Back to Journals » Clinical Ophthalmology » Volume 14
Successful Treatment of Peripheral Proliferative Vitreoretinopathy with Cryocoagulation During Retinal Detachment Repair – A New Surgical Technique
Authors Guber J, Lang C, Scholl HPN, Guber I, Valmaggia C
Received 14 February 2020
Accepted for publication 28 April 2020
Published 22 May 2020 Volume 2020:14 Pages 1413—1416
DOI https://doi.org/10.2147/OPTH.S249881
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Josef Guber,1,2 Corina Lang,1 Hendrik PN Scholl,2– 4 Ivo Guber,5 Christophe Valmaggia1
1Eye Clinic, Cantonal Hospital Sankt Gallen, Sankt Gallen, Switzerland; 2Department of Ophthalmology, University Hospital Basel, Basel, Switzerland; 3Institute of Molecular and Clinical Ophthalmology Basel (IOB), Basel, Switzerland; 4Wilmer Eye Institute, Johns Hopkins University, Baltimore, MD, USA; 5Department of Ophthalmology, University Hospital Geneva, Geneva, Switzerland
Correspondence: Josef Guber
Consultant Ophthalmic Surgeon Email [email protected]
Purpose: To evaluate the effect of extrascleral cryocoagulation for the treatment of proliferative vitreoretinopathy (PVR) during retinal detachment repair.
Methods: Patients with a rhegmatogenous retinal detachment associated with peripheral PVR Grade C star-folds were included in this study and analysed retrospectively. In all patients, PVR star-folds were treated by extrascleral cryocoagulation.
Results: A total of six patients with a rhegmatogenous retinal detachment associated with at least one peripheral PVR star-fold were included in this study. Reattachment of the retina was successfully achieved in all patients.
Conclusion: This novel and simple technique for the treatment of localized PVR using extrascleral cryocoagulation appears to be a safe and effective approach with favourable surgical success rates.
Keywords: vitrectomy, pars plana vitrectomy, retinal detachment, retinal detachment repair, proliferative vitreoretinopathy, cryotherapy
Introduction
Proliferative vitreoretinopathy (PVR) is a severe complication of rhegmatogenous retinal detachment (RRD), and is the most common reason for failure after surgical treatment.1–3 Several studies have confirmed the hypothesis that PVR occurs as a reparative process induced by retinal breaks and excessive inflammatory reaction.4 Clinically, early stages of PVR are characterized by an increased reflectance and a cellophane appearance of the inner retinal surface. Additionally, tortuosity of both small and larger vessels is regularly observed. The pathological hallmarks of advanced PVR include pre-retinal membrane formation, causing surface wrinkling and single or multifocal star-folds. In the final stages, multidirectional tractional forces produced by posterior and/or anterior PVR form a narrow or closed funnel of the detached retina.5
Recent published series suggest that the frequency of PVR remains largely unchanged in primary rhegmatogenous retinal detachment, with the incidence ranging from 5.1 to 11.7%.1–3 In particular, intraretinal PVR can be a surgical challenge as is it not possible to peel due to the lack of a preretinal membrane. In addition, these PVR folds are often located peripherally or mid-peripherally and are therefore not really suitable for retinectomy. In this case a major retinal resection, including healthy parts of the retina, has to be performed. On the other hand, if left untreated, this area could become the source of re-detachment.
In this retrospective study, we report the outcome of PVR star-folds treated with extrascleral cryocoagulation in patients who underwent pars plana vitrectomy (PPV) for RRD.
Patients and Methods
The study was approved by the local ethic committee EKOS (BASEC Nr. 2018–00104). Research adhered to the tenets of the Declaration of Helsinki. For each patient participating in the study informed consent was obtained.
A total of six consecutive eyes/patients with a RRD were included in this study and analysed retrospectively. The age ranged between 50 and 80 years with average age of 67.8 years. In five patients vitrectomy had to be performed because of primary RRD, whereas one patient had a re-detachment after previous vitrectomy. All patients presented with a detached macula and at least one star-fold (PVR Grade C) in the periphery. Patients’ characteristics are summarized in Table 1. Patients were reviewed in our outpatient’s clinic for a mean follow-up time of 12 months (range 6–17 months).
 |
Table 1 Patients’ Characteristics |
Reattachment of the retina was successfully achieved in all patients.
Surgical Technique
All surgical procedures were performed under general anaesthesia with the Stellaris System (Bausch and Lomb, New York, USA) using three 23 gauge-valved ports. A standard core and peripheral vitrectomy was performed in all patients. Retina reattachment was achieved either by direct fluid-air exchange with drainage of subretinal fluid through the main break or by using perfluorocarbon liquid followed by fluid-air exchange. Retinopexy was performed either with transconjunctival cryocoagulation or by endolaser. At the end of surgery, 12% octafluoropropane (C3F8 gas), silicon oil or high-density silicone oil (Densiron) was applied as intraocular tamponade according to the surgeon’s choice. In general, silicone oil and Densiron were removed three months postoperatively.
PVR peeling was attempted to prove that no further epiretinal PVR-membrane is present before cryotherapy. In two patients some pre-retinal PVR could be removed. Extrascleral cryotherapy (Erbokryo AE, Erbe, Germany; Temperature at −76C) was applied on the area of intraretinal PVR until a clear white spot was visible (Figure 1). Application of cryotherapy can either be performed under heavy liquid or air.
