Back to Journals » Patient Preference and Adherence » Volume 11
Swallowing difficulties with medication intake assessed with a novel self-report questionnaire in patients with systemic sclerosis – a cross-sectional population study
Authors Messerli M , Aschwanden R, Buslau M, Hersberger KE , Arnet I
Received 27 May 2017
Accepted for publication 15 August 2017
Published 28 September 2017 Volume 2017:11 Pages 1687—1699
DOI https://doi.org/10.2147/PPA.S142653
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Markus Messerli,1,2 Rebecca Aschwanden,1 Michael Buslau,2 Kurt E Hersberger,1 Isabelle Arnet1
1Pharmaceutical Care Research Group, Department of Pharmaceutical Sciences, University of Basel, Basel, Switzerland; 2European Centre for the Rehabilitation of Scleroderma, Reha Rheinfelden, Rheinfelden, Switzerland
Objectives: To assess subjective swallowing difficulties (SD) with medication intake and their practical consequences in patients suffering from systemic sclerosis (SSc) with a novel self-report questionnaire.
Design and setting: Based on a systematic literature review, we developed a self-report questionnaire and got it approved by an expert panel. Subsequently, we sent the questionnaire by post mail to SSc patients of the European Center for the Rehabilitation of Scleroderma Rheinfelden, Switzerland.
Participants: Patients were eligible if they were diagnosed with SSc, treated at the center, and were of age ≥18 years at the study start.
Main outcome measures: Prevalence and pattern of SD with oral medication intake, including localization and intensity of complaints.
Results: The questionnaire consisted of 30 items divided into five sections Complaints, Intensity, Localization, Coping strategies, and Adherence. Of the 64 SSc patients eligible in 2014, 43 (67%) returned the questionnaire. Twenty patients reported SD with medication intake (prevalence 47%), either currently (11; 26%) or in the past that had been overcome (9; 21%). Self-reported SD were localized mostly in the larynx (43%) and esophagus (34%). They were of moderate (45%) or strong to unbearable intensity (25%). Modification of the dosage form was reported in 40% of cases with SD. Adherence was poor for 20 (47%) patients and was not associated with SD (p=0.148).
Conclusion: Our novel self-report questionnaire is able to assess the pattern of complaints linked to medication intake, that is, localization and intensity. It may serve as a guide for health care professionals in selecting the most suitable therapy option, enabling tailored counseling to reduce inappropriate medication modifications.
Keywords: swallowing difficulties, medication intake, systemic sclerosis, coping behavior, self-report questionnaire, deglutition disorders
Introduction
Swallowing difficulties cause problems with the intake of solid oral dosage forms, an issue that has been reported in 9% of polypharmacy patients attending community pharmacies and 27% of a general practice population.1,2 Such problems may affect the patient’s quality of life, lead to hazardous coping strategies (splitting or crushing pills), and reduce adherence to medication regimens.1
Several questionnaires assessing dysphagia (ie, swallowing problems), in general, are available in the literature,3 but very few detect swallowing difficulties with medicine intake. Moreover, most questionnaires aim at evaluating swallowing in its detailed physiologic function4 or tend to be tantamount to diagnostic tools.5 Questionnaires that consider medication swallowing were primarily developed for research purposes and are too comprehensive to be used in practice by health care professionals.6 Further, reports mention poor linkage between patients’ complaints and diagnostic findings.7,8 We hypothesize that the “one single question fits all” approach (eg, “Do you suffer from swallowing difficulties when taking your medication?”) represents a first step for a loose detection of individual issues with medication intake, but needs further in-depth assessment.
Systemic sclerosis (SSc) is a rare multisystem autoimmune disease with a prevalence of 1–10 cases per 100,000 individuals in Europe.9 Vascular remodeling, inflammatory reaction, and abnormal fibroblast activation lead to impaired circulation and fibrosis in skin and multiple inner organs. SSc is a chronic, often progressive disease with high morbidity and mortality. Organ failure can also include the gastrointestinal (GI) tract.10 Progressive worsening of the disease often leads to swallowing problems with food and liquids11 and, therefore, probably medicines. A common comorbidity of patients suffering from SSc is the autoimmune Sicca or Sjögren’s syndrome, which may also affect the swallowing process.12,13 Since SSc cannot be cured yet, treatment of organ manifestations remains the main therapeutic strategy usually involving oral medications.14 Patient education, psychologic support, and highly specialized physical therapy are essential to the management of SSc. The European Centre for the Rehabilitation of SSc in Rheinfelden, Switzerland, serves the trinational region’s 1 million residents and offers specialized care for patients suffering from SSc.
This study aimed at developing a patient self-report questionnaire that assesses subjective swallowing difficulties with medication intake, which can be used to guide a health care professional when choosing therapy options or optimizing a patient’s medicines. The purpose of this questionnaire was not a diagnostic, but a screening approach. Pilot testing was performed in patients suffering from SSc, a very specific population at risk for swallowing disorders.
Strengths of this study
- Based on a systematic literature search, a patient self-report questionnaire assessing swallowing difficulties with medication intake was developed.
- Face validity of the initial questionnaire involved professional experts as well as patients.
- The use of a visual analog scale (VAS) to indicate the intensity and a human profile to indicate the localization of complaints ensured that answers were provided independently of language and health literacy.
