Back to Journals » Clinical Ophthalmology » Volume 14
Targeting Netrin-1 and -4 as a Novel Diagnostic Parameter and Treatment Option for Diabetic Retinopathy
Authors Zewdie KA , Ayza MA , Amare Tesfaye B , Yimer EM
Received 13 April 2020
Accepted for publication 16 June 2020
Published 25 June 2020 Volume 2020:14 Pages 1741—1747
DOI https://doi.org/10.2147/OPTH.S258044
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Kaleab Alemayehu Zewdie,1 Muluken Altaye Ayza,1 Bekalu Amare Tesfaye,1 Ebrahim M Yimer2
1Department of Pharmacology and Toxicology, School of Pharmacy, Mekelle University, Mekelle, Ethiopia; 2Department of Pharmacy, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
Correspondence: Kaleab Alemayehu Zewdie
Department of Pharmacology and Toxicology, School of Pharmacy, Mekelle University, PO Box 1871, Mekelle, Ethiopia
Tel +251921546562
Email [email protected]
Abstract: Diabetic retinopathy (DR) is a retinal vascular disorder associated with both type 1 and type 2 diabetes mellitus (DM). It is characterized by specific loss of pericytes, which leads to an augmented blood vessel permeability, and development of new blood vessels (retinal neovascularization). Moreover, stiffening of eye membrane, inflammation, and apoptosis of endothelial cells also lead to damage of the blood–retinal barrier and blindness in most cases unless it’s detected and managed early. Hence, this review was intended to assess the potential roles of Netrin-1 and -4 as new/alternative biomarkers and therapeutic options for DR. Netrin-1 and -4 have been the most known ligands and are well known for their role in neural guidance. DR has both neural and vascular components; therefore, biomarkers used for both neural and vascular retinal tissues are potentially important. According to different experimental and clinical studies, as compared to the normal groups, there was a significant increment in both retinal Netrin-1 and -4 mRNA and protein levels in the retinopathy groups. In addition, exogenous supplementation of these proteins is also used as a therapeutic agent for DR.
Keywords: diabetic retinopathy, Netrin-1, Netrin-4, biomarker, diabetes mellitus
Introduction
Diabetic retinopathy (DR) is a retinal vascular disease1 and the most prevalent microvascular complication of both type I and type II diabetes mellitus (DM).2 The prevalence is strongly correlated to the duration of DM and level of glycemic control.3 Globally, nearly 90 million people have DR, of which 21 million have diabetic macular edema (DME), 28 million have sight-threatening retinopathy, and 17 million have proliferative retinopathy.3,4 In the United States, retinopathy is the major cause of loss of sight in productive age groups.1
In patients with type II DM, retinopathy may not be detected for years until significant damage occurs. Therefore, patients are diagnosed with DR after it has caused serious damage (visual problems). Even though type I DM is mostly diagnosed in early age, it still needs early detection for prevention of related complications, including retinopathy and other complications.5
Different biological mechanisms have been associated with the pathology of hyperglycemia-induced retinopathy. Among them, polyol and hexosamine pathway activity, activation of protein kinase C isoforms, oxidative stress, and formation of advanced glycation end-product are mostly reported.3,6 DR is characterized by specific loss of pericytes, which leads to an augmented blood vessel permeability, and the development of new blood vessels (retinal neovascularization).7 In addition, stiffening of the membrane in the eye and apoptosis of endothelial cells are detected during the pathogenesis, which collectively contributes to the damage of the blood–retinal barrier. Moreover, marked loss of endothelial cells and pericytes leads to upregulation of vascular endothelial growth factor (VEGF) by activating hypoxia-inducible factor 1 (HIF-1). Likewise, phospholipase A2’s (PLA2) elevation due to hyperglycemia also prompts upregulation of VEGF.6 DME can occur at any stage of DR and cause distortion of visual images and a decrease in visual acuity.8 Individuals with DR has been a higher chance to develop other vascular complications, including diabetic nephropathy, diabetic neuropathy, and cardiovascular diseases.9
Different conventional and newer diagnostic tools including glycated hemoglobin (HbA1c), thioredoxin-interacting protein, fructosamine, and glycated albumin have been used for detection of retinopathy in diabetic patients. However, most of them are used after it causes significant damage to patients.9,10 In addition, they have limited specificity, sensitivity, and are imprecise in certain clinical conditions.11 Moreover, increased cost related to newer diagnostic tools were also a substantial challenge.12 Hence, the present review was intended to assess the potential role of Netrin-1 and -4 as a novel biomarker and therapeutic option for DR.
