Back to Journals » International Journal of Nanomedicine » Volume 14
The bactericidal effect of lysostaphin coupled with liposomal vancomycin as a dual combating system applied directly on methicillin-resistant Staphylococcus aureus infected skin wounds in mice
Authors Hajiahmadi F, Alikhani MY , Shariatifar H, Arabestani MR , Ahmadvand D
Received 5 May 2019
Accepted for publication 28 June 2019
Published 29 July 2019 Volume 2019:14 Pages 5943—5955
DOI https://doi.org/10.2147/IJN.S214521
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Fahimeh Hajiahmadi,1 Mohammad Yousef Alikhani,1,2 Hanifeh Shariatifar,3 Mohammad Reza Arabestani,1,2 Davoud Ahmadvand4,5
1Department of Microbiology, Hamadan University of Medical Sciences, Hamadan, Iran; 2Brucellosis Research Center, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran; 3Young Researches and Elite Club, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran; 4Cellular and Molecular Research Center, Iran University of Medical Sciences, Tehran, Iran; 5Department of Medical Laboratory Sciences, Faculty of Allied Medicine, Iran University of Medical Sciences, Tehran, Iran
Background and aim: Methicillin-resistant Staphylococcus aureus (MRSA) is one of the most common causes of surgical infection, and its resistance to numerous conventional antibiotics makes treatment difficult. Although vancomycin is often an effective agent for the initial therapy of MRSA, clinical failure sometimes occurs. Therefore, there is an urgent need to develop better therapies. Here, we prepared some vancomycin-loaded nanoliposomes coupled with anti-staphylococcal protein (lysostaphin) and evaluated their in vitro and in vivo efficacy as a topical MRSA therapy.
Methods: Vancomycin was encapsulated in liposomes, and the coupling of lysostaphin with the surface of liposomes was carried out through cyanuric functional groups. The bactericidal efficacies and a full characterization were evaluated. To define different nanoliposomal–bacterium interactions and their bactericidal effect, flow cytometry was employed. Finally, in vivo, the topical antibacterial activity of each formulation was measured against surgical wound MRSA infection in a mouse model.
Results: High encapsulation and conjugation efficiency were achieved for all formulations. All the formulations showed a significant reduction in bacterial counts (p<0.05). The targeted liposomes more effectively suppress bacterial infection in vitro and in vivo relative to equivalent doses of untargeted vancomycin liposome. The flow cytometry results confirmed liposome–bacterium interactions, which increased during the incubation time. The maximum binding rate and the bactericidal effect were significantly higher in targeted liposomes (p<0.05) compared with control liposomes.
Conclusion: Our data suggest a novel nano-vehicle (lysostaphin-conjugated coupled liposomal vancomycin) which could be used as a great topical antimicrobial construct for treatment of MRSA skin infections.
Keywords: MRSA, antibacterial activity, encapsulation efficiency, conjugation efficiency, lysostaphin
Introduction
For many years, broad-spectrum antibiotics have been used to combat a wide range of bacterial infections. However, antibiotic resistance is rising to dangerously high levels, and new resistance mechanisms are emerging and spreading globally.1 Of the Gram-positive bacteria, drug-resistant Staphylococcus aureus (MRSA) is of serious threat and is among the most common causes of infections associated with indwelling catheters and prosthetic devices.2 The prevalence of S. aureus from skin and soft tissue infections was reported as the most common isolate worldwide with 20–30% of MRSA in Europe and Latin America, up to 35% in North America and even higher in developing countries.3 Moreover, the prevalence of postoperative MRSA infection can occur from 1% to 33% in surgical sites.4
In the United States, approximately 11,000 people die as a result of MRSA infection each year.5,6 Antibiotics such as vancomycin, linezolid, daptomycin, tigecycline, and telavancin are primarily employed against MRSA skin, soft tissue, and postsurgical infections in hospitals.7 Although vancomycin is effective against MRSA, vancomycin intermediate-resistant S. aureus and vancomycin-resistant S. aureus have been reported in some studies.8 Usual adult dose for skin and soft tissue infection is recommended 500 mg IV every 6 hrs or 1 g IV every 12 hrs,9 while a major side effect of systemic vancomycin treatment is the development of anaphylactic reactions, including hypotension, wheezing, dyspnea, urticarial, or pruritus.10 Considering these limitations, there is an urgent need to develop better therapeutic approaches to combat MRSA skin infection. Liposomes have been applied clinically as a drug delivery vehicle for many years. Moreover, liposomes were found to be the most effective carriers for topical drug administration.11 Many studies show that topical application of liposomes can improve the therapeutic effect by prolonging the drug release during treatment and also can diminish the risk of systemic side effects. Also, it can improve the stability of the drug and prolong its impact in vivo.12 Systemic vancomycin treatment results in poorly penetration into the necrotic wounded tissue. Therefore, high vancomycin concentration requires to be used in order to achieve therapeutic concentrations at the infection sites which increase the antibiotic side effects.13,14 In most of the surgical wound, bacteria survive inside the wound and can be resistant to antibacterial therapy and subsequently they can involve deeper tissues and lead to systemic pathogenesis. The liposomal vancomycin can be used directly at the infected wound, so vancomycin can be released directly at necrotic tissue and also lead to disrupting of intracellular and extracellular MRSA.15
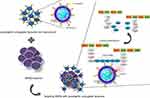
Most strains of S. aureus produce alpha-toxin (Alpha hemolysin) (Hla), a membrane disrupting toxin that through pore formation induces cell lysis and tissue damage.16 Here, we have taken advantage of this microbial process to design targeted vancomycin-encapsulated liposomes to combat skin MRSA infection. For microbial targeting, we conjugated lysostaphin to liposomes. Lysostaphin is a 27-kDa endopeptidase enzyme that binds specifically to the peptidoglycan of S. aureus and destroys its cell wall. Several studies have confirmed the antibacterial activity of lysostaphin on antibiotic-resistant S. aureus.17,18 Thus, through this targeted approach, we immobilize the pathogen, destruct its cell wall, and release vancomycin (through hla-mediated pore formation in liposomal bilayer) near bacteria and demonstrate effective antibacterial efficacy (compared with control formulations) in a validated skin MRSA infection in a murine model.