- First validation steps of the questionnaire was performed in patients with SSc, a highly specific population prone to develop swallowing difficulties.
Limitations of this study
- As SSc is a rare disease, the investigated population provided a limited number of patients.
- Construct validity (defined as placing the measure of a construct in a nomological network and establishing its relation to other variables) and criterion validity (defined as the association with other measures of the same variable) were not performed.
Methods
Systematic literature search and article eligibility
The databases PubMed, CINAHL and Embase were searched on 29th March 2014 with the terms “deglutition disorders [MeSH]” OR “swallowing difficult*” AND “drug dosage form*” AND “interview*” OR “questionnaire*”, with publication date being before February 2014 and without language restriction. Findings were reported according to the PRISMA statement (Preferred Reporting Items for Systematic reviews and Meta-Analyses).15,16 The identified abstracts were screened for eligibility according to the following inclusion criteria: 1) human population, 2) swallowing difficulties with medication intake assessed in a systematic and structured form as an outcome measure (eg, interview guide), and 3) full-text publication in English or German language. The full texts were then screened again for eligibility by two independent researchers. Discordance was resolved by consensus.
Development and validation of the questionnaire
Items from the questionnaires retrieved from the literature search were summarized, translated in German language, rephrased, and compiled into a patient self-report questionnaire. We termed the questionnaire SWAMECO for SWAllowing difficulties with MEdication intake and COping strategies. Face validation was performed with a panel of 11 experts (4 patients, 4 pharmacists, 2 speech-language pathologists, and 1 professor in pharmaceutical care). Positive statements on sections, content relevance, intelligibility, comprehensibility, impact on patient privacy, and length of a first draft were graded from 1 (totally disagree) to 4 (totally agree). The higher the value, the more positive was the judgment.
Content validation was performed with nine SSc patients (mean age 52 years; four Germans, five Swiss; six women) attending an information seminar in Rheinfelden on 29 March 2014. Completeness, comprehensibility, appropriateness and ambiguity of question wording, interpretation of the questions, ability to provide accurate answer, and length were tested with structured questions using a 4-point Likert-scale (1= fully disagree, 2= tend to disagree, 3= tend to agree, 4= fully agree). Reliability was tested with six patients by a retest procedure 2 weeks later through post mail and measured using Cohen’s Kappa.17 A value >0.80 indicates substantial test–retest reliability. Construct and criterion validation were not performed because SWAMECO does not deliver a score or a threshold that could be compared to existing questionnaires.
Study design, sample, and recruitment
The cross-sectional population study took place at the European Centre for the Rehabilitation of Scleroderma, Rheinfelden, Switzerland. All patients fulfilling the new classification criteria for SSc,18 currently being treated at the center, and of age ≥18 years were eligible. Pathophysiologic swallowing problems were not an inclusion criterion because dysphagia is not routinely diagnosed in the SSc patients attending the center (eg, by radiographic assessment or taking a medication with a standardized bolus of water).
Eligible patients were invited by letter in March 2014 to participate in the study. They received a written overview of the study, including purpose, an informed consent form (including consent to publish data), a SWAMECO self-report questionnaire, and a demographics sheet (including confounding factors that may influence swallowing difficulties, such as tobacco and alcohol consumption, unexplained weight loss [as sign of GI manifestation in SSc], and diagnosed pneumonia in the past 6 months). The participants were asked to complete and return the informed consent form, the questionnaire, and the demographics form within 4 weeks.
Reporting standards and data analysis
The authors followed the STROBE reporting standards for observational studies. Patient characteristics and answers of face validation are presented as percentages or means with standard deviation. Chi-square test was used to compare group variables. p-values <0.05 were considered significant. Statistical analysis was conducted using SPSS Version 22 (IBM Corporation, Armonk, NY, USA).
Ethical approval and trial registration
The study was approved by the local ethics committee Northwest/Central Switzerland (EKNZ 2014-013) and registered in the international clinical trial registry platform www.ClinicalTrials.gov (Identifier: NCT02105818, first entry March 28, 2014).
Results
Systematic literature search
A total of 47 articles were identified (Figure 1). After screening of titles and abstracts, 41 articles were excluded from further analysis. The remaining six articles reported results from observational studies with low level of evidence according to GRADE19 (Supplementary material, Tables S1 and S2). Four articles contained specific questionnaires.1,2,6,20 None of them was designed as a self-report form.
  | Figure 1 Flow chart of the systematic literature search. |
Development and validation of the questionnaire
The two categories “Complaints” and “Coping strategies” were retrieved from the literature search and expanded with two new sections “Localization” and “Intensity”. The initial version of the questionnaire contained 32 items fitting on four pages as a DIN A4 double-sided, color-printed brochure.
Face validity was given with a mean overall agreement of 3.7 (Table 1). The experts agreed with all items (no deletion), proposed 27 changes in the wording or the layout, 2 changes in the scales (adding the category “no answer” for 2 items), and suggested the separation of one item in two single items, the addition of one free-text item, and the inclusion of “choking” as a single item.