Netrin-1 and -4 and Their Receptors
Netrin is among axon guidance molecules with Semaphorins and Ephrins. It has been known to regulate axonal growth in the development of the nervous system, regulation of immune and inflammatory responses (inflammation of the nervous system).13,14 Netrin-1 and -4 are the most known ligands of the family and critical axonal guidance protein during morphogenesis, embryonic development, and angiogenic processes.15,16 Netrin-1 related axonal functions have been linked to two classes of receptors: the deleted in colorectal cancer (DCC) family, including DCC and its orthologue Neogenin-1, and the Unc5s family, including Unc5A17,18 while Netrin-4 performs its activity majorly through binding to the laminin γ1 chain and disrupts laminin networks and basement membranes15,16,19 (Figure 1).
 |
Figure 1 Netrin and its Receptors. |
The Role of Netrin in Retina Development
Netrin receptors have been expressed in developing retina and Netrin-neogenin signaling, which might stimulate retinal ganglion cell axon growth into and along the optic nerve.20,21 Netrin-1/UNC5B may activate retinal vessel development and they can stimulate pro-angiogenesis.22 The guidance of retinal ganglion cell axons through the optic disc is dependent on the DCC/Netrin-1 axonal guidance system.21 Furthermore, netrin-1 contributes to steering axons out of the retina.23 Under hypoxic conditions, the Netrin-1 level increases and it might be a key factor in inducing retinal neovascularization.25,26 Retinal neovascularization is the major pathological condition of several diseases including diabetic retinopathy, retinal vein occlusion, and age-related macular degeneration.8,24,27 Netrin-1 promotes retinal angiogenesis in oxygen-induced retinopathy.28,29
Netrin-4 is highly expressed in the retina and its role is as an angiogenesis modulating factor in oxygen-dependent vascular homeostasis.24 In an in vivo study, Netrin-4 is found to be a negative regulator of corneal epithelial cell proliferation and retinal vascular branching. It is also expressed in the retinal vascular basement membrane.16
Preclinical and Clinical Studies on the Level of Netrin in Diabetic Retinopathy
DR has both neural and vascular components; proteins used for both neural and vascular retinal tissues are a potentially important role. Through time the early diagnosis of retinopathy is increasing by different biomarkers related with the neural component.30,31 Among them, Netrin was studied by various experimental and clinical models.7,19,24,32,33 The level of Netrin protein in the plasma has been positively correlated with DR. As compared with the normal controls, the level of Netrin was markedly increased in the early phase of DR. As shown in Table 1, different experimental studies revealed that the level of Netrin in the cornea increased substantially at the early phase of DR (Table 1).
 |
Table 1 Role of Netrin-1 and -4 in Diabetic Retinopathy as a Biomarker and Treatment Modality for DR |
Association of Netrin-1 and -4 with Diabetic Retinopathy
DR is one of the major complications of DM and known to cause visual loss in many cases.5 It involved a neurovascular lesion that can damage blood vessels and nerves of the retina.2 Due to that protein mostly found in nerve endings were used as indicators for changes which occurred in the system.7 Among them, Netrin has been recently considered as a novel biomarker and therapeutic agent for DR. Netrin-1 and -4 has been well studied regarding their role in DR.39 Alteration of the body’s Netrin-1 and -4 level may be reflected as upcoming biological protein to identify retinopathy promptly and to decide its severity.
Angiogenesis secondary to DR causes pericytes cell injury, which is due to infiltration of neutrophils and monocytes like IFN-γ and IL-17.6 Generally, Netrin-1 regulates hypoxia-induced inflammation through COX-2/PGE2 and NFκB pathways during inflammation secondary to hypoxia. It has a protective role in DR by conversing new blood vessel formation and overwhelming inflammation in the cornea32,38 (Figure 2).
