Back to Journals » International Journal of Nanomedicine » Volume 14
Temperature and pH-responsive nano-hydrogel drug delivery system based on lysine-modified poly (vinylcaprolactam)
Authors Farjadian F , Rezaeifard S, Naeimi M, Ghasemi S, Mohammadi-Samani S , Welland ME, Tayebi L
Received 4 May 2019
Accepted for publication 31 July 2019
Published 30 August 2019 Volume 2019:14 Pages 6901—6915
DOI https://doi.org/10.2147/IJN.S214467
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Fatemeh Farjadian,1 Somayeh Rezaeifard,2 Mahsa Naeimi,1 Sahar Ghasemi,1 Soliman Mohammadi-Samani,1,3 Mark E Welland,4 Lobat Tayebi5
1Pharmaceutical Sciences Research Center, Shiraz University of Medical Sciences, Shiraz, Iran; 2Institute for Cancer Research, Shiraz University of Medical Sciences, Shiraz, Iran; 3Department of Pharmaceutics, Faculty of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran; 4The Nanoscience Centre, Department of Engineering, University of Cambridge, Cambridge, United Kingdom; 5Marquette University, School of Dentistry, Milwaukee, WI, USA
Correspondence: Fatemeh Farjadian
Pharmaceutical Sciences Research Center, Shiraz University of Medical Sciences, P.C. 71348-14336,
Shiraz, Iran
Tel +987132424127(302)
Fax +9871132424126
Email [email protected]
Lobat Tayebi
Marquette University, School of Dentistry, Milwaukee, WI 53233, USA
Tel +1 414 288 8383
Fax +2708971179
Email [email protected]
Website
tayebigroup.mu.edu
Background: Smart materials capable of responding to external stimuli are noteworthy candidates in designing drug delivery systems. In many of the recent research, temperature and pH have been recognized as the main stimulating factors in designing systems for anti-cancer drugs delivery systems.
Purpose: In this study, thermo and pH-responsive character of a nano-carrier drug delivery platform based on lysine modified poly (vinylcaprolactam) hydrogel conjugated with doxorubicin was assessed.
Methods: Poly (vinylcaprolactam) cross-linked with poly (ethyleneglycol) diacrylate was prepared via RAFT polymerization, and the prepared structure was linked with lysine through ring-opening. The anti-cancer drug doxorubicin, was linked to lysine moiety of the prepared structure via Schiff-base reaction. The prepared platform was characterized by 1,HNMR and FT-IR, while molecular weight characterization was performed by size exclusion chromatography. The temperature-responsive activity was evaluated using differential scanning calorimetry and dynamic light scattering. In vitro release pattern in simulated physiologic pH at 37°C was compared with acidic pH attributed to tumor site and elevated temperature. The anticancer efficiency of the drug-conjugated structure was evaluated in breast cancer cell line MCF-7 in 24 and 48 h, and cell uptake assay was performed on the same cell line.
Conclusion: According to the results, well-structure defined smart pH and temperature responsive nano-hydrogel was prepared. The enhanced release rates are observed at acidic pH and elevated temperature. We have concluded that the doxorubicin-conjugated nanoparticle results in higher cellular uptakes and more cytotoxicity.
Keywords: drug delivery, DOX, RAFT, PVCL, lysine, cancer
Introduction
During the past decade, nanotechnology has been focused on finding novel solutions to reduce the side effects of drugs, especially in diseases like cancer.1 Nowadays, nano-sized particles have been recruited as the carrier of therapeutic agents to deliver them to the specified target tissues, such as tumor sites.2 Some types of therapeutics loaded with nanoparticles have been categorized as nanopharmaceuticals.3 Doxil, as the commercial formulation of nano-liposome of doxorubicin (DOX), is a chemotherapeutic agent used to treat cancer. It has found a huge market size and made great benefits for patients traditionally treated with DOX by reducing common side effects, such as hair loss, heart and tissue damage and more.4
The medical application of nanotechnology, which is recognized in an emerging field called nano-medicine, especially in drug delivery, is under development. Designing an engineered sustainable nano-structured system that has complete control over releasing cargo is the golden goal in drug delivery systems (DDS).5,6 In this regard, smart materials capable of responding to external stimuli are noteworthy candidates in DDS engineering and have the potential to be applied commercially.2,7 These types of materials are mostly made by the development of smart and intelligent polymers. The external stimulus is mostly categorized as chemical and physical; chemical stimuli consisting of changes in pH8 and ionic strength9 and physical stimuli including light,10,11 temperature,12 mechanical forces10, and wavelengths.13 Due to the numerous benefits acquired in working with smart polymeric systems – including sensitivity to stimulus, long-term stabilities, biocompatibility, and high adsorption capacity – their applications are extensively studied in tissue engineering, gene delivery, and drug delivery.14 In many of the recent research, temperature and pH have been recognized as the main stimulating factors in designing DDS, given the fact that the temperature of the tumor site is higher than average body temperature, and their pH is more acidic than the normal cells (~5.8).8,12
Thermoresponsive polymeric materials could exhibit two main critical phase transitions in aqueous media at certain temperatures: the first show lower critical solution temperature (LCST) (immiscible at a temperature higher than LCST), and the second presents upper critical solution temperature (UCST) (immiscible at temperature lower than UCST).15 The hydration and dehydration reactions in the aqueous system after heating affects the dissolution process for thermoresponsive materials.16 To have a controlled DDS from a thermoresponsive nano-carrier for cancer therapy purposes, the LCST should be adjusted to a temperature higher than the normal body temperature (>37°C).17 Temperature variation in fighting infections in most vertebrates is through elevation between 1 and 5 degrees.18 To adjust the temperature in thermoresponsive polymers the addition of hydrophilic segments could increase the LCST of well-known types, such as poly(N-isopropylacrylamide) (PNIPAAm) (LCST~32°C), poly(N, N-diethylacrylamide) (LCST ranging from 25°C to 32°C) and cyclic lactam, such as poly(N-vinylcaprolactam) (PVCL) (LCST ranging from 25°C to 35°C).16,19