All changes were implemented. The final questionnaire contained 30 items (Table 2) and was redesigned as a DIN A3 landscape format and folded, to be provided as a double-sided, color-printed brochure.
  | Table 2 Sections, number of items, and type of response scales of the SWAMECO questionnaire |
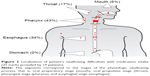
Item 1 asked for current oral medication intake (yes/no). The presence of swallowing difficulties with intake of liquid (item 2), food (item 3), or medication (item 4) was evaluated on a 3-point Likert scale (1= current, 2= past, 3= never suffered from swallowing difficulties). Complaints (items 5–14) were rated on a 4-point Likert scale (1= totally agree, 4= totally disagree) and contained four items related to the Sicca syndrome (item 6: “I have a dry mouth during daytime”).21,22 Intensity of the complaints (item 15) was rated on a VAS using pictorial representations of facial expressions (0/laughing face = no complaints, 10/weeping face = unbearable complaints). A drawing of the upper human body from head to stomach (item 16) was divided into four segments according to the physiologic swallowing process,23 that is, oral preparatory stage (mouth), oral propulsive stage (throat), pharyngeal stage (pharynx), and esophageal stage (esophagus). Patients placed a cross to mark the localization of their complaints at the corresponding site. Item 17 assessed medicines (product name, dosage, and intake interval). Position of the head while swallowing medication (item 18) was asked with three predefined answers (chin toward chest, head straight ahead, head straight back). As the chin-tuck technique, that is, to put chin toward the chest, changes pharyngeal dimensions through postural maneuver, it is recommended by speech specialists to move the bolus anterior in patients with dysphagia.24 Thus, we considered this technique as appropriate for patients reporting swallowing difficulties. Coping strategies were reported by answering open questions with free-text options or predefined answers (items 19, 20) and closed questions with dichotomous options (items 21–27). Three single items (items 28–30) to assess patients’ adherence were selected from existing cognitive services25 (“Do you sometimes forget to take your medicines” [yes/no]) and literature26 (“People sometimes miss taking their medications for reasons other than forgetting. Thinking over the past 2 weeks, were there any days when you did not take your medicine?” [yes/no] and “Have you ever cut back or stopped taking your medication without telling your doctor, because you felt worse when you took it?” [yes/no]). Patients were assessed as nonadherent when answering items 28–30 once with “yes”.
Content validation was given with a median score of 4 (range 3–4) over all criteria. The questionnaire was judged as understandable, helpful, and clear. Patients were able to fill in the questionnaire within 15 min, which was estimated as acceptable by all nine participants. Test–retest reliability showed an acceptable kappa κ=0.81.
Cross-sectional population study
Of the 64 eligible patients, 43 (67%) returned the questionnaire, 35 (81%) of them within 3 weeks. Mean age was 54.6 years (standard deviation 12.23); the majority of them were female (n=36, 84%) and Swiss (n=32), ten were Germans, and one was an Austrian.
Of the 43 returned questionnaires, a total of 46 empty fields (3.3% missing data) were irregularly disseminated over 15 questionnaires (65% fully completed questionnaires). Seventeen empty fields concerned a block of responses (“Taking oral medication triggers 1) a choking, 2) a cough, 3) nausea, 4) tightness while swallowing.”). In ten cases, questions with free-text options were left unanswered, that is, 1) “Describe how you feel the discomfort of swallowing medication(s)” and 2) “Which of your medication(s) cause swallowing difficulties?”.
Swallowing difficulties were reported by 20 patients (47%), as a current problem by 11 patients (26%), and as past difficulties that had been overcome by 9 patients (21%). Two patients left the question on swallowing difficulties with medication intake unanswered (missing data), but answered the question on swallowing difficulties with food or liquids in the negative. Thus, they were assigned to the group without complaints with medication intake for further analysis. Presence of possible confounding factors (tobacco and alcohol consumption, unplanned weight loss) was not correlated to swallowing difficulties with medication intake (data not shown).
Appropriate swallowing technique, that is, the chin-tuck technique, was mentioned in four (9%) cases. Patients with current complaints tilted their head backward as often as patients with past or no difficulties (5/11, 45% vs 11/29, 38%; three missing; p=0.467). All 43 patients support their medication intake with a sip of water, and 11 patients reported regularly choking on their medication (26%).
Nonadherence (answering items 28–30 once with “yes”) was present in 47% of all patients and did not correlate with swallowing difficulties (12/19, 63% vs 8/20, 40%; four missing values; p=0.148).
Pattern of difficulties with swallowing medication
Of 20 patients with current or past self-reported swallowing difficulties with medication intake, 19 (95%) marked their complaints on the human profile (Figure 2) with a total of 35 locations and a median number of marks per patient of 2 (range 1–4). Most marks were placed at the pharynx (n=15; 43%) and esophagus (n=12; 34%). Five marks were placed outside the GI tract.
The 20 patients indicated the intensity of complaints with a median of 4.4 (range 0.8–9.4). After repartition in tertiles, the intensity was low for six (30%) patients, moderate for nine (45%) patients, and strong for five (25%) patients. All patients but one (19 patients; 95%) reported pills or capsules stuck in the throat and could mostly name them (Figure 3). In 9 of 23 (39%) medicines involved, available drug form alternatives could have been recommended by a health care professional (Supplementary material, Table S3) according to the summaries of product characteristics currently in use in Switzerland.27
The most frequent complaints related to Sicca syndrome were ocular and nasal dryness (80%), dry mouth during daytime (80%), the need to drink water for better speech (70%), and burning sensations (35%). Four patients (20%) were afraid of taking their medication because of the complaints. Ten patients (50%) had been worried about their swallowing difficulties during the past 4 weeks (Figure 3).
