Back to Journals » Clinical Ophthalmology » Volume 13
The effect of increasing power when grooving using phacoemulsification
Authors Thomson RS, Bird BA, Stutz LA , Heczko JB, Bernhisel AA, Barlow WR , Zaugg B , Olson RJ, Pettey JH
Received 16 November 2018
Accepted for publication 14 January 2019
Published 12 April 2019 Volume 2019:13 Pages 611—615
DOI https://doi.org/10.2147/OPTH.S194731
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Video abstract presented by Rhett S Thomson
Views: 304
Rhett S Thomson,1,2 Brian A Bird,3 Lance A Stutz,4 Joshua B Heczko,1 Ashlie A Bernhisel,1 William R Barlow,1 Brian Zaugg,1 Randall J Olson,1 Jeff H Pettey1
1Department of Ophthalmology and Visual Sciences, John A Moran Eye Center, University of Utah, Salt Lake City, UT 84132, USA; 2University of Utah School of Medicine, Salt Lake City, UT 84132, USA; 3University of Nevada Reno School of Medicine, Reno, NV 89557, USA; 4University of Texas Southwestern Medical School, Dallas, TX 75390, USA
Purpose: To determine optimal power settings on the Centurion Vision System during the grooving step in cataract surgery.
Methods: Intact porcine lenses hardened by formalin and placed in a chamber designed to simulate the anterior chamber of the eye were used to test longitudinal power at 40%, 70%, and 100% and torsional power at 0%. Flow rate was set at 40 mL/min. Vacuum was set at 400 mmHg, intraocular pressure was set at 50 mmHg, and a balanced phacoemulsification tip with a 20 degree tip and a 30 degree bevel was used. Efficiency (time to groove the lens in half) was determined.
Results: Increasing longitudinal power from 40% to 70% increased efficiency by 28% (P<0.05), and by 32% (P<0.05) when increasing longitudinal power from 40% to 100%. There was no statistically significant increase in efficiency from 70% to 100%.
Conclusion: For the tested variables, a longitudinal power of 70% was determined to be most efficient during the grooving step of cataract surgery for equivalent 3–4+ nuclei. Further increases in power demonstrated no statistically significant improvement in efficiency.
Keywords: settings, longitudinal power, efficiency, porcine lens model
Plain language summary
Lens cataract surgery is performed using a phacoemulsification (phaco) machine which relies on ultrasound. This process allows the surgeon to break up and safely remove the cataract before inserting a new artificial lens. There are various settings on the machine, including adjustments for power (0%–100%). Excessively high power has the potential to damage fragile eye tissue surrounding the lens. With this in mind, knowing what level of power is most effective can equip cataract surgeons with optimal efficiency and maximum safety for their patients. Identifying the optimal power level is the purpose of this study. Our team obtained over 80 pig lenses and fixed them with formalin to resemble the hardness of cataract lenses. We then divided each lens using phaco at 40%, 70%, and 100% power, measuring how long it took for complete division. Results indicate that efficiency increases from 40% to 70% power, but does not increase at higher levels. The general trend we found in this study suggests that cataract surgeons need not exceed 70% phaco power, since higher levels do not improve efficiency and may increase risk to patients.
Introduction
Cataracts are a surgically correctable cause of vision loss. During cataract surgery surgeons commonly carve a central groove into the lens to enable division of the lens into smaller pieces that can be removed by the phacoemulsification (phaco) handpiece. A new artificial, intraocular lens (IOL) replaces the opacified crystalline lens in order to restore vision and focusing power. During the grooving step, various settings can be adjusted to allow for an optimal and efficient surgery. These settings include the aspiration flow rate, level of vacuum at the tip, and magnitude of ultrasound energy. A previous series of studies have determined the optimal settings for lens removal during the quadrant removal portions of the surgery.1–26 However, the optimal settings for the grooving step, the initial step of phaco, have not yet been elucidated.
This study is the second in a series that will determine the optimal settings for nucleus grooving. The first study found the optimal aspiration flow rate was 40 mL/min.27 This study now examines optimal longitudinal power using a porcine lens model developed in our laboratory.
Methods
Porcine lens preparation
Lenses obtained from whole pig eyes (Visiontech, Inc., Sunnyvale, TX, USA) were dissected within 48 hours of arrival at the laboratory where the procedures were conducted; immediately thereafter, the nuclei were incubated at room temperature, in 10 mL of 10% neutral buffered formalin, for 2 hours. They were rinsed three times in balanced salt solution (BSS) over 24 hours in order to increase the formalin’s hardening effect uniformity. Previous studies have determined that lenses prepared in this manner are comparable to hard (3–4+) human cataract lenses in terms of their density and behavior.28
Experimental procedure
The phaco experiments were performed with the Centurion Vision System with an Ozil handpiece (Alcon Laboratories, Inc., Fort Worth, TX, USA), using only longitudinal phaco set at 40%, 70%, and 100% for the three different arms of the study.