PVCL is among the well-known synthetic thermoresponsive polymers, which, despite PNIPAAm, do not produce toxic amide compound under acidic conditions.19 Furthermore, due to the important characteristics including as hydrophilicity, excellent biocompatibility, stability against hydrolysis, and smart behavior toward pH and temperature, it has been widely applied for biomedical applications. Various types of PVCL-based materials have been developed such as micelles, gels (i.e., nano and micro) and hybrids (i.e., core/corona) and investigated for their pharmaceutical trends in drug/gene delivery.20
Nanogels are recognized as potent carriers in medical applications.21 Injectable bio-based hydrogels were constructed for multi-mode tumor therapy.22
Development in free radical polymerization techniques, like reversible addition fragmentation/RAFT and atom transfer radical polymerization/ATRP, has enabled scientists to synthesize a polymeric nano-carrier in several forms, including self-assembled, micelles, and hydrogels.23,24 The RAFT method is recognized as an ideal and green method that could be applied in the polymer synthesis with a wide class of monomers, is also applicable to a less activated monomer (such as vinylamides) and could be successfully applied to N-vinylcaprolactam (VCL).19,25
RAFT polymerization has been successfully recruited in the synthesis of smart thermoresponsive platform with PVCL. Recently, a physically cross-linked microgel has been derived from poly(ethylene glycol) (PEG)-b-poly(VCL-co-vinyl acetate), where chain transfer agent (CTA) is a functionalized CTA-PEG and efficiently serves as macro RAFT agent.26 Biocompatible nanocapsules emanated from RAFT copolymerization of VCL and acrylic acid was developed in a vesicle templating route.27 A sustainable amino-functionalized platform with thermo and pH sensitivity was prepared from amino-VCL, which was basically synthesized from cyclic lysine and polymerized in the RAFT process.28 A polymersome nano-formulation made of tannic acid locked-PVCL-b-poly(N-vinylpyrrolidone) with vesicular morphology was prepared and applied efficiently for thermo-triggered DOX delivery.29 Nano-micellar formulation of PVCL-b-polycaprolactone, suitable for drug delivery, was synthesized.30 Furthermore, in an emulsion polymerization microgels of PVCL cross-linked by PEG diacrylate (PEGDA) was created.31 A biodegradable DOX delivery system was also created based on PVCL nanogels.32
Recently, we reported synthesis and application of smart pH-responsive DOX delivery system based on poly (hydroxyethyl methacrylate-co-dimethylaminoethyl methacrylate) nano-hydrogel.33 In this study, PVCL as a hydrophilic temperature-sensitive polymer that is well known for biocompatibility and complexation capability was selected. PEG, as an amphiphilic polymer with nontoxic metabolites and FDA approval was considered as a cross-linker of this system. L-lysine as a cationic and water soluble amino acid was selected as PVCL modifier and drug linker agent. DOX, as one of the most common models of anticancer drugs, was conjugated to polymeric-supported backbone via a Schiff-base linkage to obtain pH-responsive release. In a brief, the preparation of PVCL cross-linked (PEGDA) hydrogel via RAFT polymerization and then functionalization with L-lysine and conjugation via Schiff-base linkage with DOX as novel thermo and pH-responsive anticancer delivery system is reported.
Experimental section
Materials and methods
Cyanomethyl N-methyl N-phenyldithiocarbamate, l-lysine, VCL (98%), α,α-bisisobutyronitrile (AIBN, 98%), PEGDA(Mn 575), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), rhodamine B (Rhod), N-hydroxysuccinimide, and (1-ethyl-3-(3-dimethylaminopropyl) carbodiimide) (EDC) were bought from Sigma-Aldrich. AIBN was purified through recrystallization from methanol, VCL was recrystallized in benzene and PEGDA purified through the silica gel column by washing with dichloromethane and dried under vacuum before starting the synthesis.1,4-Dioxane (Fluka, 99.5%) was dried using CaCl2, and absolute drying was accomplished by refluxing in sodium wire and benzophenone (0.2% g/L) under nitrogen until the dark blue color appeared. DOX hydrochloride was purchased from EBEWE Pharma. MCF-7 breast cancer cell lines were purchased from Pasteur Institute (Iran). RPMI (Roswell Park Memorial Institute medium) 1640 from Biosera, France and fetal bovine serum (FBS) from Shellmax.
Instrument
1HNMR spectra were obtained using a 250 MHz instrument from Bruker Avance DPX Company, using D2O as the solvent. Fourier transform infrared spectra of the synthesized polymers were recorded on a Bruker (VERTEX70). Dynamic light scattering (DLS) was carried out by using Malvern Nano and zeta (ξ) potential determined by zeta sizer (Microtrac USA). Transmission electron microscopy (TEM) images were recorded by Philips CM 10 instrument via grid preparation. Grids were prepared by dropping a solution of 500 µg/mL of PVCL-Lys. After taking images at room temperature, the grid was incubated at 42°C and image taking was repeated. Cryo TEM was performed by FEI Titan Krios. The samples were vitrified (plunge frozen into liquid ethane) on Quantifoil R2/2 Cu 300 mesh grids. Biotrak Elisa plate reader was applied for UV-Vis records. The dialysis tubing from Sigma with 32 mm width and MWCO 12,400. Size exclusion chromatography (SEC) was performed with Agilent 1260 high-performance liquid chromatography (HPLC). The samples were lyophilized by Christ, Alpha, 2–4-ld plus/Germany freeze dryer. Microtube shaking was performed in Bioer, mixing block MB-102/China instrument. UV absorbance was recorded by CECIL CE7250 instrument. Olympus BX61/Japan Fluorescent microscope was applied for observing slides and for PVCL-Rhod particles, filter with mirror unit of U-MWLB3, dichrom mirror of DM505, excitation filter of BP460-495, and barrier filter BA510lF was applied. Flowcytometry was operated by BD FACSCalibur flowcytometer/BD USA.