Coping strategies were reported by 10 patients, who modified the dosage form (n=8; 40%) or stopped medication (n=2; 10%). Modification resulted in splitting tablets (n=8; 100%), opening capsules (n=4; 50%), dissolving medication in liquids (n=2; 25%), or crushing pills (n=1; 13%). Only one patient consulted a health care professional before applying the coping strategy.
Discussion
We retrieved from the literature questions assessing swallowing difficulties with medication, amended them, and developed a patient self-report questionnaire that screens for swallowing difficulties with medication intake. Face and content validity confirmed the completeness, clarity, and appropriateness of the questionnaire. The use of the pictorial VAS to indicate intensity and of a human profile to indicate localization ensures that answers are provided independently of language or health literacy. Pilot testing was performed in patients suffering from SSc, a specific population at high risk for swallowing disorders. We added specific items covering xerostomia and ocular or nasal dryness because these symptoms are often developed by SSc patients.28 The observed high response to these complaints (80%) in our study confirmed the influence of these specific symptoms on the swallowing process and the appropriateness of the SWAMECO questionnaire to reveal them. Generalization to other patients will be investigated in a further study.
We selected a self-report structure because patients with swallowing difficulties with medication feel a subjective complaint, which may be difficult, time-consuming, and frustrating to depict in words. In contrast to others,1,2 the SWAMECO self-report questionnaire was able to detect a heterogeneous pattern of complaints. On one hand, the human profile allows the patient to indicate precisely the subjective place of the complaints. On the other hand, a number from 0 to 10 from a psychometric response scale is able to quantify the intensity of complaints. Our questionnaire cannot be used for diagnostic purpose. Previous studies observed that the place of the complaints indicated by the patients was poorly correlated with objective findings, and concluded that the ability of patients to self-localize dysphagia symptoms is weak,7 especially in those with esophageal problems.8 Other reports similarly indicate that the intensity of symptoms is not reliable for predicting the location of the responsible lesion.29 Inversely, many functional abnormalities that are unrelated to the patients’ symptoms can be found with radiographic evaluation or video fluoroscopy.8 In summary, symptom referral varies between patients and can hardly be used as a diagnostic tool. Nevertheless, regardless of their correlation to diagnostic findings, subjective complaints during medication intake should be taken into account by health care professionals when choosing a pharmacotherapy. Thus, by using patient’s self-competencies in reporting, the SWAMECO questionnaire provides a snapshot of a patient’s experience with medication intake and their swallowing difficulties. In analogy to pain scales, intensity remains an important marker of patient’s burden with medication intake and enables tailored interventions to overcome hazardous coping strategies. The obtained answers can represent a starting point for deeper medical clarification and initiation of individual counseling, and conceivable communication difficulties become circumvented. Moreover, it may avoid time pressure when filled in advance of a consultation.
Prevalence of swallowing difficulties in patients with SSc
An unprecedented comprehensive insight into the medicine use in everyday life of SSc patients was achieved. To date, existing population-specific tools have primarily focused on the reporting of a broad spectrum of GI disorders,30,31 while issues in the deglutition of medicines hereby were described for the first time by using the SWAMECO questionnaire. In total, difficulties with swallowing medication concerned as much as 47% of the surveyed patients at some point in time. The self-reported prevalence rate of current swallowing difficulties in this population was high (26%) and in the upper range of studies performed in a more general population,1,2 while the rate of past difficulties (21%) was indicative of sustained complaints. This may be explained by the progressive nature of SSc disease that results in continuous suffering. It remains unclear whether the pattern of swallowing difficulties with medication intake in a more general population would be similar. These results highlight the need for a greater awareness of health care professionals on swallowing difficulties in this population.
Coping strategies to overcome swallowing difficulties with medication
The coping strategies used by patients in our study, that is, opening capsules or crushing pills without informing the health care providers, are of great concern. Recent studies revealed that patients are often not aware of the safety issues when they modify medication dosage forms.32 In our study, patients were asked to report their coping strategies in a free-text format. The health care provider might use this individual information for further clarification or counseling, for example, by performing an in-depth medicine use review focusing on the coping strategies in daily use, and empower the patient with recommendations for safe and appropriate medication use. However, pharmacists and physicians rarely question patients about swallowing difficulties, and very few professionals systematically ask patients about this specific drug-related problem.1 Since health care professionals claim lack of time and personal resources, new screening tools such as the SWAMECO may reduce the workload and involve patients at an early stage.
Even if all patients reported taking water to ease the swallowing process, the amount of liquid remained unclear and might be critical. Schiele et al observed that 41% of all patients in their study took their medicines with less than half a glass of water.2 Similarly, the swallowing technique of the medication-water bolus showed potential for improvement regarding the low proportion of patients (9%) with head tilted forward, the strategy regarded as the best practice.33 The use of the SWAMECO questionnaire may uncover some individual practices that might jeopardize successful swallowing.