Materials and methods
Materials
Soybean phosphatidylcholine (Phospholipon.® 90 G) (SPC) was purchased from Lipoid Inc (NJ, USA). Cholesterol, vancomycin hydrochloride, lysostaphin, propidium iodide (PI), Triton X-100, and sodium chloride were from Sigma Aldrich (MO, USA). Blood agar, mueller hinton (MH) broth, methanol, chloroform and glycine were obtained from Merck (Darmstadt, Germany). 1,2-Dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(cyanur PE) and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(carboxyfluorescein) (ammonium salt) (PE CF) were provided by Avanti Polar Lipids, Inc (AL, USA). Sepharose CL-4B was purchased from GE Healthcare (Bucks, UK). Novex SilverXpress Silver Staining Kit, Novex 4–12% Bis-Tris Gel 1.5 mm 10 well, and RNA extraction and cDNA synthesis kits were obtained from Thermo-Fisher Scientific (MA, USA). Antibiotic disks were purchased from MAST (Merseyside, UK). Gene MATRIX Quick Blood DNA Purification Kit was from EURx Ltd (Gdańsk, Poland). Phosphocholine assay kit was obtained from MTI diagnostics GmbHP (Idstein, Germany). Male NMRI mice, 6–8-week old, were purchased from Center of Experimental and Comparative Study (Iran University, Tehran). All animal experiments were carried out in accordance with the protocols approved by the Ethics Review Body of Animal Experimentation of Iran University (IR.UMSHA.REC.1394.134).
Bacterial strain
S. aureus was isolated from a 72-year-old male patient with a skin wound infection (Hamadan Hospital, Iran) and confirmed for the presence of the femA gene.19 The bacterial isolate was preserved at −70°C in MH broth supplemented with 20% (v/v) glycerol. Antimicrobial susceptibility testing using the disk diffusion method as well as the minimum inhibitory concentration (MIC) by the broth microdilution technique was performed according to the Clinical and Laboratory Standards Institute (CLSI) guidelines.20 S. aureus ATCC 25923 was used as a control strain. Template DNA was prepared using GeneMATRIX Quick Blood DNA Purification Kit and employed according to the manufacturer’s instruction. Presence of the mecA and hla genes was detected by PCR using specific primers, as previously described.21,22 Total RNA extraction and cDNA synthesis were prepared using the Thermo Fisher Scientific Kit in accordance with the manufacturer’s instruction. Real-time PCR was performed on a 7500 Real-Time PCR System (ABI, Real-time PCR Step one plus) using specific primers, as previously described.22 The gmK gene was used as an internal control. The primers used for gmK amplification were those previously described.23 Product specificity was evaluated by melting-curve analysis.
Liposome preparation and drug encapsulation
Liposomes were composed of SPC and cholesterol (mole ratio, 6.5:3.5) and prepared by the thin-film hydration method.24 When cyanur-PE was incorporated into the vesicular bilayer, SPC: cholesterol: cyanur-PE mole ratio was 5.5:3.5:1. Briefly, the lipids were dissolved in 9:1 (v/v) chloroform and methanol and a thin film was made in a round-bottom flask by evaporating the solvent under vacuum using a rotary evaporator. The lipid film was then hydrated with 10 mg/mL vancomycin containing PBS (pH: 7.4). The resulting suspension was sonicated for 3 mins using a probe sonicator (Vibra-Cell TM Jencons Scientific Ltd., USA) with pulsed duty cycle of 45 s on, 15 s off, and power delivery of 30%. The suspension was extruded 21 times through a 200-nm-pore-sized polycarbonate membrane (Avanti Polar Lipids, USA) at room temperature to obtain small unilamellar vesicles (SUVs). Free, nonencapsulated vancomycin was removed using a Sepharose CL-4B gel filtration column (24×1 cm internal diameter). To measure vancomycin encapsulation efficiency, 90 μL of the SUV preparation was added to 200 μL of 10% Triton X-100. Following incubation at 37°C for 5 mins and vortexing. Vancomycin content was analyzed by HPLC with the column C18 (Knauer, Italy). beatify, 0.1 ml of unloaded vancomycin and vancomycin standard solutions were prepared and monitored at a wavelength of 280 nm. The mobile phase was composed of 0.575% ammonium acetate in methanol: water (450/550, v/v) (pH 7.4) at a flow rate of 0.8–1.0 mL/min.25 Triplicate samples were used from each injected material and the area under the peak was evaluated for each run. The assays for each experimental system were all run on the same day.
