larger numbers of patients who are carriers of HLA-A*31:01 are required to characterize the causal pathways.
Pharmacogenetic testing for HLA-A*31:01 prior to CBZ therapy
Pharmacogenetic testing for HLA-B*15:02 is recommended before initiation of CBZ therapy in patients of Asian origin.17 The utility of genotype testing for HLA-B*15:02 has been confirmed in a prospective study in a Taiwanese population where pre-prescription genotyping reduced the incidence of CBZ-induced SJS/TEN from 10 expected cases to 0.16 No prospective genotyping studies for HLA-A*31:01 have been published, although one is currently being undertaken in Japan.
Three systematic reviews have examined the association between HLA-A*31:01 and CBZ-induced hypersensitivity in multiple ethnic groups.3,22,44 A pooled analysis of all phenotypes of CBZ hypersensitivity and carriage of HLA-A*31:01 reported a pooled OR=9.45 (95% CI: 6.41–13.93; P<0.00001).3 The second systematic review analyzed the association between HLA-A*31:01 and CBZ-induced HSS and SJS/TEN separately.22 A strong pooled OR=13.2 (95% CI: 8.4–20.8; P<0.00001) was reported for the association with HSS, whereas a weaker pooled OR=3.9 (95% CI: 1.4–11.5; P=0.01) was reported for SJS/TEN.22 No patients with MPE were included in the second review. In the third systematic review, carriage of HLA-A*31:01 was reported to increase the risk of CBZ-induced HSS in Caucasian and Japanese/Korean patients by 14- and 10-fold, respectively.44 Susceptibility to all phenotypes of CBZ hypersensitivity was increased by 7-fold in Caucasians and 8-fold in Japanese patients positive for HLA-A*31:01.44
Estimates for sensitivity, specificity, positive predictive value, negative predictive value (NPV) and the number needed to test (NNT) to prevent a CBZ hypersensitivity reaction have been generated from the two large GWAS (Table 3).7,8 In Japanese patients, the NNT to prevent one CBZ hypersensitivity reaction is 67 based on an incidence of CBZ hypersensitivity reaction of 2.9%.8 The incidence of CBZ hypersensitivity in European patients was estimated to be 10%1 meaning the NNT to prevent one case of CBZ hypersensitivity in Caucasians is 47.3 In Han Chinese patients, HLA-A*31:01 was significantly associated with CBZ-induced HSS.22 The NNT was 5000 in order to prevent one case of HSS as the incidence of CBZ-induced HSS was estimated as 0.05%.22
A single study has examined the cost-effectiveness of pharmacogenetic screening for HLA-A*31:01 prior to the initiation of CBZ therapy in the UK.45 The authors concluded that routine testing for HLA-A*31:01 in order to reduce the incidence of hypersensitivity reactions in patients being prescribed CBZ for epilepsy is likely to be cost-effective. The cost-effectiveness model predicted a reduction in cases from 780 to 700 per 10,000 patients with an incremental cost-effectiveness ratio of £12,808 per quality-adjusted life-year (QALY) gained, which is below the threshold used by the UK National Institute for Health and Care Excellence to judge cost-effectiveness (£20,000–30,000/QALY).
A study of patient and physician expectations of pharmacogenetic testing prior to CBZ therapy revealed that patients were willing to accept a less effective anticonvulsant drug if the treatment had less risk of harm; whereas neurologists placed emphasis on higher NPVs for pharmacogenetic testing in order to reduce the likelihood of false negative tests.46 Based on actual rates of CBZ hypersensitivity and the characteristics of HLA-A*31:01 testing, patients preferred testing and prescription of an alternative anticonvulsant, such as lamotrigine (conditional on test result) compared with current standard of care (no pharmacogenetic testing).