In our study, the majority of medications reported for causing swallowing difficulties were essential therapeutic medications for the treatment of SSc (calcium channel blocker/PDE5 [phosphodiesterase type 5] receptor inhibitor) or for the prevention and treatment of gastroesophageal reflux disease (proton pump inhibitor/H2 receptor antagonists). For many of the involved products, available drug form alternatives could have been recommended. Continuous and appropriate use of the medicines is mandatory to slow down the progression of the disease. Consequently, any factor that may influence their efficacy needs the attention of the involved health care providers.
Adherence to medication
Nonadherence was self-reported by almost half of our patients (47%). Compared to other diseases with similar characteristics such as noticeable symptoms, chronicity, and evolution with degradation, our result is much lower than the 91% of outpatients with rheumatoid arthritis,34 or the 91% of elderly patients with asthma who indicated nonadherence.35 We expected a higher proportion of nonadherent patients when reporting swallowing difficulties. As the participating patients were rather young, with full cognitive capabilities and high motivation to take their medicines as prescribed, we can hypothesize that the observed overall higher adherence to medication results from intense care and self-empowerment provided by the specialized center.
Further development of the questionnaire
When patients were asked to localize their complaints (Figure 2), four dots were placed in an indicating triangle instead of the GI tract. It remains unclear if the corresponding patients were confused by the triangles representing a segment, or the difficulty really occurred at this place. Further development should also evaluate indicating signs. Also, next validation steps should focus on clinical examination and confirmation of swallowing difficulties with video fluoroscopy. Finally, further studies should investigate a larger cohort in a more general population and evaluate the clinical implication of the questionnaire in daily practice, that is, patient counseling.
Strengths and limitations
Our study has several strengths. First, face validity of the initial questionnaire involved both professional experts and patients, who commented predominantly the wording of individual items. They made a significant contribution to the comprehensiveness of the questions, and thus, to the acceptance of the questionnaire and the feasibility of the study. This may explain the high response rate of 67% without the use of any reminders. Second, the patient-oriented language may explain that the majority of missing values concerned personal items. We presume that patients did not wish to answer the questions, rather than failing to answer because of understanding difficulties. Third, we investigated heterogeneous symptoms in a highly homogenous population in regards to the underlying disease. Consequently, our questionnaire may be seen as able to catch all symptoms of swallowing disorders.
We acknowledge some limitations. First, our results are patient-reported outcomes, and thus, subjective information. We did not confirm the findings with clinical diagnosis of the swallowing process or of GI disorders. Consequently, a correlation between the reported swallowing difficulties and a clinical implication is not possible. The SWAMECO questionnaire remains inconclusive on the cause of the symptoms, but offers initial opportunity for further and targeted investigations. Second, the European Centre for the Rehabilitation of Scleroderma Rheinfelden is a leading center in the German-speaking region of Europe and takes care of a considerable number of SSc patients. However, since SSc is a rare disease, the investigated population provided a limited number of patients. Third, the investigated population was recruited in a highly specialized center where patients are under regular and specific surveillance. Therefore, some answers might have been influenced by this unique situation, such as the questions regarding communication with health care professionals. Fourth, nonadherence was assessed using a nonvalidated approach. To assess this issue from a more comprehensive perspective, the use of validated outcome measures independently from the self-report should be considered. Also, a general quality of life instrument that is, SF-36 (36-Item Short Form survey) or EQ-5D (European Quality of Life-5 Dimensions questionnaire) could be used to describe health-related quality of life in patients with SSc.36
Conclusion
Through self-report questionnaires, patients can efficiently provide individual information that can be used for relevant counseling and tailored interventions. We developed a first self-report questionnaire assessing swallowing difficulties with medication intake that entirely relies on patients’ impressions and not on detailed physiologic functions. Pilot testing of the SWAMECO questionnaire in patients with SSc, a highly specific population prone to develop swallowing difficulties, showed feasibility and acceptance of patients. Prevalence of swallowing difficulties with medication intake was remarkably high in the investigated population. Reported localization and intensity of complaints as well as potentially hazardous coping strategies indicated the need for in-depth counseling by health care professionals. Further validation of the SWAMECO self-report questionnaire should be continued in the general population, including evaluation of its complementary value in patient care.
Data sharing statement
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
Transparency statement
The corresponding author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported, no important aspects of the study have been omitted, and any discrepancies from the study as planned have been explained.
Acknowledgments
We thank all participating patients from the European Centre for the Rehabilitation of Scleroderma, Rheinfelden, Switzerland. Furthermore, we are grateful to Christian Rutschmann (Business Images AG, Switzerland) for his support as a graphic designer.
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf. The authors report no conflicts of interest in this work and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years, and no other relationships or activities that could appear to have influenced the submitted work.