Lysostaphin was conjugated to cyanur-PE. Before the conjugation step, blank and vancomycin-encapsulated liposomes were suspended in borate buffer (0.15 M boric acid, 0.1 M EDTA), pH 8.3. Next, lysostaphin (500 μg, in 50 μL 10 mM sodium acetate solution) was added dropwise to the liposome suspension. The mixture was incubated while shaking for 16 hrs at 4°C in the dark. Unreacted cyanuric groups were quenched with glycine (50 mM) for 1 hr at room temperature. Free lysostaphins were removed by an Amicon Ultra 15 mL centrifugal filter device with a 100,000 MWCO Ultracel membrane (Merck, Germany). The mixture was diluted with 2 mL PBS (pH 7.4) and centrifuged at 4000 rpm for 1 hr in an Eppendorf 5702R maintained at 4°C (buffer exchange was done three times). Uncoupled lysostaphin was concentrated by 3000 MWCO Ultracel membrane. Finally, concentrated free lysostaphin was measured to determine the coupling efficiency level using HPLC (C18 column) analysis.26 Briefly, the mobile phase consisted of acetonitrile, water, and 0.01% trimethylamine (v/v). The eluting conditions were at a flow rate of 1 mL/min and monitored at 280 nm. For uncoupled lysostaphin analysis, standard curves were constructed in PBS with known amounts of lysostaphin. Quantitation was achieved by measurement of the peak area ratios of the lysostaphin to reference standards.
Liposome characterization
Vesicular size distribution and polydispersity index (PDI) were determined by dynamic light scattering (DLS) analysis (Brookhaven Instrument, Holtsville, New York) at 25°C with refractive index 1.3332, viscosity 0.8878, and scattering intensity 11,480. The number of vesicles/mL was estimated by considering a cross-sectional area of 0.71 nm2 for the phospholipid head group and 0.19 nm2 for cholesterol, respectively, and considering a bilayer thickness of 5 nm.27 A commercially available phosphocholine assay was used for the determination of liposomal phospholipid content. Lysostaphin coupling to liposomes was qualitatively assessed by SDS-PAGE on a 4–12% NuPAGE Bis-Tris gel. Gels were stained using Novex Silver Xpress Staining Kit according to manufacturer’s instruction.
Antimicrobial susceptibility test
The MIC of free vancomycin, free lysostaphin, and lysostaphin-conjugated liposomal formulations was determined in a liquid growth inhibition assay in accordance with the established CLSI guidelines.20 The MIC was regarded as the lowest concentration of the drug that inhibited the visible growth of bacteria after 16–18 hrs of incubation at 35°C.
Liposome–bacterial interaction
Flow cytometry was used to evaluate liposome binding to bacteria. To follow liposome–bacterial interaction, liposomes were labeled with 1% carboxy-fluorescein-PE. Liposomes were mixed with the bacterial suspension (1×106 CFU/mL) at 35°C with agitation based on MIC dose. Labeled empty liposomes were applied as the control. At selected time intervals (5 and 120 mins), aliquots were washed with 1 mL of PBS to remove free liposomes using centrifugation at 4000–6000 RPM for 5 mins. The pellet was including the bacteria and liposomes that bound to bacteria and supernatant was contains free liposomes. The bacterial pellet was diluted in 200 µL of PBS for flow cytometry analysis. Bacterial viability was followed through PI labeling.
Liposomal vancomycin releasing study
To determine the hla-triggered vancomycin release from LV and V formulations, liposomes were incubated with and without MRSA (1×106 CFU/mL) at 35°C for 2 and 18 hrs. After incubation, free vancomycin was separated from the mixture by an Amicon Ultra 15 mL centrifugal filter device with a 100,000 MWCO Ultracel membrane (4000 rpm, 1 hr) and measured by HPLC.25
Cytotoxicity test
The effect of liposomal formulations on mammalian cell viability was investigated using MTT assay and the human epidermoid carcinoma epithelial cell line A431 (ATCC® CRL-1555™). Briefly, cells were seeded in a 96-well plate at a density of 5×103 cells/well for subconfluency and incubated for 24 hrs at 37°C. Next, cells were treated with all formulations for 24 hrs, then washed twice with PBS, replaced with fresh RPMI medium, and MTT added to each well. Following a period of 4-hr incubation, dimethyl sulfoxide was added and the absorbance at λλ =570 nm was measured in a microplate reader (Bio Rad; Model 680).