Twenty-four hours later, nuclei were randomly selected and individually placed in the “groover”, a small plastic chamber specifically developed in our lab to simulate the anterior chamber anatomy of the eye during the grooving step of phacoemulsification (Figure 1). This base is pentagon-shaped, with a clear plastic dome on top simulating the cornea and a circular opening at the edge of the dome which is just large enough to permit insertion of the phaco needle, while maintaining a closed system.
For each trial, after a single lens was inserted into the groover and fixed in place by a drop of cyanoacrylate adhesive, a single operator (RST) filled the chamber with BSS and sealed the rubber lid, thereby creating a closed system. The same operator inserted the handpiece through a small hole in the circular rubber piece on the groover front. He then grooved the lens in half, using a handheld stopwatch to measure and record the time from start of phacoemulsification to complete division of the nucleus. A second investigator (BAB) operated a digital stopwatch to time each run. After removing the divided lens, the chamber was cleaned before beginning another run. A third investigator (AAB) provided oversight and input to ensure consistency in performing the grooving procedures.
Statistical analysis
Efficiency was defined as the number of seconds that ultrasound was used for lens fragment removal. Efficiency times were averaged and the SDs were calculated. Previous studies1–3,5–17,19–22 considered all data points more than two SDs from the mean as outliers and removed them. Utilizing these same two SDs as cutoff was considered necessary for this study because research shows that outside of two SDs, data points likely represent very hard lenses that require more time to emulsify.28 One outlier from each arm was removed except for arm 2 (70% power), which had two outliers removed. The remaining efficiency times were averaged and new means were calculated. Finally, efficiency times for each variable longitudinal power setting were compared using one-way ANOVA. When a significant P-value was found, Student’s t-tests were performed to identify distinct relationships between the variables tested. A P-value of ≤0.05 was considered significant. Statistical analyses were performed using Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, USA).
Results
The average time for each lens to be divided with phaco was found for 40%, 70%, and 100% longitudinal power (Table 1). As longitudinal power increased from 40% to 70% to 100%, the average time to groove the lens decreased. At a 40% power setting, the average time was 19.83 seconds, at 70% the average time was 15.25 seconds, and at 100% the average time was 14.92 seconds. Increasing power from 40% to 70% revealed an increase in efficiency of 28% (P<0.05). Increasing longitudinal power from 40% to 100% revealed an increase in efficiency by 32% (P<0.05). There was no statistically significant increase in efficiency from 70% to 100% (Figure 2).
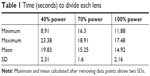  | Table 1 Time (seconds) to divide each lens |
  | Figure 2 Average grooving time in seconds at 40%, 70%, and 100% longitudinal power. |
Discussion
During phacoemulsification, safety and efficiency are paramount. Previous studies have demonstrated that increased total ultrasound energy and on time can potentially increase the risk of damage to fragile eye structures via delivered ultrasound energy, thermal energy, and instrument exposure.29–31 Flow rate, vacuum, and power are settings that can be adjusted to optimize safety and efficiency. Longitudinal power, specifically, can cause cavitation bubbles and free radical formation which may damage the corneal epithelium.32 Cavitation bubbles are produced at levels around 30%–50% and increase at higher levels.33,34 Identifying the most efficient power setting can potentially reduce the unnecessary risk of damage, which is the purpose of this study. Our previous work has identified ideal settings and parameters for quadrant or small particle removal after grooving.3–6 This study is the second in a series seeking to identify optimal settings during the grooving step. The first study determined that for dense nuclei, the flow rate is optimized at 40 mL/min.27 The data in this study indicate optimal longitudinal power settings at 70% during the grooving stage of cataract surgery when compared to 40% and 100% longitudinal power, and somewhat surprisingly that higher power settings have no additional benefit in this setting. Furthermore, the increase in efficiency in going from 40% to 70% (4.6 seconds) is of relatively small magnitude and may not warrant the increased inherent risk of using higher overall ultrasound energy for such a small time savings.
As this is a new line of research, the reason as to why efficiency does not directly correlate with energy used is not clear. The relationship between power and efficiency is clearly non-linear, as our results show a relative plateau from 70% to 100% power settings. In a previous work evaluating fragment removal, we have theorized that the loss of a linear relationship between power used and efficiency is commonly due to chatter and microchatter events.7 With the nucleus glued in place, it is hard to posit chatter as the cause; however, possibly the repulsive force still repels the nuclear material enough to result in loss of contact between the tip and the nucleus with a subsequent decrease in efficiency. We will gain better understanding as we delve further into this new line of inquiry.