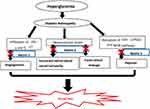 |
Figure 2 Relation of Netrin-1 and -4 with Diabetic Retinopathy. |
Different studies also proved that the level of Netrin in the cornea significantly increased at the early stage of DR. So considering this protein as a biomarker is imperative to detect the disease as timely before it progresses and causes severe damage.26,32,34 According to Miloudi et al, Netrin-1 level has been increased in the inflamed retina. In addition, degradation of Netrin-1 by collagenases and MMPs generated fragments, such as the VI–V peptide, that exacerbate retinal edema. Moreover, these data further suggest that cells of the ganglion layer undergo diabetes-induced retinopathy.32
Netrin-4 also has a role in vascular remodeling such as an increased retinal blood vessel tortuosity, persistence of the hyaloid artery in adult mice and spontaneous focal retinal leakage.36,40 Studies done on knockout mice (Ntn-4-/-) confirmed that lack of Netrin-4 has a major role in the pathophysiology of neovascularization due to ischemia but it does not relate to inflammatory neovascularization24 (Figure 2).
Unlike other biomarkers, various preclinical/clinical studies proved that administration of exogenous Netrin (Netrin-1and -4) has a therapeutic effect (Table 1).19,25,36,39 According to Crespo-Garcia et al, exogenous administration of Netrin-1 and -4 into the vitreous of diabetic rats reduced retinal vascular permeability. These effects may relate to the potential role of Netrin-1 in vascular repair, inhibition of angiogenesis, and preferentially preserves the remaining normal vasculature to supply ischemic tissue during retinopathy. In addition, Netrin-4 acts as an antiangiogenic factor through the regulation of both endothelial and perivascular cells in the retina.40 Netrin-4 decreased human umbilical vein endothelial cells (HUVECs) viability in a dose dependent manner by regulating HUVEC proliferation and apoptosis. It also prevented corneal neovascularization, enhanced the regression of corneal neovascularization, and inhibited apoptosis in alkali-burned rat corneas. Another study done by Prieto et al38 revealed that Netrin aroused endothelial cell migration in vitro at minimal concentrations while at higher concentrations it tended to promote endothelial cell migration to a smaller degree.
Conclusion and Future Perspectives
The review revealed that Netrin-1 and -4 are a potential to be an alternative biomarker for DR, and exogenous supplementation of these proteins can be used as a therapeutic agent. Change of the retinal/corneal Netrin-1 and -4 levels may be reflected as upcoming biological protein to identify DR promptly, which in turn becomes eye-opening for novel drug discovery for this upsetting disease condition. Further detailed animal and human studies are required to advance the role of Netrin as a novel biomarker for DR. Additionally, further investigations are needed to determine the mechanisms by which Netrin is used as a therapeutic agent for the treatment of DR.
Abbreviations
DCC, deleted in colorectal cancer receptors; DM, diabetes mellitus; DME, diabetic macular edema; DR, diabetic retinopathy; HIF-1, hypoxia-inducible factor 1; HUVECs, human umbilical vein endothelial cells; KO, knockout; OIR, oxygen-induced retinopathy; UNC5, uncoordinated 5 receptors; VEGF, vascular endothelial growth factor.
Author Contributions
All authors contributed to data analysis, drafting, or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there is no conflict of interest.
References
1. Vislisel J, Oetting T Diabetic retinopathy for medical students. EyeRounds.org - Ophthalmology - The University of Iowa; The University of Iowa. (2016). Available from: https://webeye.ophth.uiowa.edu/eyeforum/tutorials/Diabetic-Retinopathy-Med-Students/index.htm.
2. Solomon SD, Chew E, Duh EJ, et al. Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(3):412–418. doi:10.2337/dc16-2641
3. Heng LZ, Comyn O, Peto T, et al. Diabetic retinopathy: pathogenesis, clinical grading, management and future developments. Diabetic Med. 2013;30(6):640–650. doi:10.1111/dme.12089
4. Rosenblatt BJ, Benson WE, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366(13):1227–1239. doi:10.1056/NEJMra1005073
5. Song SJ, Wong TY. Current concepts in diabetic retinopathy. Diabetes Metab J. 2014;38(6):416–425. doi:10.4093/dmj.2014.38.6.416