Animal studies
The MRSA culture was prepared 1 day prior to infection in accordance with procedures of Wen et al.28 Male NMRI mice aged between 6 and 8 weeks were used for this study. Mice were kept in sterile cages individually on a 12-hr light/dark cycle with free access to sterile chow and water ad libitum. After 1-week acclimatization, initially, the MRSA doses leading to mortality in a murine model of the surgical wound were determined. A full-thickness wound was created by biopsy punch (7 mm) and the wound wasinfected with various colony-forming unit (CFU) counts of MRSA including 10,7 108, and 10.9 The survival of all infected mice was monitored daily. Treatment protocol was based on 7 groups of mice (9 mice per group) and each wound was inoculated with 10 μL of broth containing 107 CFU of MRSA: mice given bacteria and liposomal vancomycin, mice given bacteria and lysostaphin conjugated to blank liposomes, mice given bacteria and lysostaphin conjugated to vancomycin-encapsulated liposomes, mice given bacteria and free vancomycin, mice given bacteria and free lysostaphin, mice given bacteria and free lysostaphin combined with free vancomycin, and mice treated with PBS and liposome with carbapol gel. Treatment was carried out based on MIC dose and transdermal delivery was applied with carbapol gel. Briefly, all the developed liposomes and free drugs were mixed into 1% (W/W) 90 NF carbopol gel in order to enhance and improve the drug permeability.29 Nonliposomal drugs were administered in two daily doses while liposomal drugs were administered as a single daily dose. Thirty minutes after treatment, the wound was covered with a bandage. On the 3rd, 9th, and 14th days, mice were anesthetized and sacrificed. After homogenizing lesion skin specimens in sterile PBS, bacterial counts (in CFU) determined by serial dilution.
Statistical analysis
The significant difference between groups (p<0.05) was determined using ANOVA and Tukey Test. All experiments were repeated three times.
Results and discussion
Antimicrobial susceptibility test
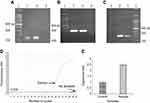
The S. aureus strain isolated from the wound was confirmed through conventional biochemical tests and the existence of the femA gene (Figure S1A). Antimicrobial susceptibility testing showed (Table 1) that it was resistant to multiple classes of antibiotics, including cefoxitin but not vancomycin. The presence of mecA gene verified methicillin resistance (Figure S1B). The PCR result indicated that it had hla gene and also a high α-toxin expression (Figure S1C–E).
 |
Table 1 Bacterial antibiotic susceptibility test of methicillin-resistant Staphylococcus aureus strain |
Liposome characterization
Physiochemical parameters of each formulation, including the average particle size, PDI, encapsulation, and conjugation efficiency, were measured (Table 2). High entrapment of vancomycin was achieved (~60%) in compression with the study done by Liu et al.25 The higher encapsulation efficiency is likely due to the difference in the liposome composition and construction method. DLS measurement showed that all liposomes except LysLV were less than 200 nm size (Table 2). Particle sizes of unconjugated liposome were slightly smaller than those of lysostaphin-conjugated vancomycin liposomes. The PDIs of all liposomes were less than 0.2, indicating a relatively homogenous size distribution. Lysostaphin-conjugated liposomes contained approximately 52 and 49 Lys/vesicle in LysLV and LysL, respectively.
 |
Table 2 Physicochemical parameters of liposomes |
Conjugated liposomes
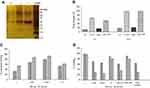
Coupling of lysostaphin with the surface of liposomes was carried out through cyanuric functional groups. In this method, lysostaphin was coupled with the surface of liposome through covalent bond and confirmed by a gel electrophoresis analysis with silver nitrate staining (Figure 1A). Conjugation of lysostaphin to lipid was observed as a band shift (~1 kDa) due to increased molecular weight, in comparison with untreated lysostaphin, in SDS-PAGE.
Liposomal antimicrobial activity
We explored the antibacterial activity of free vancomycin, free lysostaphin, free lys/van, LV, LysL, and LysLV against MRSA and S. aureus (ATCC 25923) (Table 3). Entrapment of vancomycin in liposomes did not enhance its antibacterial effect on MRSA compared to free vancomycin. These results were different from the results obtained by Sande et al, that this could be due to the difference in the liposome composition.30 Since the increased use of vancomycin can lead to increased vancomycin resistance, we decided to use vancomycin and lysostaphin as combination therapy.8 Several studies have shown the effectiveness of lysostaphin against MRSA31–33 and it has been successfully evaluated as an anti-staphylococcal agent in wound dressing materials.34,35 The MIC values of free lysostaphin were lower with MRSA compared to LysL, which could be due to inactivation of the antibacterial activity of some of the lysostaphins during the process of conjugation. So, LysLV had more antibacterial activity than the other formulations except for free lys/van. Since lysostaphin bind specifically to the S. aureus cell wall, it can be used to target liposomes to the surface of MRSA.36 Lysostaphin also destroys the Staphylococcal cell wall,36 and so it can potentially also contribute a therapeutic benefit to the liposomes. Simultaneously, vancomycin releases on MRSA surface locally, while the MRSA cell wall has been destroyed by lysostaphin (Figure 2).