Limitations
Limitations of this study include the fact that we tested only three distinct longitudinal power settings and one single lens density. It was our goal to evaluate the general trend rather than to prescribe a specific power for uniform clinical use. However, we recognize that it would have improved our precision to test additional power settings between 40% and 100% and different lens densities which would have given a more complete picture of the findings. However, given the lack of data for grooving settings for any lens density, an experimental design with three distinctly different power settings with a single density that is consistent with previous studies investigating phacoemulsification efficiency both informs and allows for a prudent starting point for future evaluations.1–3,28 In future work, we will evaluate the role of torsional phaco and variability of aspiration and vacuum in the grooving step. We also recognize the limitations of having a single surgeon unblinded to the power setting. Unfortunately, the tactile differences between 40%, 70%, and 100% power are obvious to the surgeon, and blinding to this variable was not possible. The potential for systematic error due to possible insufficient flow may be of concern. A follow-up trial to our previous studies,3 which would evaluate high flow and vacuum levels, could document that flow rate is not a limiting factor in this study. Another weakness is the in vitro nature of the study. While a clinical trial would be ideal, it is not feasible to perform a number of trials while controlling for all of the individual variables to allow reproducible and reliable data. We believe our study sufficiently replicated the surgical procedure and allowed us to reliably investigate the impact flow would have when grooving in a clinical setting.
Conclusion
For longitudinal phaco, a power of 70% was determined to be the most efficient during the grooving step of cataract surgery when compared to 40% and 100%. Further increases in power demonstrated no statistically significant improvement in efficiency and a small increase in efficiency (4.6 seconds) in going from 40% to 70% power may not warrant the increased risk of the additional ultrasound power used. This is the first series to address the efficiency trends for the grooving phase of phaco, and as we evaluate additional variables, the accumulated data will fill in the gaps of knowledge to date.
Ethics approval and informed consent
Since no human subjects were involved, approval from the University of Utah Institutional Review Board was not obtained.
Acknowledgments
Susan Schulman provided writing assistance. This study was supported in part by an unrestricted grant from Research to Prevent Blindness, Inc., New York, NY, USA, to the Department of Ophthalmology and Visual Sciences, University of Utah, Salt Lake City, Utah, USA.
Disclosure
Dr Olson is on the scientific advisory board of EyeGate Pharmaceuticals and of Perfect Lens. The other authors report no conflicts of interest in this work.
References
Boulter T, Jensen JD, Christensen MD, et al. Comparison of a torsional and a standard tip with a monitored forced infusion phacoemulsification system. J Cataract Refract Surg. 2016;42(4):613–617. | ||
Jensen JD, Boulter T, Lambert NG, et al. Intraocular pressure study using monitored forced-infusion system phacoemulsification technology. J Cataract Refract Surg. 2016;42(5):768–771. | ||
Jensen JD, Shi DS, Robinson MS, et al. Torsional power study using CENTURION phacoemulsification technology. Clin Exp Ophthalmol. 2016;44(8):710–713. | ||
Gilbert M, Zaugg B, Stagg B, Olson RJ. Safety profile of Venturi versus peristaltic phacoemulsification pumps in cataract surgery using a capsular surrogate for the human lens. Am J Ophthalmol. 2015;160(1):179–184. | ||
Shi DS, Jensen JD, Kramer GD, et al. Comparison of vacuum and aspiration on phacoemulsification efficiency and Chatter using a monitored forced infusion system. Am J Ophthalmol. 2016;169:162–167. | ||
Gupta I, Cahoon JM, Gardiner G, et al. Effect of increased vacuum and aspiration rates on phacoemulsification efficiency. J Cataract Refract Surg. 2015;41(4):836–841. | ||
DeMill DL, Zaugg BE, Pettey JH, et al. Objective comparison of 4 nonlongitudinal ultrasound modalities regarding efficiency and chatter. J Cataract Refract Surg. 2012;38(6):1065–1071. | ||
Garff K, Jensen JD, Cahoon J, et al. Impact of micropulsed ultrasound power settings on the efficiency and chatter associated with lens-fragment removal. J Cataract Refract Surg. 2015;41(6):1264–1267. | ||