Synthesis of nano-hydrogel (PVCL-PEG)
The experimental procedure employed in this study was a RAFT polymerization.34 The details are explained here, briefly. Initially, VCL (9 mmol), cyanomethyl N-methyl N-phenyl dithiocarbamate (0.045 mmol) as RAFT agent, AIBN (3 mg, 0.0182 mmol) as initiator and PEGDA (0.27 mmol) as a cross-linker were dissolved in dried dioxane (5 mL). In the next step, the mixture was placed in a Schlenk reaction tube and degassed under nitrogen and freeze-thawed five times. Then, the tube was sealed and stirred at 90°C for 48 hrs. Afterward, the reaction was chilled and washed with dried n-hexane three times. Finally, the product was dried by using freeze-drying machine for 48 hrs.
Synthesis of lysine-modified PVCL-PEG (PVCL-Lys)
Synthesized PVCL-PEG (0.1 g) and L-lysine (20 mg) were dissolved in 20 mL deionized (DI) water and stirred for 24 hrs at room temperature. Then, to remove excess and unreacted lysine, the mixture dialyzed in DI water for 24 hrs and finally freeze-dried.
DOX conjugation to PVCL-LYs (PVCL-DOX)
DOX conjugation was accomplished through Schiff-base reaction and prepared using the following method. Briefly, PVCL-Lys (80 mg), DOX (8 mg), and triethylamine (Et3N) (8 µL) were dissolved in methanol (20 cc) and stirred for 48 hrs at room temperature in a dark location. Afterward, to remove unconjugated DOX, the mixture was dialyzed in DI water for 6 hrs and then freeze-dried.
Rhodamine conjugation to Pvcl-Lys (PVCL-Rhod)
To have fluorescence-tagged particles, rhodamine B (Rhod) was conjugated to PVCL-Lys. To do so, Rhod (0.06 mmole) was activated in aqueous solution (4 mL) of N-hydroxysuccinimide (0.12 mmole) and EDC (0.06 mmole) for 4 hrs at 40°C. After that, PVCL-Lys (40 mg) and Et3N (0.14 mmole) were admixed with activated Rhod, and the mixture stirred for 24 hrs at the same temperature. Afterward, to remove unconjugated Rhod, the mixture dialyzed in DI water for 6 hrs and then freeze-dried.
Size exclusion chromatography
To define the molecular weight (MW), SEC was performed on HPLC system with a Tsk gel G 3000 SWXL column. Epidermal growth factor with 6 KDa was utilized as the standard sample. The instrument was equipped with UV/Vis detector and detected samples at 200 nm. Fifty microliters aqueous solution (1 mg/mL) of PVCL-Rhod was injected onto the column. Elution buffer was 9 g/L NaH2PO4, 9 g/L Na2HPO4, and orthophosphoric acid 85% was applied to adjust pH of aqueous solution in 6.5. The adjusted flow rate was 0.5 mL/min.
Determination of DOX and Rhod content
To define the amount of DOX conjugated to PVCL, samples containing 100 µg/mL of PVCL-DOX were acidified by hydrochloride acid (pH=1.2, 2 mL). Then, they were stirred for 48 hrs at 40°C, afterward the absorbance at 478 nm was recorded using Elisa plate reader. Finally, absorbance of the samples was subtracted from the absorbance of PVCL-Lys solution with similar concentration. The same procedure was performed to determine Rhod conjugation percent of PVCL-Rhod. Finally, the Rhod amount was determined using standard calibration curve of Rhod.
Release profile of PVCL-DOX
Release profile experiment assessment was performed by considering two factors, pH (5 and 7.4) and temperature (37°C and 40°C). The set of four experiments were performed in microtubes (2 mL). Samples of 100 µg/mL PVCL-DOX in physiological-simulated pH of 7.4 with two temperatures of 37°C and 40°C in PBS medium, and simulated tumor pH of 5 with two temperatures of 37°C and 40°C in HCL adjusted pH were prepared. The profile of release was measured within different time intervals of 0.5, 1, 2, 3, 6, 12, 24, 48, 72 hrs by detecting the absorbance of free DOX using UV/Vis spectrophotometer at 478 nm and subtracting from the absorbance of the solution of PVCL-Lys with similar concentration.
In vitro cellular cytotoxicity assessment
To investigate cytotoxic effects, MTT assay was used according to the reported procedure.35 First, the MCF-7 cells were cultured in RPMI 1640-supplemented medium with 10% FBS at 37°C (5% CO2) incubator. Briefly, 10×104 cells in 100 μL of completed medium were seeded on each well of 96-well microplates. After 24 hrs, different concentrations of DOX and PVCL-Lys in various concentrations (0.5, 1.25, 2.5, 3.75, 5 µg/mL) and PVCL-DOX concentrations (10, 25,50, 75, 100 µg/mL) corresponding to DOX concentrations of (0.5, 1.25, 2.5, 3.75, 5 µg/mL) were added to the wells in triplicate. The cells were incubated further for 24 and 48 hrs, and the medium was then recharged with 100 μL of diluted MTT solution. Following 4 hrs of incubation and MTT solution aspiration, 150 μL of dimethyl sulfoxide was added to the wells. Finally, the absorption of each well was measured after 30 mins by spectrophotometry in the wavelength of 492 nm, and then 50% inhibitory concentration (IC50) was determined by Curve Expert 1.4 software. The statistical analysis was performed by analysis of variance method.