6. Wang W, Lo ACY. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci. 2018;19:1816.
7. Yimer EM, Zewdie KA, Hishe HZ. Netrin as a novel biomarker and its therapeutic implications in diabetes mellitus and diabetes-associated complications. J Diabetes Res. 2018;2018:1–21. doi:10.1155/2018/8250521
8. Fong DS, Aiello L, Gardner TE, et al. Diabetic Retinopathy. Diabetes Care. 2003;26 Suppl 1:S99-S102.doi:10.2337/diacare.26.2007.s99
9. Jenkins AJ, Joglekar MV, Hardikar AA, et al. Biomarkers in diabetic retinopathy. Rev Diabet Stud. 2015;12(1–2):159–195. doi:10.1900/RDS.2015.12.159
10. Wondafrash DZ, Nire’a AT, Tafere GG, et al. Thioredoxin-interacting protein as a novel potential therapeutic target in diabetes mellitus and its underlying complications. Diabetes Metab Syndr Obes. 2020;13:43–51. doi:10.2147/DMSO.S232221
11. Dorcely B, Katz K, Jagannathan R, et al. Novel biomarkers for prediabetes, diabetes, and associated complications. Diabetes Metab Syndr Obes. 2017;10:345–361. doi:10.2147/DMSO.S100074
12. Laakso M. Biomarkers for type 2 diabetes. Mol Metab. 2019;27:S139–S146. doi:10.1016/j.molmet.2019.06.016
13. Lee WS, Lee W-H, Bae YC, Suk K. Axon guidance molecules guiding neuroinflammation. Exp Neurobiol. 2019;28(3):311. doi:10.5607/en.2019.28.3.311
14. Nakayama H, Kusumoto C, Nakahara M, Fujiwara A, Higashiyama S. Semaphorin 3F and Netrin-1: the novel function as a regulator of tumor microenvironment. Front Physiol. 2018;9:1–11. doi:10.3389/fphys.2018.01662
15. Reuten R, Patel TR, McDougall M, et al. Structural decoding of netrin-4 reveals a regulatory function towards mature basement membranes. Nat Commun. 2016;7(1):1–17. doi:10.1038/ncomms13515
16. Li YN, Pinzón-Duarte G, Dattilo M, et al. The expression and function of netrin-4 in murine ocular tissues. Exp Eye Res. 2012;96(1):24–35. doi:10.1016/j.exer.2012.01.007
17. Vosberg DE, Leyton M, Flores C. The Netrin-1/DCC guidance system: dopamine pathway maturation and psychiatric disorders emerging in adolescence. Mol Psychiatry. 2019. doi:10.1038/s41380-019-0561-7
18. Ranganathan P, Jayakumar C, Navankasattusas S, et al. UNC5B receptor deletion exacerbates tissue injury in response to AKI. J Am Soc Nephrol. 2014;25(2):239–249. doi:10.1681/ASN.2013040418
19. Han Y, Shao Y, Liu T, et al. Therapeutic effects of topical Netrin-4 inhibits corneal neovascularization in alkali-burn rats. PLoS One. 2015;10(4):e0122951. doi:10.1371/journal.pone.0122951
20. Livesey FJ, Hunt SP. Netrin and Netrin receptor expression in the embryonic mammalian nervous system suggests roles in retinal, striatal, nigral, and cerebellar development. Mol Cell Neurosci. 1997;8(6):417–429. doi:10.1006/mcne.1997.0598
21. Gad JM, Keeling SL, Shu T, Richards LJ, Cooper HM. The spatial and temporal expression patterns of Netrin receptors, DCC and Neogenin, in the developing mouse retina. Exp Eye Res. 2000;70(6):711–722. doi:10.1006/exer.2000.0823
22. Wu W, Tang L, Lei H. The role of netrin-1 in diabetic retinopathy: a promising therapeutic strategy. Discov Med. 2017;23.