Although the majority of hypersensitivity reactions with CBZ present as the milder MPE phenotype; it is not possible to distinguish the patients who will progress from MPE to the more severe systemic and blistering conditions. Therefore, patients are currently advised to stop CBZ on first occurrence of cutaneous eruption because early discontinuation of culprit drug reduces the risk of progression to more severe disease and death.47 Patients who test positive for HLA-A*31:01 can be prescribed alternative antiepileptic drug (AED) therapy, such as lamotrigine, which has not been associated with hypersensitivity in HLA-A*31:01 carriers.48 In patients positive for HLA-A*31:01 who still require CBZ therapy, for example, when alternative treatments have failed or are unavailable, pharmacogenetics testing can still help alert clinicians to patients at greater risk of hypersensitivity and to monitor these patients more closely.
One study has explored the potential for using a combined HLA-A*31:01 and HLA-B*15:02 pharmacogenetic test to prevent CBZ hypersensitivity in a Han Chinese population.22 Using a combined test, the NNT to prevent one case was 455 with 94 out of 1000 patients being unnecessarily denied CBZ. The potential clinical utility and cost-effectiveness of the combined test will need to be evaluated in further clinical studies.
If pretreatment testing for HLA-A*31:01 is adopted into clinical practice it is important that physicians receive education regarding pharmacogenetic testing. In Hong Kong, introduction of HLA-B*15:02 testing led to the unintended consequence of reduction in the prescription of CBZ from 16.2% to 2.6% of all new AEDs, with a switch to other AEDs such as phenytoin and lamotrigine, which are also associated with SJS-TEN.49 Thus, while the incidence of CBZ-induced SJS-TEN decreased in those patients tested for HLA-B*15:02, the overall incidence of SJS-TEN did not change, as patients were put on other drugs associated with SJS-TEN, where no recommendation for HLA screening has been mandated (because no strong HLA associations have been demonstrated). It is known that there is cross-reactivity between different aromatic anticonvulsants, such as phenytoin, where a weaker association between SJS/TEN and HLA-B*15:02 has been reported.50
The Canadian Pharmacogenomics Network for Drug Safety recommend pharmacogenetic testing for HLA-A*31:01 before initiation of CBZ in patients of all ancestries to reduce the incidence of hypersensitivity reactions. It also recommends testing in patients who have had a previous hypersensitivity reaction where CBZ may have been the culprit drug or where reinitiation of CBZ is being considered. A positive test would increase the likelihood of the previous hypersensitivity reaction being related to CBZ.44
Conclusion
There is no doubt that there is an association between HLA-A*31:01 and CBZ hypersensitivity, in particular to HSS, in many different ethnic groups. This association may also be relevant for MPE, but the association is confounded by the fact that causality can be due to other factors, which are not easily distinguished from CBZ. There is also an association with SJS-TEN, albeit weaker than with HSS, in many populations, but not in South East Asians, where the prevalent HLA-B*15:02 allele has an extremely strong association with CBZ-induced SJS-TEN. It is important to stress that the association with HLA-B*15:02 is limited to CBZ-induced SJS-TEN, whereas the association with HLA-A*31:01 may be important for all CBZ hypersensitivity phenotypes,3 but the molecular mechanisms and pathways underlying these distinct clinical manifestations are unclear.
Currently, the CBZ label/SmPC mandates testing for HLA-B*15:02 before the use of CBZ in certain ethnic groups, but mentions HLA-A*31:01 for information only (Figure 3). Arguably, this could be considered to be appropriate in regulatory terms because 1) the association with HLA-B*15:02 and an immune-mediated reaction with CBZ is stronger than that seen with HLA-A*31:01;3 2) it prevents the most serious reaction associated with CBZ (i.e., SJS-TEN); and 3) the importance of pre-prescription genotyping has been shown in a prospective study.16 Conversely, there are also arguments in favor of harmonizing the SmPC for CBZ to mandate pre-prescription genetic testing for all patients for both HLA-B*15:02 and HLA-A*31:01 (in keeping with the Canadian Pharmacogenomics Network for Drug Safety recommendations).44 These include:
- Testing for some groups based on ethnicity while ignoring the rest is likely to lead to health inequalities.
- The association of HLA-A*31:01 with CBZ hypersensitivity has been replicated in many populations, and it is now widely accepted that for precision medicine to succeed, we need to look at all forms of evidence, rather than relying on the usual paradigm of prospective studies or randomized trials.51 The CBZ SmPC (Figure 3) currently states that “There are insufficient data supporting a recommendation for HLA-A*31:01 screening before starting CBZ treatment”, but it is not clear what data would be regarded as being sufficient.