Uptake assay using fluorescence microscopy
For uptake assay using fluorescence microscopy, 3×105 cells in 2 mL of completed medium were seeded on sterile glass slides already put into each well of six well microplates. After 24 hrs incubation for attaching the cells to the slides, different concentrations of DOX (0.3, 0.6, and 1.2 ppm), PVCL-DOX (15, 30, and 60 ppm) and PVCL-Rhod (15, 30, and 60 ppm) were added to the wells. After 24 and 48 hrs, the glass slides were removed from each well, one drop of glycerol on the glass slides was added; then, a new glass slide was placed on the back on the slides, which were observed by fluorescence microscope.
Uptake assay using flow cytometry assay
For uptake assay using flow cytometry method, 5×104 cells in 500 μL of completed medium were cultured on the each well of 24 well microplates. After 24 hrs, DOX (0.3, 0.6, and 1.2 µg/mL), PVCL-DOX (15, 30, and 60 µg/mL) and the same concentration of PVCL-Rhod were added to the wells. The cells were incubated for 4, 24, and 48 hrs, then harvested and transferred to flow cytometry tubes.
Results
Synthesis, structural characterization, and MWdetermination
PVCL is a promising candidate as a thermoresponsive carrier for drug delivery purposes. In this work, novel lysine-modified hydrogel was synthesized, characterized and successfully recruited for DOX delivery. The synthetic route based on RAFT polymerization technique is illustrated in Figure 1.
 |
Figure 1 Schematic and structural illustration of the synthetic pathway in preparation of PVCL-DOX nano-hydrogel. Abbreviations: PVCL, poly(N-vinylcaprolactam); DOX, doxorubicin. |
VCL is copolymerized with PEGDA, which acts as a cross-linker via RAFT polymerization technique. CTA acts as the main precursor in RAFT reactions, and for n-vinylamide monomers, such as VCL, cyanomethyl N-methyl N-phenyl dithiocarbamate is an appropriate candidate.36 The initiator (i.e., AIBN) starts the polymerization, and the dormant chains are capped with dithiocarbamate end group, which can be activated in the second run of polymerization and is useful in preparation of block copolymers with controlled chains length.37 PEGDA, as an amphiphilic cross-linker with PEG chains, was selected to develop the hydrogel property and biocompatibility. Herein, to provide a specific site for the conjugation of drug to synthesized structure of PVCL-PEG, L-lysine was conjugated. To characterize the structure of PVCL-PEG and PVCL-Lys, 1HNMR spectroscopy was performed (Figure 2). The characteristic peaks of PVCL and PEG appeared in their spectra, as shown in Figure 2. The degree of polymerization was determined by 1HNMR spectroscopy through determining the integration of CTA characteristic peak while compared with characteristic peak of PVCL and PEG.
In this regard, dithiocarbamate moiety (N-CH3) at end group of each polymeric chain was confirmed by the peak at 4.83 µg/mL and hydrogen of methane group of PVCL from (CH2-N) at 4–4.5 µg/mL and methylene groups of PEG (-O-CH2-CH2-O-) at 3.8 µg/mL. The integrated area ratio of CTA/PVCL/PEG is about 1/40/1.5, close to feed ratio amount (1:47:3), and the MW is found to be 6652 D. In the next step, L-lysine was added to open PVCL ring and start branches functionalized with lysine. The 1HNMR spectrum (Figure 2) is shown and, in comparison to PVCL-PEG, the peak multiplexing of PVCL occurs in the region of 1.1–1.9, 2–2.5, and 3.18–3.3 µg/mL, while a characteristic band of lysine moiety (COOH-CH-NH) appears at 4.5 µg/mL. The amount of CTA/Lysine-modified branches are about 1/5, indicating that 5 out of 40 PVCL rings were opened. After that, the PVCL-Lys were reacted with DOX to have (PVCL-DOX) through Schiff-base reaction.
 |
Figure 2 1HNMR spectra of PVCL-PEG and PVCL-Lys. Abbreviations: PVCL, poly(N-vinylcaprolactam); PEWG, poly(ethylene glycol). |
Furthermore, the structures of synthesized nanogels were characterized by FT-IR spectroscopy (Figure S1). As depicted in the spectrum, the absorption at 1420 and 1470 cm−1 can be attributed to amide I and II of PVCL. The C–H stretching vibration appears at 2937 and 2910 cm−1. The characteristic band of C–O appears at 1070–1200 cm−1, related to PEG group of cross-linker. Also, after PVCL-PEG was modified with lysine, the N–H vibrational absorption peak of ammonium chloride group appears at 1718 cm−1 due to successful modification with lysine.
To confirm the accurate MW of the prepared sample, SEC was carried out. As the HPLC detector was UV/Vis, the PVCL-Lys sample was tagged with fluorescent dye (Rhod) to create PVCL-Rhod. The MW was determined to be 6035 D, which is close to 1HNMR measurement.
Phase transition behavior
The phase transition behaviors of synthesized materials were determined by calorimetric measurements, as they are dependent on temperature (Figure S2). The DSC curves show the phase transition changes of solid materials of PVCL-PEG and PVCL-Lys at temperatures of 32°C and 35°C, respectively. This provides evidence that the LCST changes at higher temperatures when PVCL-PEG is modified with lysine.
However, the phase transition behavior was monitored by particle size changes via recording DLS as a function of temperature (Figure S3). Within a temperature range of (30–45°C), the Z-average diameters of PVCL-PEG and PVCL-Lys in aqueous solution were monitored and within transition temperature, the sizes dropped dramatically. The decrease in sizes is observed for PVCl-PEG and PVCL-Lys at about 35°C and 38°C, respectively, which were in correlation with what was observed in test tubes through mild temperature changes that caused the medium to become turbid. There were about 3°C differences between the phase transition temperatures recorded by DSC and DLS. This observation could be rationalized by successful hydrogen bonding, which induces an increase in phase transition temperature in an aqueous solution. On the other hand, there was no change in LCST while DOX was conjugated to PVCL-Lys due to the low concentration of DOX.