 |
Table 3 Minimum inhibitory concentration (MIC) (μg/mL) for free and liposomal formulations |
Liposome–bacterial binding using flow cytometry
Binding of fluorescent labeled liposomes to the bacteria (in the green channel) and cell viability (in red channel) were demonstrated by flow cytometry (Figure 1). Labeled liposomes without vancomycin and lysostaphin were utilized to avoid bacterial death and use as a control. The binding signal was increased in all the formulations after 2 hrs (p<0.05). LysL and LysLV reached 67% and 78.3% at 5 mins and 86.5% and 91.3% at 2 hrs, respectively, while, the empty liposome (L) reached 31.8% and 53.5% in 5 mins and 2 hrs, respectively. These results showed a maximum binding level of LysLV and LysL in comparison with other formulations which is due to the targeting of liposomes with lysostaphin (p<0.05) (Figure 1C). Further, bactericidal effect of all the formulations using flow cytometry in present PI was showed that they could lead to the death of bacteria (p<0.05) in compression with control liposome. Bacteria effect of free lysostaphin could lead to the death of bacteria to 65.8% after 2 hrs. When lysostaphin was conjugated with liposomal vancomycin, its bactericidal effect increased. On the other hand, vancomycin and LV needed more than 2 hrs to kill the most of the bacteria (Figure 1D). The comparison with the bacterial effect of all formulations confirmed an effect of dual attacking to the bacteria. When lysostaphin bounded to the bacteria surface, lysing bacteria cell wall occurred and then vancomycin releasing was facilitated, so vancomycin’s effect could accelerate in comparison with the other formulations.
In vitro liposomal vancomycin release
To study the mechanism of vancomycin releasing from different formulations of liposomes and the effect of α-toxin pore-forming activity on phospholipids membranes which involved in the interaction between α-toxin and liposome, we evaluated the membrane-damaging effect of α-toxin on LV and LysLV formulations in the presence and absence of MRSA bacteria. α-Toxin is one of the most common and important pore-forming toxins secreted by S. aureus which makes pores in biological or artificial membranes.16 Susceptibility of liposomes containing phosphatidylcholine and cholesterol to α-toxin has been previously reported by Pornpattananangkul et al.37 In our study, LV and LysLV formulations were incubated with MRSA bacteria (1×106 CFU/mL) at 35°C for 2 and 18 hrs. In the presence of MRSA, the vancomycin release in both formulations was significantly higher in comparison with the absence of MRSA (p<0.05) (Figure 1B). The vancomycin releasing and real-time PCR results indicated that the employed MRSA bacteria had a high α-toxin expression. This confirms that likely vancomycin was released from liposomes due to pores forming on the liposomal membrane which was made by α-toxin secreted by MRSA bacteria. The hla monomers bound to phosphatidylcholine head group and cholesterol then assemble as a heptamer form to make pores in liposome membranes, resulting in increased membrane permeability following increase in the vancomycin release.37 In the presence of MRSA, the vancomycin releasing from LV formulations was higher than LysLV in comparison with the absence of MRSA bacteria condition. Actually, the presence of MRSA did not affect the increasing vancomycin release on LysLV formulations which might be because of the following two reasons: 1) coupling of lysostaphin on the liposomal surface could make strict barriers on the surface of liposome which leads to less pores forming on this kind of liposomes and 2) while 90% of bacteria has died before 2 hrs (Figure 1D), which they could not normally react against inappropriate conditions and secret α-toxin. Thus, the secreted α-toxin was probably less than the same condition which all bacteria were alive.
Cell viability assay
The results of cell viability assay using MTT dye are shown in Figure 3. The formulations free Lys/Van* till ≤5.85 μg/mL, free vancomycin and LysLV* till ≤23.4 μg/mL, and LV till ≤46.87 μg/mL did not show toxicity after 24 hrs of treatment (Figure 3A). The formulations free Lys, LysL, LysLV**, and free Lys/Van** also have not shown toxicity till ≤10.9≤21.8, ≤2.7, and ≤0.68 μg/mL, respectively (Figure 3B). This shows that all the formulations were noncytotoxic till 4–6× MIC dosage.