23. Ming G, Poo M, Tessier-lavigne M, Hemmati-brivanlou A, Holt CE. Turning of retinal growth cones in a Netrin-1 gradient mediated by the Netrin receptor DCC. Neuron. 1997;19(6):1211–1224. doi:10.1016/S0896-6273(00)80413-4
24. Kociok N, Crespo-Garcia S, Liang Y, et al. Lack of Netrin-4 modulates pathologic neovascularization in the eye. Sci Rep. 2016;6(1):1–13. doi:10.1038/srep18828
25. Liu D, Xiong S-Q, Shang L, et al. Expression of netrin-1 receptors in retina of oxygen-induced retinopathy in mice. BMC Ophthalmol. 2014;14(1):1–10. doi:10.1186/1471-2415-14-102
26. Tian XF, Xia X-B, Xiong S-Q, et al. Netrin-1 overexpression in oxygen-induced retinopathy correlates with breakdown of the blood-retina barrier and retinal neovascularization. Ophthalmologica. 2011;226(2):37–44. doi:10.1159/000324474
27. Nguyen QD, De Falco S, Behar-Cohen F, et al. Placental growth factor and its potential role in diabetic retinopathy and other ocular neovascular diseases. Acta Ophthalmol. 2018;96(1):e1–e9. doi:10.1111/aos.13325
28. Zhao -R-R, Xu X-C, Xu F, et al. Metformin protects against seizures, learning and memory impairments and oxidative damage induced by pentylenetetrazole-induced kindling in mice. Biochem Biophys Res Commun. 2014;448(4):414–417. doi:10.1016/j.bbrc.2014.04.130
29. Ke T, Wu Y, Li L, et al. Netrin-1 ameliorates myocardial infarction-induced myocardial injury: mechanisms of action in rats and diabetic mice. Human Gene Ther. 2014;25(9):787–797. doi:10.1089/hum.2014.021
30. Ting DSW, Tan K-A, Phua V, et al. Biomarkers of diabetic retinopathy. Curr Diab Rep. 2016;16(12). doi:10.1007/s11892-016-0812-9.
31. Sen S, Chakraborty R, De B. Diabetes mellitus: general consideration. In: Diabetes Mellitus in 21st Century. Springer Science+Business Media Singapore, editors. Singapore: Springer;2016:13–22. doi:10.1007/978-981-10-1542-7_2
32. Miloudi K, Binet F, Wilson A, et al. Truncated Netrin-1 contributes to pathological vascular permeability in diabetic retinopathy. J Clin Invest. 2016;126(8):3006–3022. doi:10.1172/JCI84767
33. Liu C, Ke X, Wang Y, et al. The level of Netrin-1 is decreased in newly diagnosed type 2 diabetes mellitus patients. BMC Endocr Disord. 2016;16(1):1–5. doi:10.1186/s12902-016-0112-z
34. Liu J, Xia X, Xiong S, Le Y, Xu H. Intravitreous high expression level of Netrin-1 in patients with proliferative diabetic retinopathy. Eye Sci. 2011;26(2):85–90,120. doi:10.3969/j.issn.1000-4432.2011.02.017
35. Zhang X, Liu J, Xiong S, Xia X, Xu H. Expression of Netrin-1 in diabetic rat retina. Eye Sci. 2013;28(3):148–152.
36. Lejmi E, Bouras I, Camelo S, et al. Netrin-4 promotes mural cell adhesion and recruitment to endothelial cells. Vasc Cell. 2014;6(1):1–13. doi:10.1186/2045-824X-6-1
37. Yu Y, Zou J, Han Y, et al. Effects of intravitreal injection of Netrin-1 in retinal neovascularization of streptozotocin-induced diabetic rats. Drug Des Dev Ther. 2015;9:6363–6377. doi:10.2147/DDDT.S93166
38. Prieto CP, Ortiz MC, Villanueva A, et al. Netrin-1 acts as a non-canonical angiogenic factor produced by human Wharton’s jelly mesenchymal stem cells (WJ-MSC). Stem Cell Res Ther. 2017;8(1):1–15. doi:10.1186/s13287-017-0494-5
39. Cao B, Meng X, Fu Y, et al. Neuron-derived netrin-1 and netrin-4 proteins are additional effective targets in diabetic retinopathy beyond VEGF. Int J Clin Exp Pathol. 2017;10(8):8174–8186.
40. Crespo-Garcia S, Reichhart N, Wigdahl J, et al. Lack of netrin-4 alters vascular remodeling in the retina. Graefes Arch Clin Exp Ophthalmol. 2019;257(10):2179–2184. doi:10.1007/s00417-019-04447-3
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