Morphology observation
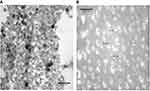
To observe size changes of the prepared materials in aqueous solution, TEM images were recorded below and above LCST temperatures of PVCL-Lys (Figure 3). The nanogel formation was vivid in TEM images below and above LCST. In the room temperature, particles are formed in sizes around 50 nm, and, with short sonication, nanoparticles become immiscible in aqueous solution (Figure 7A). At 42°C, nano-hydrogels lose their hydrophobic interaction with water molecules and form nanocapsules; this dehydration process is observable in TEM images. The size of nanocapsules’ length ranges between 45 and 72 nm, with a width around 18 nm (Figure 3B).
To have better observation of PVCL-Lys without aggregation which was apparent in Figure 3A, the PVCL-Lys sample was scanned by Cryo TEM (Figure 4). The separate nano-hydrogel particles showed to have sizes less than 20 nm.
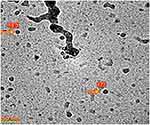 |
Figure 4 Cryo TEM image of PVCL-Lys nano-hydrogel. Abbreviations: TEM, transmission electro microscopy; PVCL, poly(N-vinylcaprolactam). |
DOX conjugation and release profile
Taking into account the aforementioned sections, the PVCL-Lys can serve as a platform for drug delivery system. The zeta-potential of this system is measured at 2.4 mV. The amine group of lysine as nucleophilic site could be applied for the conjugation of drugs with electrophilic groups. DOX was selected to evaluate the application of this platform in drug delivery. The conjugation of DOX was carried out through Schiff-base reaction (Figure 1). Schiff-base bond would provide pH sensitivity to this delivery system.38 After chemical reaction, the synthesized material (PVCL-DOX) was dialyzed to remove physically attached particles. The amount of DOX was determined by UV-Vis spectroscopy to be 5% in PVCL-DOX. The synthesized material (PVCL-DOX), which has characteristics similar to PVCL-Lys, acts as a thermo and pH-responsive platform for DOX delivery. The release profile was determined in the simulated medium of physiological pH and temperature (7.4°C and 37°C) and tumor site with pH and temperature changes (5°C and 40°C, respectively) (Figure 5). The lowest and moderate releases are observed at 37°C in pH of 7.4 within the time interval of 72 hrs of about 37% and changes occurred to about 50% while pH reduced to 5, which made Schiff-base bond susceptible to hydrolysis. The highest amounts of release (80%) is recorded at 40°C and pH of 5 within 72 hrs, which could be considered as proof of pH and thermoresponsive properties of PVCL-DOX (Figure 5).
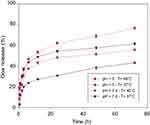 |
Figure 5 Release profile of PVCL-DOX in regard to temperature and pH changes. Abbreviations: PVCL, poly(N-vinylcaprolactam); DOX, doxorubicin. |
In vitro toxicity evaluation
In an efficient anticancer delivery system, the biocompatibility of the nano-carrier and the toxicity of drug-loaded nano-carrier are of great importance. To evaluate the biocompatibility of PVCL-Lys and the anticancer efficiency of PVCL-DOX, the MTT assay was performed on breast cancer cell line/MCF-7. The cultured medium of MCF-7 was treated with DOX, PVCL-Lys, and PVCL-DOX in various concentrations within two time intervals of 24 and 48 hrs (Figure 6). The results reveal that PVCL-DOX is an effective anticancer agent with a toxicity level comparable to free DOX (and even more so after 24 hrs); however, the anti-proliferation activity of PVCL-Lys demonstrates a minor toxicity. The results of PVCL-DOX inhibition could be more effective if the temperature as a factor of sensitivity was evaluated. The amount of IC50 is calculated for DOX and PVCL-DOX to be 13.5 and 6.72 µg/mL in 24 hrs, and 0.6 and 1.49 µg/mL in 48 hrs, respectively.
Uptake assay using fluorescence microscopy and flow cytometry
Identifying the cellular entrance pathway of drug-loaded nanoparticles is an important way to understand their efficiency in drug delivery. Size, shape, charge, functional groups, and the presence of targeting ligands on nanoparticles are some of the confirmed influential factors in the cellular entrance of nanoparticles. Dye-tagging technique was used to check the cellular accumulation of PVCL-DOX and PVCL-Lys. The fluorescence microscopy images were taken after 24 and 48 hrs treatment of MCF-7 cells with DOX, PVCL-DOX, and PVCL-Rhod (Figure 7).
Cellular penetration of DOX was observed after 24 hrs incubation of cells by its low (III) and high (I) concentrations (Figure 6). On the other hand, due to cellular death, no significant fluorescence is observed after 48 hrs incubation of cells with a high concentration of DOX (I) (Figure 7A and D). The results indicate the ability of the PVCL-DOX in cellular death induction, even after the treatment of cells by its low concentration (III).
In order to eliminate the effect of DOX in cellular death and examine whether the PVCL-Lys could penetrate into cells, images from PVCL-Rhod were taken in the same concentration as PVCL-DOX (Figure 7C and F). Based on the observations, after 24 hrs incubation of cells with PVCL-Rhod, nano-hydrogels are dispersed only in the cytoplasm of cells. However, after 48 hrs, the fluorescence dye is observed in both cytoplasm and nucleus of the cells (Figure 7F).