In vivo study and MRSA mortality
We investigate the efficiency of all the formulations against MRSA in a mouse surgical wound model. Mice receiving 10,9 108, and 107 FU, died after 2, 3, and 4 days, respectively. After their death, MRSA was isolated from blood, liver, and spleen of all groups, indicating sepsis. Mice that were not infected stayed alive (Figure S2). According to these findings, 107 CFU of MRSA was employed in subsequent experiments to infect all groups of mice. Finally, the effect of each formulation on MRSA infection was evaluated. All mice treated with various formulations were treated at the end of the 14th day in comparison with the 1st day (p<0.05). The most effective results belonged to the mice treated with LysLV and then free Lys/Van (Table 4). LysLV significantly reduced the number of bacteria in the surgical site in compression with other formulations at the end of the 9th and 14th days (p<0.05). But there was no significant reduction in the number of bacteria in compression with free lysostaphin and free Lys/Van groups at the end of the 4th day. After treatment, no bacteria were isolated from blood, spleen, and liver in all the groups, indicating effective prevention of postsurgical infection. We propose that targeting of the cell wall by lysostaphin maximizes exposure of the bacteria to the liposomal vancomycin. Additionally, the lysostaphin component may contribute to antibacterial activity. The nanoliposomal delivery vehicle used in the present work has several attractive features. Applied topically, it can penetrate the infected area, eliminating the need for systemic drug administration, thereby reducing adverse side effects.38 Moreover, it can improve the therapeutic effect by prolonging vancomycin release during treatment and also it leads to better vancomycin penetration into the eukaryote cells in compassion with the other formulation leading to killing of all intracellular and extracellular MRSA. The antibacterial activity of free lysostaphin was more effective than liposome-conjugated lysostaphin (LysL) in both in vitro and in vivo (Tables 3 and 4) indicating that the antibacterial properties of some of the lysostaphins became inactivated during the process of conjugation. The presence of functional groups of cyanur-PE on the surface of liposomes could have interfered with some of the lysostaphins binding to the S. aureus cell wall, or the lysostaphin active site could have been blocked by them. Further research could focus on improving the lysostaphin conjugating method to minimize the loss of lysostaphin activity. That means the lysostaphin-binding site and active site remain intact during conjugation. Vancomycin resistance in MRSA strains has been reported,39,40 and mutations in the femA gene confer lysostaphin resistance.41–43 A special nanoparticle construction, such as the one described here, including both lysostaphin and vancomycin, may encounter less clinical resistance than a single drug.
 |
Table 4 Bacterial counts (CFU/g) of mice skin section at 4, 9, and 14 days after treatment. |
Conclusions
We developed vancomycin nanocarriers which their surface decorated with lysostaphin as a new platform to be efficiently and safely applied for topical administration to treat skin or surgical site infections. We showed that liposomes loaded with vancomycin and targeted with the anti-staphylococcal protein lysostaphin are superior to other formulations and its confirmed the synergetic effect of dual attacking to the bacteria. When lysostaphin bounded to the bacteria surface, lysing bacteria cell wall occurred while, vancomycin releasing was facilitated by MRSA bacteria α-toxins that make pore on liposomal membrane, so vancomycin’s effect could accelerate in comparison with other formulations. And also, we believe that the results of this study could lead to less resistance in clinical MRSA strains.
Abbreviations list
LV, liposomal vancomycin; LysLV, lysostaphin-conjugated liposomal vancomycin, LysL, lysostaphin-conjugated liposome; Lys/Van, lysostaphin combined with vancomycin.
Ethical approval
This study was approved by the Ethics Review Body of Animal Experimentation of Iran University (IR.UMSHA.REC.1394.134).
Acknowledgments
The authors gratefully thank the vice-chancellor of Hamadan University of Medical Sciences and Iran University of Medical Sciences for funding support. The authors would like to thank Dr. Shapiro for reading, editing and giving some helpful comments to improve the manuscript. This work was supported by research grants from the Hamadan University of Medical Sciences (grant/award number: 94-10-29-6134) and the Iran University of Medical Sciences (grant/award number: 94-05-31-26873).
Disclosure
The authors declare no conflicts of interest in this study.
References
1. Munita JM, Arias CA. Mechanisms of antibiotic resistance. Microbiol Spectr. 2016;4(2). doi:10.1128/microbiolspec.VMBF-0016-2015.
2. Katakura T, Yoshida T, Kobayashi M, Herndon D, Suzuki F. Immunological control of methicillin‐resistant Staphylococcus aureus (MRSA) infection in an immunodeficient murine model of thermal injuries. J Clin Exp Immunol. 2005;142(3):419–425.
3. Moet GJ, Jones RN, Biedenbach DJ, Stilwell MG, Fritsche TR. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998–2004). Diagn Microbiol Infect Dis. 2007;57(1):7–13. doi:10.1016/j.diagmicrobio.2006.05.009
4. Gurusamy KS, Koti R, Toon CD, Wilson P, Davidson B. Antibiotic therapy for the treatment of methicillin‐resistant Staphylococcus aureus (MRSA) infections in surgical wounds. Cochrane Database Syst Rev. 2013;8:CD009726.