- Testing for HLA-A*31:01 before prescribing CBZ has been shown to be cost-effective,45 and at present, there is a disconnect between health technology assessment and regulatory advice.
- There are alternative drugs available for patients who test positive for HLA-A*31:01. However, even when CBZ is the preferred alternative, a test that shows a patient is positive for HLA-A*31:01 would allow for closer monitoring and stopping the drug quickly when a patient presents with signs of hypersensitivity. Our initial study showed that application of the test would increase the posttest probability to 26% from the current 5% without the test.7
- Although testing for HLA-A*31:01 will largely prevent the milder cutaneous reactions, we cannot at present predict which patients with mild reactions will progress to the more serious reactions such as HSS and SJS/TEN, and thus by default serious reactions will also be prevented.
  | Figure 3 A comparison of the wording in the carbamazepine summary of product characteristics approved by the European Medicines Agency for testing for HLA alleles. Notes: Adapted from Electronic Medicines Compendium website. Available from https://www.medicines.org.uk/emc/medicine/24201.65 Abbreviations: AGEP, acute generalized exanthematous pustulosis; DRESS, drug reaction with eosinophilia and systemic symptoms; HLA, human leukocyte antigen; SJS, Stevens–Johnson syndrome; TEN, toxic epidermal necrolysis. |
Given the above arguments, on balance, we would favor that patients starting on CBZ are genotyped for both HLA-B*15:02 and HLA-A*31:01. The success of this approach will depend on the availability of HLA testing, rapid turnaround times for the test (so that patients are not kept waiting to start their treatment), education of the prescribers, preferably accompanied by decision support to enable correct interpretation of the test results, and warnings that avoiding genetic testing and prescribing alternatives may have unintended consequences as was seen in Hong Kong.49
Acknowledgments
VLMY is a MRC Clinical Training Fellow supported by the North West England Medical Research Council Fellowship Scheme in Clinical Pharmacology and Therapeutics, which is funded by the Medical Research Council (grant number G10000417), ICON, GlaxoSmithKline, AstraZeneca, and the Medical Evaluation Unit. MP is a National Institute for Health Research senior investigator. The authors also wish to thank the MRC Centre for Drug Safety Science for support.
Disclosure
The authors report no conflicts of interest in this work.
References
Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369(9566):1000–1015. | ||
Baftiu A, Johannessen Landmark C, Rusten IR, Feet SA, Johannessen SI, Larsson PG. Changes in utilisation of antiepileptic drugs in epilepsy and non-epilepsy disorders-a pharmacoepidemiological study and clinical implications. Eur J Clin Pharmacol. 2016;72(10):1245–1254. | ||
Yip VL, Marson AG, Jorgensen AL, Pirmohamed M, Alfirevic A. HLA genotype and carbamazepine-induced cutaneous adverse drug reactions: a systematic review. Clin Pharmacol Ther. 2012;92(6):757–765. | ||
Sekula P, Dunant A, Mockenhaupt M, et al. Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Invest Dermatol. 2013;133(5):1197–1204. | ||
Quirke KP, Beck A, Gamelli RL, Mosier MJ. A 15-year review of pediatric toxic epidermal necrolysis. J Burn Care Res. 2015;36(1):130–136. | ||
Chung W-H, Hung S-I, Hong H-S, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428(6982):486. | ||
McCormack M, Alfirevic A, Bourgeois S, et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med. 2011;364(12):1134–1143. | ||