In further analysis, the presence of DOX, PVCL-DOX, and PVCL-Rhod in the MCF-7 cells was checked quantitatively by flow cytometry. MCF-7 cells were treated with DOX, PVCL-DOX, and PVCL-Rhod, and after 4, 24, and 48 hrs, the cells were analyzed. The obtained flow cytometry histograms are shown in Figure 7. The mean fluorescent intensity (MFI) of the MCF-7 cells in each experiment is shown in the histograms. The MFI results reveal the easy and high penetrations of DOX molecules into the cells (Figure 8A). A high fluorescence intensity is observed in the cells treated with DOX after 24 hrs incubation. However, the fluorescence intensity decreases after 48 hrs incubation due to the cellular death. Fluorescence intensity of the cells treated with a low concentration of PVCL-DOX (15 µg/mL) is not high after 4 hrs incubation, indicating the lower cellular penetration of these particles (Figure 8B).
However, promising results are observed after 24 and 48 hrs of incubation, where the PVCL-DOX nanoparticles are able to induce more cellular death (lower MFIs). PVCL-Rhod particles are able to penetrate into the cells roughly after 4 hrs. A concentration-dependent increase in MFI of the PVCL-Rhod treated cells is observed after 24 hrs incubation. However, after 48 hrs incubation, there is a decrease in the MFI, which could be a result of cellular death (Figure 8C).
Discussion
A novel type of lysine-modified nano-hydrogel based on PVCL with PEGDA cross-linker was synthesized and characterized. RAFT polymerization technique, as a powerful method in controlled living polymerization, was utilized. As an important feature of controlled radical polymerization technique is that the crosslinking could be controlled while monomer and cross-linker (divinyl compound) are copolymerized simultaneously.21 1HNMR and SEC results show the expected MWs are close to feed ratio amounts. Lysine could act as a modifying agent of thermo-sensitivity and offers several other advantages such as being non toxic agent, increasing positive charge, improving the interaction of the nano-carrier with cell membrane ligands and providing functional groups (i.e., NH2 and COOH) for the conjugation of therapeutic agents, all of which could make the structure appropriate for drug and gene delivery.39 DSC and SEC prove a thermo responsive feature of the synthesized particles and lysine modification, which could successfully tune the LCST in aqueous solution up to 38°C. As prepared, the material could disperse in aqueous solution and make stable and transparent medium. Upon encountering LCST, stable colloidal solution is formed. Based on DLS and TEM results, the PVCL-Lys form hydro-colloidal water miscible microparticles. Morphology and sizes of these particles can change in temperature higher than LCST (≥38°C), and nanoparticles with sizes less than 200 nm in DLS is observed (Figure 3 SI). TEM images also show nano-capsule formation with less than 100 nm length. The engineered nanoparticles possess the expected and essential criteria, which are necessary for a nano-carrier for therapeutic agent delivery.
In this work, delivery of DOX was evaluated. The presence of lysine more specifically, its amine groups in the polymeric matrix of nanoparticles provides special sites for DOX conjugation via pH-responsive Schiff-base linkages. The developed platform shows pH- and temperature-responsive features, which are suitable for delivery of drug molecules to tumor sites. According to the internalization route of nanoparticles, temperature would be the primary stimulus in cell entrance of nanoparticles. The systems would realize pH changes gradually from early endosomes processes.5 The results of release assessment show the highest release percent (~80%) at 40°C and pH of 5, where the release was directly in response to temperature and pH sensitivity. In vitro cellular evaluation in breast cancer cell line/MCF-7 exhibits a high toxicity by PVCL-DOX; comparable to free DOX and even more after 24 hrs. However, the temperature factor could not be involved in in vitro cellular analysis, and all tests were performed at 37°C. It is expected that the PVCL-DOX would be more efficient in vivo due to the higher temperature of the tumor sites (40°C). Cellular uptake assay via fluorescence microscopy demonstrates a higher toxicity induction by PVCL-DOX when compared to free DOX, even in 24 hrs. It is assumed that the crossing of PVCL-DOX through cell membrane bypasses the apically located efflux transporter, such as pg-protein and facilitated the cellular accumulation of DOX. After the evaluation of the PVCL-Rhod, it appears that nanoparticles could accumulate in cytoplasm in first 24 hrs and then distributed in nucleus, too. The flow cytometry results demonstrate a gradual cell penetration process of the nanoparticles, compared to the free DOX molecules (in 4 hrs). However, the induction of cellular death by PVCL-DOX increases during the time (in 24 and 48 hrs). This result could support to the time dependency of cellular entrance by PVCL-DOX. Based on this observation, compared to the mechanism of cellular entrance by free DOX molecules, it seems that the cellular entrance of PVCL-DOX nanoparticles is occurring via cellular membrane penetration. According to the suggested cellular route of internalization of nanoparticles which encompasses endocytosis, temperature would be the first stimulus that DDS encounters in tumor site.5
The PVCL-Lys has the potential to be considered as a potent carrier in therapeutic delivery. Gene delivery of the aforementioned particles is also under investigation and will be the topic of our next report. It is anticipated that PVCL-Lys being degraded in cellular medium due to PEGDA hydrolysis, and both cargo delivery and the clearance route of carrier will be facilitated. In this experiment, we introduced and evaluated the PVCL-Rhod as dye-tagged particle, which could be efficiently applied in the determination of some biological phenomenon pathways. In vivo investigation of the aforementioned platform will be considered in future studies.
Conclusion
Herein, a novel temperature and pH-responsive DOX delivery system based on hydrogel was developed. The primary structure was based on PVCL cross-linked with PEGDA modified with lysine, and in the final step, DOX was conjugated. Successful RAFT polymerization technique was performed for the skeletal synthesis of nano-hydrogel resulting in network formation with MW of about 6022 Da. The thermoresponsive properties were evaluated and lysine-modified structure (PVCL-Lys) proved to be tuned up to 38°C. TEM images show that particles that have swelled below LCST could form separated nanoparticles at a temperature higher than LCST. Conjugation of DOX to PVCL-Lys took place via pH-responsive Schiff-base linkage. The release profile showed that about 80% release could be obtained at 40°C and pH of 5, which is in accordance with simulated condition of cancer site. In vitro cellular studies on MCF-7 cell line showed a high potential of PVCL-DOX in killing cells. The uptake assay indicates that nanoparticles’ entrance route to cells might be through cellular membrane penetration. PVCL-DOX acts more efficiently than free DOX and has a potential to be considered as an anti cancer agent.