References
Marquis J, Schneider MP, Payot V, et al. Swallowing difficulties with oral drugs among polypharmacy patients attending community pharmacies. Int J Clin Pharm. 2013;35(6):1130–1136. | ||
Schiele JT, Quinzler R, Klimm HD, Pruszydlo MG, Haefeli WE. Difficulties swallowing solid oral dosage forms in a general practice population: prevalence, causes, and relationship to dosage forms. Eur J Clin Pharmacol. 2013;69(4):937–948. | ||
Speyer R, Cordier R, Kertscher B, Heijnen BJ. Psychometric properties of questionnaires on functional health status in oropharyngeal dysphagia: a systematic literature review. Bio Med Res Int. 2014;2014:458678. | ||
Dwivedi RC, St Rose S, Roe JW, et al. Validation of the Sydney Swallow Questionnaire (SSQ) in a cohort of head and neck cancer patients. Oral Oncol. 2010;46(4):e10–e14. | ||
Antonios N, Carnaby-Mann G, Crary M, et al. Analysis of a physician tool for evaluating dysphagia on an inpatient stroke unit: the modified Mann Assessment of Swallowing Ability. J Stroke Cerebrovasc Dis. 2010;19(1):49–57. | ||
Kelly J, D’Cruz G, Wright D. Patients with dysphagia: experiences of taking medication. J Adv Nurs. 2010;66(1):82–91. | ||
Roeder BE, Murray JA, Dierkhising RA. Patient localization of esophageal dysphagia. Dig Dis Sci. 2004;49(4):697–701. | ||
Smith DF, Ott DJ, Gelfand DW, Chen MY. Lower esophageal mucosal ring: correlation of referred symptoms with radiographic findings using a marshmallow bolus. AJR Am J Roentgenol. 1998;171(5):1361–1365. | ||
Ranque B, Mouthon L. Geoepidemiology of systemic sclerosis. Autoimmun Rev. 2010;9(5):A311–A318. | ||
Coral-Alvarado P, Pardo AL, Castano-Rodriguez N, Rojas-Villarraga A, Anaya JM. Systemic sclerosis: a world wide global analysis. Clin Rheumatol. 2009;28(7):757–765. | ||
Vischio J, Saeed F, Karimeddini M, et al. Progression of esophageal dysmotility in systemic sclerosis. J Rheumatol. 2012;39(5):986–991. | ||
Ebert EC. Esophageal disease in scleroderma. J Clin Gastroenterol. 2006;40(9):769–775. | ||
Pierce J, Tanner K, Merrill R, Miller K, Kendall K, Roy N. Swallowing disorders in Sjögren’s syndrome: prevalence, risk factors, and effects on quality of life. Dysphagia. 2016;31(1):49–59. | ||
Pope J, Harding S, Khimdas S, Bonner A, Baron M. Agreement with guidelines from a large database for management of systemic sclerosis: results from the Canadian Scleroderma Research Group. J Rheumatol. 2012;39(3):524–531. | ||
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. | ||
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. | ||
Altman DG. Practical Statistics for Medical Research. London: Chapman and Hall; 1991. | ||
van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. 2013;72(11):1747–1755. | ||
Schunemann HJ, Oxman AD, Brozek J, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336(7653):1106–1110. | ||
Wright D. Medication administration in nursing homes. Nurs Stand. 2002;16(42):33–38. | ||
Fox RI, Saito I. Criteria for diagnosis of Sjogren’s syndrome. Rheum Dis Clin North Am. 1994;20(2):391–407. | ||
Daniels TE, Criswell LA, Shiboski C, et al. An early view of the international Sjogren’s syndrome registry. Arthritis Rheum. 2009;61(5):711–714. | ||
Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: normal and abnormal. Phys Med Rehabil Clin N Am. 2008;19(4):691–707, vii. | ||
Saconato M, Chiari BM, Lederman HM, Goncalves MI. Effectiveness of Chin-tuck Maneuver to Facilitate Swallowing in Neurologic Dysphagia. Int Arch Otorhinolaryngol. 2016;20(1):13–17. | ||
Messerli M, Blozik E, Vriends N, Hersberger KE. Impact of a community pharmacist-led medication review on medicines use in patients on polypharmacy – a prospective randomised controlled trial. BMC Health Serv Res. 2016;16(1):145. | ||
Arnet I, Metaxas C, Walter PN, Morisky DE, Hersberger KE. The 8-item Morisky Medication Adherence Scale translated in German and validated against objective and subjective polypharmacy adherence measures in cardiovascular patients. Journal of Evaluation in Clinical Practice. 2015;21(2):271–277. | ||
Swiss agency for therapeutic products (Swissmedic). Product information; 2015. Available from: http://swissmedicinfo.ch/Accept.aspx?Lang=EN. Accessed December 12, 2015. | ||
Vitali C, Borghi E, Napoletano A, et al. Oropharyngolaryngeal disorders in scleroderma: development and validation of the SLS scale. Dysphagia. 2010;25(2):127–138. | ||
Edwards DAW. Discriminatory value of symptoms in the differential diagnosis of dysphagia. Clin Gastroenterol. 1976;5(1):49–57. | ||
Spiegel BMR, Hays RD, Bolus R, et al. Development of the NIH patient-reported outcomes measurement information system (PROMIS) gastrointestinal symptom scales. Am J Gastroenterol. 2014;109(11):1804–1814. | ||