Ozeki T, Mushiroda T, Yowang A, et al. Genome-wide association study identifies HLA-A*3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum Mol Genet. 2011;20(5):1034–1041. | ||
The M. H. C. Sequencing Consortium. Complete sequence and gene map of a human major histocompatibility complex. Nature. 1999;401(6756):921–923. | ||
Caron E, Kowalewski DJ, Chiek Koh C, Sturm T, Schuster H, Aebersold R. Analysis of major histocompatibility complex (MHC) immunopeptidomes using mass spectrometry. Mol Cell Proteomics. 2015;14(12):3105–3117. | ||
Man CBL, Kwan P, Baum L, et al. Association between HLA-B*1502 allele and antiepileptic drug-induced cutaneous reactions in Han Chinese. Epilepsia. 2007;48(5):1015–1018. | ||
Wu XT, Hu FY, An DM, et al. Association between carbamazepine-induced cutaneous adverse drug reactions and the HLA-B*1502 allele among patients in central China. Epilepsy Behav. 2010;19(3):405–408. | ||
Tassaneeyakul W, Tiamkao S, Jantararoungtong T, et al. Association between HLA-B*1502 and carbamazepine-induced severe cutaneous adverse drug reactions in a Thai population. Epilepsia. 2010;51(5):926–930. | ||
Then SM, Rani ZZM, Raymond AA, Ratnaningrum S, Jamal R. Frequency of the HLA-B*1502 allele contributing to carbamazepine-induced hypersensitivity reactions in a cohort of Malaysian epilepsy patients. Asian Pac J Allergy Immunol. 2011;29(3):290–293. | ||
Mehta TY, Prajapati LM, Mittal B, et al. Association of HLA-B*1502 allele and carbamazepine-induced Stevens-Johnson syndrome among Indians. Indian J Dermatol Venereol Leprol. 2009;75(6):579–582. | ||
Chen P, Lin J-J, Lu C-S, et al. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med. 2011;364(12):1126–1133. | ||
Ferrell PB, McLeod HL. Carbamazepine, HLA-B*1502 and risk of Stevens–Johnson syndrome and toxic epidermal necrolysis: US FDA recommendations. Pharmacogenomics.2008;9(10):1543–1546. | ||
Leckband SG, Kelsoe JR, Dunnenberger HM, et al. Clinical pharmacogenetics implementation consortium guidelines for HLA-B genotype and carbamazepine dosing. Clin Pharmacol Ther. 2013;94(3):324–328. | ||
Hung S-I, Chung W-H, Jee S-H, et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet Genomics. 2006;16(4):297–306. | ||
Kashiwagi M, Aihara M, Takahashi Y, et al. Human leukocyte antigen genotypes in carbamazepine-induced severe cutaneous adverse drug response in Japanese patients. J Dermatol. 2008;35(10):683–685. | ||
Niihara H, Kakamu T, Fujita Y, Kaneko S, Morita E. HLA-A31 strongly associates with carbamazepine-induced adverse drug reactions but not with carbamazepine-induced lymphocyte proliferation in a Japanese population. J Dermatol. 2012;39(7):594–601. | ||
Genin E, Chen DP, Hung SI, et al. HLA-A*31:01 and different types of carbamazepine-induced severe cutaneous adverse reactions: an international study and meta-analysis. Pharmacogenomics J. 2014;14(3):281–288. | ||
Kim S-H, Lee KW, Song W-J, et al. Carbamazepine-induced severe cutaneous adverse reactions and HLA genotypes in Koreans. Epilepsy Res. 2011;97(1–2):190–197. | ||
Amstutz U, Ross CJD, Castro-Pastrana LI, et al. HLA-A*31:01 and HLA-B*15:02 as genetic markers for carbamazepine hypersensitivity in children. Clin Pharmacol Ther. 2013;94(1):142–149. | ||
Anjum N, Polak ME, Ardern-Jones M, Cooper HL. Presence of the HLA-A*3101 allele in a familial case of drug reaction with eosinophilia and systemic symptoms, secondary to carbamazepine. Clin Exp Dermatol. 2014;39(3):307–309. | ||