Acknowledgment
This project was supported by Shiraz University of Medical Sciences under grant NO. 94-01-36-9333.
Fatemeh Farjadian, as the principle investigator of this work, would like to dedicate it to prof. Bahman Tamami. He is known as a pioneer in the field of Polymer Chemistry in Iran (since 1974 till now, a faculty member at Shiraz University). He was the Ph.D supervisor of Fatemeh (2007-2012). His attitude toward education and research has inspired Fatemeh in her carrier.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nikalje AP. Nanotechnology and its applications in medicine. Med Chem. 2015;5(2):81–89. doi:10.4172/2161-0444
2. Hosseini M, Farjadian F, Makhlouf ASH. Smart stimuli-responsive nano-sized hosts for drug delivery. Industrial Applications for Intelligent Polymers and Coatings: Springer, Cham, Switzerland; 2016:1–26. doi:10.1007/978-3-319-26893-4_1.
3. Farjadian F, Ghasemi A, Gohari O, Roointan A, Karimi M, Hamblin MR. Nanopharmaceuticals and nanomedicines currently on the market: challenges and opportunities. Nanomedicine. 2019;14(1):93–126. doi:10.2217/nnm-2018-0120
4. Weissig V, Pettinger TK, Murdock N. Nanopharmaceuticals (part 1): products on the market. Int J Nanomedicine. 2014;9:4357. doi:10.2147/IJN
5. Farjadian F, Roointan A, Mohammadi-Samani S, Hosseini M. Mesoporous silica nanoparticles: synthesis, pharmaceutical applications, biodistribution, and biosafety assessment. Chem Eng J. 2019;359:684–705. doi:10.1016/j.cej.2018.11.156
6. Farjadian F, Moghoofei M, Mirkiani S, et al. Bacterial components as naturally inspired nano-carriers for drug/gene delivery and immunization: set the bugs to work? Biotechnol Adv. 2018;36(4):968–985. doi:10.1016/j.biotechadv.2018.02.016
7. Holowka EP, Bhatia SK. Smart Drug Delivery Systems. Drug Delivery: Springer, New York, NY; 2014;265–316. doi:10.1007/978-1-4939-1998-7_7
8. Karimi M, Eslami M, Sahandi-Zangabad P, et al. pH-Sensitive stimulus-responsive nanocarriers for targeted delivery of therapeutic agents. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2016;8(5):696–716.
9. Karimi M, Moosavi Basri S, Vossoughi M, Pakchin PS, Mirshekari HR, Hamblin M. Redox-sensitive smart nanosystems for drug and gene delivery. Curr Org Chem. 2016;20(28):2949–2959. doi:10.2174/1385272820666160510154557
10. Karimi M, Sahandi Zangabad P, Baghaee-Ravari S, Ghazadeh M, Mirshekari H, Hamblin MR. Smart nanostructures for cargo delivery: uncaging and activating by light. J Am Chem Soc. 2017;139(13):4584–4610. doi:10.1021/jacs.6b08313
11. Guo B, Zhao J, Wu C, et al. One-pot synthesis of polypyrrole nanoparticles with tunable photothermal conversion and drug loading capacity. Colloids Surf B Biointerfaces. 2019;177:346–355. doi:10.1016/j.colsurfb.2019.02.016
12. Karimi M, Sahandi Zangabad P, Ghasemi A, et al. Temperature-responsive smart nanocarriers for delivery of therapeutic agents: applications and recent advances. ACS Appl Mater Interfaces. 2016;8(33):21107–21133. doi:10.1021/acsami.6b00371
13. Pitt WG, Husseini GA, Staples BJ. Ultrasonic drug delivery–a general review. Expert Opin Drug Deliv. 2004;1(1):37–56. doi:10.1517/17425247.1.1.37
14. Hosseini M, Makhlouf ASH. Industrial Applications for Intelligent Polymers and Coatings. Springer, Cham, Switzerland; 2016.
15. Hoogenboom R. Chapter 2 - Temperature-Responsive Polymers: Properties, Synthesis, and Applications. In: Aguilar MR, San Román J, eds Smart Polymers and their Applications (Second Edition): Elsevier, Woodhead Publishing; 2019:13–44.