Baron M, Hudson M, Steele R, Lo E. Validation of the UCLA scleroderma clinica trial gastrointestinal tract Instrument version 2.0 for systemic sclerosis. J Rheumatol. 2011;38(9):1925–1930. | ||
Lau ETL, Steadman KJ, Mak M, Cichero JAY, Nissen LM. Prevalence of swallowing difficulties and medication modification in customers of community pharmacists. J Pharm Pract Res. 2015;45(1):18–23. | ||
Schiele JT, Schneider H, Quinzler R, Reich G, Haefeli WE. Two techniques to make swallowing pills easier. Ann Fam Med. 2014;12(6):550–552. | ||
Gadallah MA, Boulos DN, Gebrel A, Dewedar S, Morisky DE. Assessment of rheumatoid arthritis patients’ adherence to treatment. Am J Med Sci. 2015;349(2):151–156. | ||
Bozek A, Jarzab J. Adherence to asthma therapy in elderly patients. J Asthma. 2010;47(2):162–165. | ||
Gualtierotti R, Ingegnoli F, Scalone L, et al. Feasibility, acceptability and construct validity of EQ-5D in systemic sclerosis. Swiss Med Wkly. 2017;146:w14394. |
Supplementary materials
References
Aitichou M, Skalli S, Faudel A, et al. Crushing pills, an easy practice of an old problem? Evaluation of crushing practices in a geriatric long term care unit. International Journal of Clinical Pharmacy. 2012;34(1):244–245. | ||
Andersen O, Zweidorff OK, Hjelde T, Rødland EA. Problems when swallowing tablets. A questionnaire study from general practice. Tidsskrift for den Norske Laegeforening. 1995;115(8):947–949. | ||
Baker R, Tsou VM, Tung J, et al. Clinical results from a randomized, double-blind, dose-ranging study of pantoprazole in children aged 1 through 5 years with symptomatic histologic or erosive esophagitis. Clinical Pediatrics. 2010;49(9):852–865. | ||
Dabade TS, Kane SV, Howden CW, et al. T1066 Proton Pump Inhibitor Compliance Does Not Impact GERD Symptom Resolution. Gastroenterology. 136(5):A-492. | ||
Fallon A, Sloane P, Coffey M, Craig A. An analysis of the impact of xerostomia on the quality of life of head and neck cancer patients receiving radiotherapy. Radiotherapy and Oncology. 2011;99:S319. | ||
Focken P, Cohen L, Edmundowicz SA, et al. Prospective randomized controlled trial of an injectable esophageal prosthesis versus a sham procedure for endoscopic treatment of gastroesophageal reflux disease. Surgical Endoscopy and Other Interventional Techniques. 2010;24(6):1387–1397. | ||
Gawron AJ, Martinovich Z, Thompson M, et al. Esophageal hypervigilance: A construct for reflux and dysphagia symptoms based on patient reported outcomes. Gastroenterology. 2013;144(5):S267. | ||
Go J, Fields J, Rao SS, Schulze KS. Problems with swallowing pills commonly relates to properties like size. Gastroenterology. 2013;144(5):S502. | ||
Gonçalves MI, Radzinsky TC, Da Silva NS, Chiari BM, Consonni D. Speech-language and hearing complaints of children and adolescents with brain tumors. Pediatric Blood and Cancer. 2008;50(3):706–708. | ||
Hanawa T, Tokutake N, Oguchi T. Questionnaire survey of air extruded jelly dosage form (I) -Oral condition of elder patients and applicability of air extruded jelly formulation. Yakugaku Zasshi. 2012;132(12):1461–1466. | ||
Hanssens Y, Woods D, Alsulaiti A, Adheir F, Al-Meer N, Obaidan N. Improving oral medicine administration in patients with swallowing problems and feeding tubes. Annals of Pharmacotherapy. 2006;40(12):2142–2147. | ||
Iwase S, Yamaguchi T, Miyaji T, Terawaki K, Inui A, Uezono Y. The clinical use of Kampo medicines (traditional Japanese herbal treatments) for controlling cancer patients’ symptoms in Japan: A national cross-sectional survey. BMC Complementary and Alternative Medicine. 2012;12. | ||
Kalf H, Bloem B. Swallowing disorders in parkinson’s disease: As frequent and severe as you think? Dysphagia. 2013;28(2):314. | ||
Kalf J, Bloem B, De Swart B. Difficulty with pill swallowing in PD. Dysphagia. 2011;26(4):469. | ||
Kalf JG, Munneke M, van den Engel-Hoek L, et al. Pathophysiology of diurnal drooling in Parkinson’s disease. Movement Disorders. 2011;26(9):1670–1676. | ||
Lazebnik LB, Masharova AA, Vasnev OS, Bordin DS, Valitova ER, Ianova OB. [Gastroesophageal reflux disease in the elderly patients: epidemiology, clinical features, therapy]. Ėksperimental’naia i klinicheskaia gastroėnterologiia = Experimental & clinical gastroenterology. 2010;(12):10–16. | ||
Lucia M, Takase E, Crespo A. Analysis of pharyngeal phase of swallowing hard gelatin pills in asymptomatic adults. Dysphagia. 2010;25(4):394. | ||
Martínez De Haro LF, Munitiz V, Ortiz Á, De Angulo DR, Navarro MD, Parrilla P. Outpatient monitoring of oesophageal pH with a catheter-free pH-meter (Bravo® system). A study of tolerance, safety and efficacy. Cirugia Espanola. 2008;84(4):201–209. | ||
Márton I, Jenei A, Ungvári T, Sándor J, Kiss C. Evaluation of oral mucositis in children receiving intensive chemotherapy using proms questionnaire. Pediatric Blood and Cancer. 2011;57(5):834. | ||