Shirzadi M, Thorstensen K, Helde G, Moen T, Brodtkorb E. Do HLA-A markers predict skin-reactions from aromatic antiepileptic drugs in a Norwegian population? a case control study. Epilepsy Res. 2015;118:5–9. | ||
Pirmohamed M, Friedmann PS, Molokhia M, et al. Phenotype standardization for immune-mediated drug-induced skin injury. Clin Pharmacol Ther. 2011;89(6):896–901. | ||
Schrijvers R, Gilissen L, Chiriac AM, Demoly P. Pathogenesis and diagnosis of delayed-type drug hypersensitivity reactions, from bedside to bench and back. Clin Transl Allergy. 2015;5:31. | ||
May TW, Korn-Merker E, Rambeck B. Clinical pharmacokinetics of oxcarbazepine. Clin Pharmacokinet. 2003;42(12):1023–1042. | ||
Dam M, Ekberg R, Loyning Y, Waltimo O, Jakobsen K. A double-blind study comparing oxcarbazepine and carbamazepine in patients with newly diagnosed, previously untreated epilepsy. Epilepsy Res. 1989;3(1):70–76. | ||
Buggy Y, Layton D, Fogg C, Shakir SA. Safety profile of oxcarbazepine: results from a prescription-event monitoring study. Epilepsia. 2010;51(5):818–829. | ||
Moon J, Kim TJ, Lim JA, et al. HLA-B*40:02 and DRB1*04:03 are risk factors for oxcarbazepine-induced maculopapular eruption. Epilepsia. 2016;57(11):1879–1886. | ||
Pichler WJ. Delayed drug hypersensitivity reactions. Ann Intern Med. 2003;139(8):683–693. | ||
Park BK, Naisbitt DJ, Gordon SF, Kitteringham NR, Pirmohamed M. Metabolic activation in drug allergies. Toxicology. 2001;158(1–2):11–23. | ||
Pichler WJ, Beeler A, Keller M, et al. Pharmacological interaction of drugs with immune receptors: the p-i concept. Allergol Int. 2006;55(1):17–25. | ||
Illing PT, Vivian JP, Dudek NL, et al. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature. 2012;486(7404):554–558. | ||
Pichler WJ. Pharmacological interaction of drugs with antigen-specific immune receptors: the p-i concept. Curr Opin Allergy Clin Immunol. 2002;2(4):301. | ||
Yip V, Maggs J, Meng X, Marson A, Park K, Pirmohamed M. Covalent adduction of carbamazepine 10, 11-epoxide with human serum albumin and glutathione S-transferase pi: implications for carbamazepine hypersensitivity. Lancet. 2014;383:S114. | ||
Wei CY, Chung WH, Huang HW, Chen YT, Hung SI. Direct interaction between HLA-B and carbamazepine activates T cells in patients with Stevens-Johnson syndrome. J Allergy Clin Immunol. 2012;129(6):1562.e5–1569.e5. | ||
Zhou P, Zhang S, Wang Y, Yang C, Huang J. Structural modeling of HLA-B*1502/peptide/carbamazepine/T-cell receptor complex architecture: implication for the molecular mechanism of carbamazepine-induced Stevens-Johnson syndrome/toxic epidermal necrolysis. J Biomol Struct Dyn. 2016;34(8):1–33. | ||
Ko TM, Chung WH, Wei CY, et al. Shared and restricted T-cell receptor use is crucial for carbamazepine-induced Stevens-Johnson syndrome. J Allergy Clin Immunol. 2011;128(6):1266.e11–1276.e11. | ||
Chung WH, Hung SI, Yang JY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nature medicine. 2008;14(12):1343–1350. | ||
Lichtenfels M, Farrell J, Ogese MO, et al. HLA restriction of carbamazepine-specific T-Cell clones from an HLA-A*31:01-positive hypersensitive patient. Chem Res Toxicol. 2014;27(2):175–177. | ||
Amstutz U, Shear NH, Rieder MJ, et al. Recommendations for HLA-B*15:02 and HLA-A*31:01 genetic testing to reduce the risk of carbamazepine-induced hypersensitivity reactions. Epilepsia. 2014;55(4):496–506. | ||
Plumpton CO, Yip VL, Alfirevic A, Marson AG, Pirmohamed M, Hughes DA. Cost-effectiveness of screening for HLA-A*31:01 prior to initiation of carbamazepine in epilepsy. Epilepsia. 2015;56(4):556–563. | ||