16. Zhang Q, Weber C, Schubert US, Hoogenboom R. Thermoresponsive polymers with lower critical solution temperature: from fundamental aspects and measuring techniques to recommended turbidimetry conditions. Mater Horiz. 2017;4(2):109–116. doi:10.1039/C7MH00016B
17. Meeussen F, Nies E, Berghmans H, Verbrugghe S, Goethals E, Du Prez F. Phase behaviour of poly (N-vinyl caprolactam) in water. Polymer. 2000;41(24):8597–8602. doi:10.1016/S0032-3861(00)00255-X
18. Repasky EA, Evans SS, Dewhirst MW. Temperature matters! And why it should matter to tumor immunologists. Cancer Immunol Res. 2013;1(4):210–216. doi:10.1158/2326-6066.CIR-13-0118
19. Ramos J, Imaz A, Forcada J. Temperature-sensitive nanogels: poly (N-vinylcaprolactam) versus poly (N-isopropylacrylamide). Polym Chem. 2012;3(4):852–856. doi:10.1039/C2PY00485B
20. Rao KM, Rao KSVK, Ha C-S. Stimuli responsive poly (vinyl caprolactam) gels for biomedical applications. Gels. 2016;2(1):6. doi:10.3390/gels2010006
21. Neamtu I, Rusu AG, Diaconu A, Nita LE, Chiriac AP. Basic concepts and recent advances in nanogels as carriers for medical applications. Drug Deliv. 2017;24(1):539–557. doi:10.1080/10717544.2016.1276232
22. Wu C, Zhao J, Hu F, et al. Design of injectable agar-based composite hydrogel for multi-mode tumor therapy. Carbohydr Polym. 2018;180:112–121. doi:10.1016/j.carbpol.2017.10.024
23. Boyer C, Bulmus V, Davis TP, Ladmiral V, Liu J, Perrier S. Bioapplications of RAFT polymerization. Chem Rev. 2009;109(11):5402–5436. doi:10.1021/cr9001403
24. Keddie DJ. A guide to the synthesis of block copolymers using reversible-addition fragmentation chain transfer (RAFT) polymerization. Chem Soc Rev. 2014;43(2):496–505. doi:10.1039/c3cs60290g
25. Semsarilar M, Perrier S. ‘Green’reversible addition-fragmentation chain-transfer (RAFT) polymerization. Nat Chem. 2010;2(10):811. doi:10.1038/nchem.853
26. Etchenausia L, Khoukh A, Lejeune ED, Save M. RAFT/MADIX emulsion copolymerization of vinyl acetate and N-vinylcaprolactam: towards waterborne physically crosslinked thermoresponsive particles. Polym Chem. 2017;8(14):2244–2256. doi:10.1039/C7PY00221A
27. Aguirre G, Ramos J, Heuts JP, Forcada J. Biocompatible and thermo-responsive nanocapsule synthesis through vesicle templating. Polym Chem. 2014;5(15):4569–4579. doi:10.1039/C4PY00297K
28. Jia F, Wang S, Zhang X, Xiao C, Tao Y, Wang X. Amino-functionalized poly (N-vinylcaprolactam) derived from lysine: a sustainable polymer with thermo and pH dual stimuli response. Polym Chem. 2016;7(46):7101–7107. doi:10.1039/C6PY01487A
29. Kozlovskaya V, Liu F, Xue B, et al. Polyphenolic polymersomes of temperature-sensitive poly (N-vinylcaprolactam)-block-Poly (N-vinylpyrrolidone) for anticancer therapy. Biomacromolecules. 2017;18(8):2552–2563. doi:10.1021/acs.biomac.7b00687
30. Yu YC, Kang HU, Youk JH. Synthesis and micellar characterization of thermosensitive amphiphilic poly(ε-caprolactone)-b-poly(N-vinylcaprolactam) block copolymers. Colloid Polym Sci. 2012;290(12):1107–1113. doi:10.1007/s00396-012-2630-1
31. Imaz A, Forcada J. N-vinylcaprolactam based microgels: effect of the concentration and type of cross linker. J Polym Sci A Polym Chem. 2008;46(8):2766–2775. doi:10.1002/(ISSN)1099-0518
32. Wang Y, Zheng J, Tian Y, Yang W. Acid degradable poly (vinylcaprolactam)-based nanogels with ketal linkages for drug delivery. J Mater Chem B. 2015;3(28):5824–5832. doi:10.1039/C5TB00703H
33. Roointan A, Farzanfar J, Mohammadi-Samani S, Behzad-Behbahani A, Farjadian F. Smart pH responsive drug delivery system based on poly(HEMA-co-DMAEMA) nanohydrogel. Int J Pharm. 2018;552(1):301–311. doi:10.1016/j.ijpharm.2018.10.001
34. Ghasemi S, Harandi ZA. Thermo-responsive poly (N-isopropylacrylamide)-block-poly (ionic liquid) of pyridinium sulfonate immobilized Pd nanoparticles in C–C coupling reactions. RSC Adv. 2018;8(26):14570–14578. doi:10.1039/C8RA01303A
35. Farjadian F, Ghasemi S, Mohammadi-Samani S. Hydroxyl-modified magnetite nanoparticles as novel carrier for delivery of methotrexate. Int J Pharm. 2016;504(1):110–116. doi:10.1016/j.ijpharm.2016.03.022
36. Nakabayashi K, Mori H. Recent progress in controlled radical polymerization of N-vinyl monomers. Eur Polym J. 2013;49(10):2808–2838. doi:10.1016/j.eurpolymj.2013.07.006
37. Shao L, Hu M, Chen L, Xu L, Bi Y. RAFT polymerization of N-vinylcaprolactam and effects of the end group on the thermal response of poly(N-vinylcaprolactam). React Funct Polym. 2012;72(6):407–413. doi:10.1016/j.reactfunctpolym.2012.04.002
38. Farjadian F, Schwark S, Ulbricht M. Novel functionalization of porous polypropylene microfiltration membranes: via grafted poly (aminoethyl methacrylate) anchored Schiff bases toward membrane adsorbers for metal ions. Polym Chem. 2015;6(9):1584–1593. doi:10.1039/C4PY01521E
39. Tao Y, Chen X, Jia F, et al. New chemosynthetic route to linear ε-poly-lysine. Chem Sci. 2015;6(11):6385–6391. doi:10.1039/c5sc02479j
Supplementary materials
 |
Figure S1 FT-IR spectra of PVCL-PEG and PVCL-Lys. Abbreviations: PVCL, poly(N-vinylcaprolactam); PEWG, poly(ethylene glycol). |
 |
Figure S2 DSC graph of PVCL-PEG and PVCL-Lys. Abbreviations: PVCL, poly(N-vinylcaprolactam); PEWG, poly(ethylene glycol); DSC, differential scanning calorimetry. |
 |
Figure S3 Dynamic light scattering (DLS) records according to Z-average (nm) changes in regard to temperature rise (1°C) and ranges between 30 and 45°C. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.