5. Mohammad H, Thangamani S, Seleem NM. Antimicrobial peptides and peptidomimetics-potent therapeutic allies for Staphylococcal infections. Curr Pharm Des. 2015;21(16):2073–2088.
6. Stryjewski ME, Chambers HF. Skin and soft-tissue infections caused by community-acquired methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46(Supplement_5):S368–S377. doi:10.1086/533593
7. NOW MM, COMMON BSAS. Skin and soft-tissue infections: classifying and treating a spectrum. Cleve Clin J Med. 2012;79(1):57. doi:10.3949/ccjm.79a.11044
8. Boswihi SS, Udo EE. Methicillin-resistant Staphylococcus aureus: an update on the epidemiology, treatment options and infection control. J Curr Med Res Pract. 2018;8(1):18–24. doi:10.1016/j.cmrp.2018.01.001
9. Wong M, Chapman MG, Malhotra S, Mirzanejad Y, Deans GD. Experience with high dose once-daily vancomycin for patients with skin and soft-tissue infections in an ambulatory setting. Open Forum Infect Dis. 2017;4(Suppl 1):S338.
10. Bruniera F, Ferreira F, Saviolli L, et al. The use of vancomycin with its therapeutic and adverse effects: a review. Eur Rev Med Pharmacol Sci. 2015;19(4):694–700.
11. Touitou E, Junginger H, Weiner N, Nagai T, Mezei M. Liposomes as carriers for topical and transdermal delivery. J Pharm Pharm. 1994;83(9):1189–1203.
12. Weiner N, Lieb L, Niemiec S, Ramachandran C, Hu Z, Egbaria K. Liposomes: a novel topical delivery system for pharmaceutical and cosmetic applications. J Drug Target. 1994;2(5):405–410. doi:10.3109/10611869408996816
13. Shanmugasundaram N, Uma T, Ramyaa Lakshmi T, Babu M. The Japanese society for biomaterials, biomaterials TASf, biomaterials tKSf. Efficiency of controlled topical delivery of silver sulfadiazine in infected burn wounds. J Biomed Mater Res A. 2009;89(2):472–482. doi:10.1002/jbm.a.31997
14. Teo EY, Ong S-Y, Chong MSK, et al. Polycaprolactone-based fused deposition modeled mesh for delivery of antibacterial agents to infected wounds. Biomaterials. 2011;32(1):279–287. doi:10.1016/j.biomaterials.2010.08.089
15. Surewaard BG, Deniset JF, Zemp FJ, et al. Identification and treatment of the Staphylococcus aureus reservoir in vivo. J Exp Med. 2016;213(7):1141–1151.
16. Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991;55(4):733–751.
17. Chen C, Fan H, Huang Y, et al. Recombinant lysostaphin protects mice from methicillin-resistant Staphylococcus aureus pneumonia. Biomed Res Int. 2014;2014:602185.
18. Nithya S, Nimal T, Baranwal G, et al. Preparation, characterization and efficacy of lysostaphin-chitosan gel against Staphylococcus aureus. Int J Biol Macromol. 2018;110:157–166. doi:10.1016/j.ijbiomac.2018.01.083
19. Kateete DP, Kimani CN, Katabazi FA, et al. Identification of Staphylococcus aureus: dNase and mannitol salt agar improve the efficiency of the tube coagulase test. Ann Clin Microbiol Antimicrob. 2010;9(1):23. doi:10.1186/1476-0711-9-23
20. Clinical Institute LS. Performance Standards for Antimicrobial Susceptibility Testing. Document M100-S15. Wayne, PA: Clinical and Laboratory Standards Institute; 2016.
21. Heydari N, Alikhani M, Jalilian F, Tahmasebi H, Arabestani M. Evaluation of real time PCR for detection of clinical isolates of Staphylococcus aureus and methicillin-resistance strains based on melting curve analysis method. Koomesh. 2017;19(4):877–886.
22. Jarraud S, Mougel C, Thioulouse J, et al. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect Immun. 2002;70(2):631–641. doi:10.1128/iai.70.2.631-641.2002
23. Eleaume H, Jabbouri S. Comparison of two standardisation methods in real-time quantitative RT-PCR to follow Staphylococcus aureus genes expression during in vitro growth. J Microbiol Methods. 2004;59(3):363–370. doi:10.1016/j.mimet.2004.07.015
24. Zhang H. Thin-film hydration followed by extrusion method for liposome preparation. In: Liposomes. Springer; 2017:17–22.
25. Liu J, Wang Z, Li F, Gao J, Wang L, Huang G. Liposomes for systematic delivery of vancomycin hydrochloride to decrease nephrotoxicity: characterization and evaluation. Asian J Pharm Clin Res. 2015;10(3):212–222.
26. Labella C, Lelario F, Bufo SA, Musto M, Freschi P, Cosentino C. Optimization and validation of a chromatographic method for quantification of lysozyme in jenny milk. Nutr Res. 2016;55(3):263–269.