Powell G, Holmes EA, Plumpton CO, et al. Pharmacogenetic testing prior to carbamazepine treatment of epilepsy: patients’ and physicians’ preferences for testing and service delivery. Br J Clin Pharmacol. 2015;80(5):1149–1159. | ||
Garcia-Doval I, LeCleach L, Bocquet H, Otero XL, Roujeau JC. Toxic epidermal necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol. 2000;136(3):323–327. | ||
McCormack M, Urban TJ, Shianna KV, et al. Genome-wide mapping for clinically relevant predictors of lamotrigine- and phenytoin-induced hypersensitivity reactions. Pharmacogenomics. 2012;13(4):399–405. | ||
Chen Z, Liew D, Kwan P. Effects of a HLA-B*15:02 screening policy on antiepileptic drug use and severe skin reactions. Neurology. 2014;83(22):2077–2084. | ||
Illing PT, Mifsud NA, Purcell AW. Allotype specific interactions of drugs and HLA molecules in hypersensitivity reactions. Curr Opin Immunol. 2016;42:31–40. | ||
The Academy of Medical Sciences. Realising the Potential of Stratified Medicines. London: The Academy of Medical Sciences; 2013. | ||
Mallal S, Nolan D, Witt C, et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet. 2002;359(9308):727–732. | ||
Hetherington S, Hughes AR, Mosteller M, et al. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet. 2002;359(9312):1121–1122. | ||
Hung S-I, Chung W-H, Liou L-B, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005;102(11):4134–4139. | ||
Kaniwa N, Saito Y, Aihara M, et al. HLA-B locus in Japanese patients with anti-epileptics and allopurinol-related Stevens–Johnson syndrome and toxic epidermal necrolysis. Pharmacogenomics. 2008;9(11):1617–1622. | ||
Hautekeete ML, Horsmans Y, Van Waeyenberge C, et al. HLA association of amoxicillin-clavulanate-induced hepatitis. Gastroenterology. 1999;117(5):1181–1186. | ||
Lucena MI, Andrade RJ, Stephens C, et al. Susceptibility to amoxicillin-clavulanate-induced liver injury is influenced by multiple HLA class i and II alleles. Gastroenterology. 2011;141(1):338–347. | ||
Daly AK, Donaldson PT, Bhatnagar P, et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet. 2009;41(7):816–819. | ||
Kazeem GR, Cox C, Aponte J, et al. High-resolution HLA genotyping and severe cutaneous adverse reactions in lamotrigine-treated patients. Pharmacogenet Genomics. 2009;19(9):661. | ||
Martin AM, Nolan D, James I, et al. Predisposition to nevirapine hypersensitivity associated with HLA-DRB1 0101 and abrogated by low CD4 T-cell counts. AIDS. 2005;19(1):97–99. | ||
Chantarangsu S, Mushiroda T, Mahasirimongkol S, et al. HLA-B 3505 allele is a strong predictor for nevirapine-induced skin adverse drug reactions in HIV-infected Thai patients. Pharmacogenet Genomics. 2009;19(2):139. | ||
Carr DF, Chaponda M, Jorgensen AL, et al. Association of human leukocyte antigen alleles and nevirapine hypersensitivity in a Malawian HIV-infected population. Clin Infect Dis. 2013;56(9):1330–1339. | ||
Locharernkul C, Loplumlert J, Limotai C, et al. Carbamazepine and phenytoin induced Stevens-Johnson syndrome is associated with HLA-B*1502 allele in Thai population. Epilepsia. 2008;49(12):2087–2091. | ||
Gonzalez-Galarza FF, Takeshita LY, Santos EJ, et al. New features for HLA epitopes, KIR and disease and HLA adverse drug reaction associations. Nucleic Acid Research. 2015; 28: D784-788. Available from: http://www.allelefrequencies.net/default.asp. Accessed October 31, 2016. | ||
Tegretol Prolonged Release 200 mg and 400 mg Tablets (formerly Tegretol retard). Camberley: Electronic Medicines Compendium website; 2011 [updated September 14, 2016]. Available from: http://www.medicines.org.uk/emc/medicine/24201. Accessed December 2, 2016. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.