Kirk KR, Ronquillo C, Jensen JD, et al. Optimum on-time duty cycle for micropulse technology. J Cataract Refract Surg. 2014;40(9):1545–1548. | ||
Gupta I, Cahoon JM, Shi D, et al. The impact of tip bevel angulation on phacoemulsification efficiency and chatter. New Front Ophthalmol. 2017;1:3. | ||
Gupta I, Zaugg B, Stagg BC, et al. Phacoemulsification efficiency with a radiused phaco tip. J Cataract Refract Surg. 2014;40(5):818–821. | ||
Gardiner GL, Garff K, Gupta I, et al. Effect of pulsing ultrasound on phacoemulsification efficiency. J Cataract Refract Surg. 2015;41(11):2560–2564. | ||
Cahoon JM, Gupta I, Gardiner G, et al. Comparison of Venturi and peristaltic vacuum in phacoemulsification. J Cataract Refract Surg. 2015;41(2):428–432. | ||
Farukhi AM, Stagg BC, Ronquillo C, et al. Effect of phaco tip diameter on efficiency and chatter. J Cataract Refract Surg. 2014;40(5):811–817. | ||
Jensen JD, Kirk KR, Gupta I, et al. Determining optimal ultrasound off time with micropulse longitudinal phacoemulsification. J Cataract Refract Surg. 2015;41(2):433–436. | ||
Ronquillo CC, Zaugg B, Stagg B, et al. Determining optimal torsional ultrasound power for cataract surgery with automatic longitudinal pulses at maximum vacuum ex vivo. Am J Ophthalmol. 2014;158(6):1262–1266. | ||
Stagg BC, Gupta I, Cahoon J, et al. Bent versus straight tips in micropulsed longitudinal phacoemulsification. Can J Ophthalmol. 2015;50(5):354–359. | ||
Henriksen BS, Gardiner G, Garff K, et al. Thermal evaluation of two phacoemulsification systems. Can J Ophthalmol. 2016;51(1):14–18. | ||
Boulter T, Christensen MD, Jensen JD, et al. Optimization and comparison of a 0.7 mm tip with the 0.9 mm tip on an active-fluidics phacoemulsification platform. J Cataract Refract Surg. 2017;43(12):1591–1595. | ||
Wright AJ, Thomson R, Bohner A, et al. Optimization of Venturi mode phacoemulsification settings of the Abbott Medical Optics WhiteStar signature pro in a porcine lens model. Int J Ophthalmol Clin Res. 2017;4(3):077. | ||
Wright AJ, Thomson RS, Bernhisel AA, et al. Effect of chamber stabilization software on efficiency and chatter in a porcine lens model. J Cataract Refract Surg. 2017;43(11):1464–1467. | ||
Wright DD, Wright AJ, Boulter TD, et al. Optimization of transversal phacoemulsification settings in peristaltic mode using a new transversal ultrasound machine. J Cataract Refract Surg. 2017;43(9):1202–1206. | ||
Bohner AD, Wright AJ, Ta BT, et al. Optimum on-time duty cycle for a transversal ultrasound machine. J Cataract Refract Surg. 2018;44(9):1140–1143. | ||
Kabbara SW, Heczko J, Ta B, et al. Determining optimal ultrasound percent on time with long-pulse torsional phacoemulsification. Can J Ophthalmol. In press 2018. DOI: https://doi.org/10.1016/j.jcjo.2018.07.005 | ||
Kabbara S, Heczko JB, Bernhisel AA, et al. Effect of high vacuum and aspiration on phacoemulsification efficiency and chatter using a transversal ultrasound machine. J Cataract Refract Surg. 2018;44(11):1378–1383. | ||
Ha L, Wright A, Wright DD, et al. High vacuum and aspiration on phacoemulsification efficiency and chatter for Centurion. Can J Ophthalmol. 2019;21(1):136–138. DOI: 10.1016/j.jcjo.2018.03.009. | ||
Bird BA, Thomson RS, Stutz LA, et al. Effect of increasing flow when grooving during phacoemulsification. J Cataract Refract Surg. 2018;44(5):623–626. | ||
Oakey ZB, Jensen JD, Zaugg BE, et al. Porcine lens nuclei as a model for comparison of 3 ultrasound modalities regarding efficiency and chatter. J Cataract Refract Surg. 2013;39(8):1248–1253. | ||
Payne M, Waite A, Olson RJ. Thermal inertia associated with ultrapulse technology in phacoemulsification. J Cataract Refract Surg. 2006;32(6):1032–1034. | ||
Peck CM, Joos ZP, Zaugg BE, et al. Comparison of the corneal endothelial protective effects of Healon-D and Viscoat. Clin Exp Ophthalmol. 2009;37(4):397–401. | ||
Meyer JJ, Kuo AF, Olson RJ. The risk of capsular breakage from phacoemulsification needle contact with the lens capsule: a laboratory study. Am J Ophthalmol. 2010;149(6):882–886. | ||
Zacharias J. Role of cavitation in the phacoemulsification process. J Cataract Refract Surg. 2008;34(5):846–852. | ||
Takahashi H. Free radical development in phacoemulsification cataract surgery. J Nippon Med Sch. 2005;72(1):4–12. | ||
Takahashi H. Corneal endothelium and phacoemulsification. Cornea. 2016;35(Suppl 1):S3–S7. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