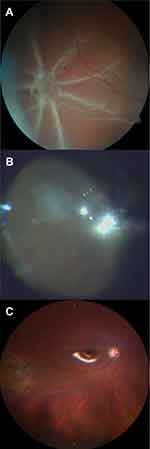 |
Figure 1 Pre- (A), intra- (B) and postoperative (C) fundus photography of PVR star-fold treated with cryocoagulation. |
Discussion
Surgical success rates for PVR have improved as vitrectomy techniques and instruments evolved. The introduction of ancillary techniques such as longer-acting gases and long-term vitreous substitutes like silicone oil have elevated the success rate from 35% to 40% to approximately 60–75% at 6 months postoperatively.1 Despite these advances, more than one-fourth of initially successful cases result in re-detachment due to recurrent retinal traction. One of the major causes for re-detachment is still PVR.2 Recently, efforts have been directed toward the chemical inhibition of cellular proliferation and membrane contractions in PVR.6 However, none of these agents has had a sufficient impact on the prevention of PVR and consequently have not been adopted by vitreoretinal surgeons. In conclusion, PVR still remains a surgical challenge.
Herein, we present a novel technique for the treatment of localized PVR using extrascleral cryocoagulation. In particular, intraretinal PVR is difficult to manage as no preretinal membrane exists and therefore peeling of the PVR is not possible.
Pathophysiologically, we assume that treating the intraretinal PVR with cryocoagulation results in destruction of all potential PVR–forming cells producing chorioretinal atrophy. This prevents further proliferation of PVR and reduces the risk of re-detachment caused by traction. However, one concern must be addressed, although we did not experience any re-detachment caused by PVR and despite localized atrophy, in the long term there may be significant subretinal cell migration which can subsequently produce PVR and re-detach the retina again.
However, this technique is not intended for diffuse or extensive PVR where a proper retinectomy is mandatory to achieve retinal reattachment. Furthermore, PVR peeling should be attempted to prove that no epiretinal PVR-membrane is present.
In conclusion, this technique appears to be a safe and effective approach for localized intraretinal PVR with favourable long-term surgical success rates.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Compliance with Ethical Standards
The study was approved by the local ethic committee and was performed as a part of departmental quality control (EKOS (Ethikkommission Ostschweiz), BASEC (Business Administration System for Ethics Committees) number 2018-00104). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all individual participants included in the study.
Acknowledgments
We thank Dr. sc. nat. Sabine Güsewell for her assistance in statistical analysis.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Dr. Hendrik PN Scholl is supported by the Swiss National Science Foundation, National Center of Competence in Research Molecular Systems Engineering “Molecular Systems Engineering”, the Wellcome Trust, and the Foundation Fighting Blindness Clinical Research Institute. Dr. Scholl is member of the Scientific Advisory Board of: Astellas Institute for Regenerative Medicine; Gensight Biologics; Ionis Pharmaceuticals, Inc.; Gyroscope Therapeutics Ltd.; Janssen Research & Development, LLC (Johnson & Johnson); Pharma Research & Early Development (pRED) of F. Hoffmann-La Roche Ltd; Novartis Pharma AG (CORE); and Retinagenix LLC. Dr. Scholl is paid consultant of: Boehringer Ingelheim Pharma GmbH & Co; Gerson Lehrman Group; and Guidepoint. Dr. Scholl is member of the Data Monitoring and Safety Board/Committee of ReNeuron Group Plc/Ora Inc. and member of the Steering Committee of Novo Nordisk (FOCUS trial). Dr. Scholl is co-director of the Institute of Molecular and Clinical Ophthalmology Basel (IOB) which is constituted as a non-profit foundation and receives funding from the University of Basel, the University Hospital Basel, Novartis, and the government of Basel-Stadt. These arrangements have been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. Johns Hopkins University and Bayer Pharma AG have an active research collaboration and option agreement. These arrangements have also been reviewed and approved by the University of Basel (Universitätsspital Basel, USB) in accordance with its conflict of interest policies. Dr. Hendrik Scholl is principal investigator of grants at the USB sponsored by the following entity: IVERIC bio (Ophthotech Corporation); Kinarus AG; and Novartis Pharma AG. Grants at USB are negotiated and administered by the institution (USB) which receives them on its proper accounts. Individual investigators who participate in the sponsored project(s) are not directly compensated by the sponsor but may receive salary or other support from the institution to support their effort on the project(s). The authors received no other financial supports for the research, authorship, and/or publication of this article.
References
1. Pastor JC. Proliferative vitreoretinopathy: an overview. Surv Ophthalmol. 1998;43:3–18. doi:10.1016/S0039-6257(98)00023-X
2. Bentivoglio M, Valmaggia C, Scholl HPN, Guber J. Comparative study of endolaser versus cryocoagulation in vitrectomy for rhegmatogenous retinal detachment. BMC Ophthalmol. 2019;19(1):96. doi:10.1186/s12886-019-1099-9
3. Guber J, Bentivoglio M, Sturm V, Scholl HPN, Valmaggia C. Combined pars plana vitrectomy with phacoemulsification for rhegmatogenous retinal detachment repair. Clin Ophthalmol. 2019;13:1587–1591. doi:10.2147/OPTH.S215352
4. Garweg JG, Tappeiner C, Halberstadt M. Pathophysiology of proliferative vitreoretinopathy in retinal detachment. Surv Ophthalmol. 2013;58(4):321–329. doi:10.1016/j.survophthal.2012.12.004
5. Thompson JT. Proliferative vitreoretinopathy. In: Ryan SJ, editor. Retina. St. Louis, MO: Mosby; 2006:2283–2309.
6. Sadaka A, Giuliari GP. Proliferative vitreoretinopathy: current and emerging treatments. Clin Ophthalmol. 2012;6:1325–1333. doi:10.2147/OPTH.S27896
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