27. Ordóñez-Gutiérrez L, Posado-Fernández A, Ahmadvand D, et al. ImmunoPEGliposome-mediated reduction of blood and brain amyloid levels in a mouse model of Alzheimer’s disease is restricted to aged animals. Biomaterials. 2017;112:141–152. doi:10.1016/j.biomaterials.2016.07.027
28. Tseng CW, Sanchez-Martinez M, Arruda A, Liu GY. Subcutaneous infection of methicillin resistant Staphylococcus aureus (MRSA). J Vis Exp. 2011;(48). doi:10.3791/2528.
29. Vyas LK, Tapar KK, Nema RK, Parashar AK. Development and characterization of topical liposomal gel formulation for anti-cellulite activity. International Journal of Pharmacy and Pharmaceutical Sciences. 2013;5:512–516.
30. Sande L, Sanchez M, Montes J, et al. Liposomal encapsulation of vancomycin improves killing of methicillin-resistant Staphylococcus aureus in a murine infection model. J Antimicrob Chemothe. 2012;67(9):2191–2194. doi:10.1093/jac/dks212
31. Desbois AP, Gemmell CG, Coote PJ. In vivo efficacy of the antimicrobial peptide ranalexin in combination with the endopeptidase lysostaphin against wound and systemic meticillin-resistant Staphylococcus aureus (MRSA) infections. Int J Antimicrob Agents. 2010;35(6):559–565. doi:10.1016/j.ijantimicag.2010.01.016
32. Yang X-Y, Li C-R, Lou R-H, et al. In vitro activity of recombinant lysostaphin against Staphylococcus aureus isolates from hospitals in Beijing, China. J Med Microbiol. 2007;56(1):71–76. doi:10.1099/jmm.0.46788-0
33. Narasimhaswamy N, Bairy I, Manuel A, Mathew J, Shenoy G, Bairy L. In-vitro bactericidal activity of recombinant lysostaphin on biofilm producing methicillin resistant Staphylococcus aureus. Int J Curr Microbiol App Sci. 2015;4(1):822–830.
34. Cui F, Li G, Huang J, et al. Development of chitosan-collagen hydrogel incorporated with lysostaphin (CCHL) burn dressing with anti-methicillin-resistant Staphylococcus aureus and promotion wound healing properties. J Drug Deliv Sci Technol. 2011;18(3):173–180.
35. Miao J, Pangule RC, Paskaleva EE, et al. Lysostaphin-functionalized cellulose fibers with antistaphylococcal activity for wound healing applications. Biomaterials. 2011;32(36):9557–9567. doi:10.1016/j.biomaterials.2011.08.080
36. Gründling A, Schneewind O. Cross-linked peptidoglycan mediates lysostaphin binding to the cell wall envelope of Staphylococcus aureus. J Bacteriol. 2006;188(7):2463–2472. doi:10.1128/JB.188.7.2463-2472.2006
37. Pornpattananangkul D, Zhang L, Olson S, et al. Bacterial toxin-triggered drug release from gold nanoparticle-stabilized liposomes for the treatment of bacterial infection. J Am Chem Soc. 2011;133(11):4132–4139. doi:10.1021/ja111110e
38. Nguyen HM, Graber CJ. Limitations of antibiotic options for invasive infections caused by methicillin-resistant Staphylococcus aureus: is combination therapy the answer? J Antimicrob Chemother. 2009;65(1):24–36. doi:10.1093/jac/dkp377
39. Hasan R, Acharjee M, Noor R. Prevalence of vancomycin resistant Staphylococcus aureus (VRSA) in methicillin resistant S. aureus (MRSA) strains isolated from burn wound infections. Ci Ji Yi Xue Za Zhi. 2016;28(2):49–53. doi:10.1016/j.tcmj.2016.03.002
40. Loomba PS, Taneja J, Mishra B. Methicillin and vancomycin resistant S. aureus in hospitalized patients. J Infect Dis. 2010;2(3):275.
41. Kusuma C, Jadanova A, Chanturiya T, Kokai-Kun JF. Lysostaphin-resistant variants of Staphylococcus aureus demonstrate reduced fitness in vitro and in vivo. Antimicrob Agents Chemother. 2007;51(2):475–482. doi:10.1128/AAC.00786-06
42. Stranden AM, Ehlert K, Labischinski H, Berger-Bächi B. Cell wall monoglycine cross-bridges and methicillin hypersusceptibility in a femAB null mutant of methicillin-resistant Staphylococcus aureus. J Bacteriol. 1997;179(1):9–16. doi:10.1128/jb.179.1.9-16.1997
43. De Jonge B, Sidow T, Chang Y-S, et al. Altered muropeptide composition in Staphylococcus aureus strains with an inactivated femA locus. J Bacteriol. 1993;175(9):2779–2782. doi:10.1128/jb.175.9.2779-2782.1993
Supplementary materials
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.