Mayadev AS, Weiss MD, Jane Distad B, Krivickas LS, Carter GT. The Amyotrophic Lateral Sclerosis Center: A Model of Multidisciplinary Management. Physical Medicine and Rehabilitation Clinics of North America. 2008;19(3):619–631. | ||
McNally D, Shephard A, Field E. Randomised, double-blind, placebo-controlled study of a single dose of an amylmetacresol/2,4-dichlorobenzyl alcohol plus lidocaine lozenge or a hexylresorcinol lozenge for the treatment of acute sore throat due to upper respiratory tract infection. Journal of Pharmacy and Pharmaceutical Sciences. 2012;15(2):281–294. | ||
Moretó M, Ojembarrena E, Barturen A, Casado I. Treatment of achalasia by injection of sclerosant substances: A long-term report. Digestive Diseases and Sciences. 2013;58(3):788–796. | ||
Nishimura N, Nakano K, Ueda K, et al. Prospective evaluation of incidence and severity of oral mucositis induced by conventional chemotherapy in solid tumors and malignant lymphomas. Supportive Care in Cancer. 2012;20(9):2053–2059. | ||
Nito T, Imai N, Yamauchi A, Ueha R, Yamasoba T. Surgical management of intractable aspiration. Dysphagia. 2013;28(2):302. | ||
Obasan AA, Showande SJ, Fakeye TO. Assessment of compliance to treatment among ambulatory asthmatic patients in a secondary health care facility in Nigeria. International Journal of Pharmaceutical Sciences and Research. 2012;3(2):483–489. | ||
Ogata I, Yamasaki K, Tsuruda A, et al. Some problems for dosage form based on questionnaire surveying about compliance in patients taken tamsulosin hydrochloride. Yakugaku Zasshi. 2008;128(2):291–297. | ||
Payot V, Cordonier AC, Marquis J, et al. Prevalence of patients’ difficulties in swallowing solid oral dosage forms. International Journal of Clinical Pharmacy. 2011;33(2):402. | ||
Peterson KA, Thomas KL, Hilden K, Emerson LL, Wills JC, Fang JC. Comparison of esomeprazole to aerosolized, swallowed fluticasone for eosinophilic esophagitis. Digestive Diseases and Sciences. 2010;55(5):1313–1319. | ||
Sakellariou V, Walshe M. Medication swallowing difficulties reported by adults with idiopathic parkinson’s disease and oropharyngeal dysphagia. Dysphagia. 2013;28(2):314–315. | ||
Sasaki CT, Leder SB. Comments on selected recent dysphagia literature. Dysphagia. 2013;28(2):271–277. | ||
Seo HG, Oh BM, Han TR. Longitudinal changes of the swallowing process in subacute stroke patients with aspiration. Dysphagia. 2011;26(1):41–48. | ||
Simons AJ. Munich dysphagia test-parkinson’s disease (MDT-PD): A new clinical questionnaire for early assessment of dysphagia in Parkinson’s disease. Dysphagia. 2013;28(2):288. | ||
Thinrungroj N, Pisespongsa P, Kijdamrongthum P, et al. Alginate accelerates healing of post-endoscopic variceal ligation ulcers: A randomized-controlled trial. Gastrointestinal Endoscopy. 2012;75(4):AB457–AB458. | ||
Truter I. An approach to dyspepsia, forthe pharmacist. SA Pharmaceutical Journal. 2012;79(8):9–16. | ||
Valenza V, Castaldi P, Silvestri G, et al. Role of oro-pharyngo-oesophageal scintigrapgy in the evaluation of swallowing disorders in patients with myotonic dystrophy type 1 (DM1). Medizinische Genetik. 2009;21(3):449. | ||
Verin E, Maltete D, Ouahchi Y, et al. Submental sensitive transcutaneous electrical stimulation (SSTES) at home in neurogenic oropharyngeal dysphagia: A pilot study. Annals of Physical and Rehabilitation Medicine. 2011;54(6):366–375. | ||
Zibetti M, Merola A, Artusi CA, et al. Levodopa/carbidopa intestinal gel infusion in advanced Parkinson’s disease: a 7-year experience. European Journal of Neurology. 2014;21(2):312–318. | ||
Kelly J, D’Cruz G, Wright D. Patients with dysphagia: experiences of taking medication. Journal of Advanced Nursing. 2010;66(1):82–91. | ||
Márquez-Contreras E, Gil V, Lopez J, et al. Pharmacological compliance and acceptability of lansoprazole orally disintegrating tablets in primary care. Current Medical Research and Opinion. 2008;24(2):569–576. | ||
Marquis J, Schneider MP, Payot V, et al. Swallowing difficulties with oral drugs among polypharmacy patients attending community pharmacies. International Journal of Clinical Pharmacy. 2013;35(6):1130–1136. | ||
Mehuys E, Dupond L, Petrovic M, et al. Medication management among home-dwelling older patients with chronic diseases: possible roles for community pharmacists. The Journal of Nutrition, Health & Aging. 2012;16(8):721–726. | ||
Schiele JT, Quinzler R, Klimm HD, Pruszydlo MG, Haefeli WE. Difficulties swallowing solid oral dosage forms in a general practice population: prevalence, causes, and relationship to dosage forms. European Journal of Clinical Pharmacology. 2013;69(4):937–948. | ||
Wright D. Medication administration in nursing homes. Nurs Stand. 2002;16(42):33–38. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.