Back to Journals » Clinical Interventions in Aging » Volume 15
Spaced Retrieval and Episodic Memory Training in Alzheimer’s Disease
Authors Small JA , Cochrane D
Received 12 December 2019
Accepted for publication 8 March 2020
Published 17 April 2020 Volume 2020:15 Pages 519—536
DOI https://doi.org/10.2147/CIA.S242113
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Jeff A Small, Diana Cochrane
School of Audiology and Speech Sciences, University of British Columbia, Vancouver, BC, Canada
Correspondence: Jeff A Small Email [email protected]
Introduction: This study replicated and extended the findings from the author’s previous pilot study to further explore how a spaced retrieval (SR) memory training program might be effectively applied to help persons with Alzheimer’s disease (AD) improve both short- and long-term recall of recent episodic events.
Methods: A quasi-experimental within-subject group study was conducted with 15 participants with a diagnosis of AD.
Results: Compared to a control condition, all participants were able to spontaneously recall significantly more specific details about trained events, and their recall was significantly enhanced when they were provided with cues. Although the findings indicated that people with AD were able to encode information during training, recall gains diminished by the end of the maintenance period.
Discussion: This study provides evidence that individuals with mild to moderate AD can learn and recall new episodic information through SR training. These findings support the use of SR as an intervention tool to help individuals maintain their functioning in episodic recent memory. However, more research into maintaining the long-term recall of recent episodic events is warranted.
Keywords: spaced retrieval, Alzheimer’s disease, memory, training, rehabilitation, episodic
Alzheimer’s disease (AD) is the leading cause of dementia in older adults, accounting for 60% to 80% of all cases.1 AD is a neurodegenerative disease that results in a slow and progressive decline in cognitive functioning affecting learning, thinking and memory. People with AD typically experience impairments in memory, executive functioning, language, visuospatial functioning, attention and affect. Of these disturbances, one of the most noticeable and earliest symptoms in AD is episodic recent memory impairment.2,3,4
The episodic memory system is responsible for the conscious retrieval of autobiographical events, remembering specific details of times, places, associated emotions, and the more contextual knowledge that makes experience unique to the individual.5 Episodic memory allows the individual the opportunity to “relive” previous meaningful experiences, whether they be from the recent or remote past. As people spend much of their time reminiscing about the past and sharing stories of recent personal experiences with others, the episodic memory system plays a critical role in communication and connection with both the self and others.
Impairment to episodic recent memory disrupts daily functioning of people with AD, causing them to forget or misplace things, experience difficulty recalling the details of conversations and recent events, and lose track of time or place.1 When combined with semantic memory deficits (word-finding problems), these challenges lead to patterns of communication where the person with AD repeats questions and comments or stops speaking mid conversation without being able to start up again. The person with AD is often left feeling confused, disoriented, fearful or anxious when pushed beyond their comfort zones, which may lead to low self-confidence or feelings of shame and embarrassment, and withdrawal from social activities that they previously enjoyed. As episodic memory loss progresses, family and friends also experience feelings of loss, grief and sadness as they lose the ability to connect and communicate with their loved one in the ways that they used to.
Developing and testing interventions aimed at enhancing memory functioning in people with AD continues to be a priority for researchers and health-care providers. Much of the previous research has focused on providing education and training to support caregivers or to facilitate the use of external memory aids to compensate for memory losses.6,7,8,9,10 Contrary to the common stereotype that memory loss is a symptom of AD that cannot be improved, recent research provides evidence that people with cognitive impairments are able to successfully learn new information and revitalize lost memory capacity through cognitive rehabilitation programs (e.g.,11,12,13,14,15,16). The premise for engaging persons with dementia in cognitive rehabilitation is that although they have difficulty encoding information into memory, if given support during encoding and retrieval, they can eventually establish long-term memories. For example, Clare et al11 trained a person with AD on face-name associations that were relevant to the individual’s daily life. They reported that his recall of the trained names increased from 20% to 100% after 9 months, and remained relatively stable for another 2 years. Other researchers have extended cognitive rehabilitation to the area of episodic recent memory in AD17,18 For example, Silva et al17 employed lifelogging and Sensecam technology as a tool to assist persons with dementia to recall recent episodic information from their daily lives. Their findings indicate that reviewing photographic images of daily events can lead to improved recall of these events. Although their and other Sensecam research has documented positive gains in recall when using Sensecam compared to low-tech memory aids [for a review see15], this research has not typically included in their training a systematic rehearsal over time of the recorded episodic information by the participants (with the exception of the Memo+ cognitive comparison condition used by Silva and colleagues). Thus, it is not known whether the gains observed could have been enhanced further through the use of a spaced retrieval training procedure. Moreover, it is unclear whether the benefits of using a stream of still-shot images in providing encoding support would be as effective as those from reviewing real-time video recordings of an event, which seamlessly capture a video (including nonverbal behaviours) and audio record of the event.
Spaced retrieval (SR) has emerged as a promising memory training technique for use with people who have AD).13,19,18,20,21 SR is an evidence-based mnemonic technique that promotes information retention and retrieval.22 SR supports the learning of new information through repeated recall of target information over expanding intervals. Spacing intervals close together at the beginning of training ensures the successful learning and retrieval of the target information. Gradually increasing subsequent intervals following successful recall strengthens memory of that information.23 In the event of an error, patients are immediately provided with corrective feedback to encourage success in subsequent intervals, and then re-directed to recall at their last successful interval. This process applies the principles of errorless learning during the acquisition phase of SR training.24,25,26 Minimizing errors may also reduce the negative feelings associated with failure and the impact on future recall.27 Instead, the use of performance-adjusted intervals enables the patient to learn and retain information, which promotes feelings of achievement and self-efficacy.28
Rehearsing target information over expanding time intervals facilitates the encoding of information into long-term memory, making it easier to recall, and less likely to be forgotten. Vance and colleagues found that after 16 mins of SR training, target information is consolidated into long-term memory and considered successfully learned.16,29 In fact, people with AD may be able to recall target information for weeks or months following training, particularly when the training includes follow-up maintenance11 or booster sessions.92
SR training engages the implicit memory system, which is associated with motor skills, routine habits and automatic and unconscious processing of information. Implicit memory is more preserved in persons with AD compared to their more marked explicit memory deficits.30 By activating the implicit memory system, SR actively engages the person with AD while requiring little cognitive effort.7,28 This is an important factor when working with people who are aware of their compromised cognitive capacity, as it is helpful in preventing disappointment and stress that can occur when they are unsuccessful in “trying” to consciously memorize target information.
The target information to be remembered in SR training can be customized to meet the specific needs of the individual, supporting a diverse range of meaningful and functional tasks (examples provided below). The time between training intervals is typically filled with activities or conversation to help the person with AD feel at ease, engaged with the trainer, and deterred from explicitly memorizing the target material.31
Numerous studies have applied SR to semantic memory to help people with AD remember various types of information: the name, face and/or role associations of unfamiliar people and/or personally relevant people (eg, family members and care staff) . ),25,32,33,19,35,34,36,37,18,21 common personal and household objects,38,39,40 names of medication,41,42 and word lists.43
SR has also been effective in supporting procedural memory in AD, including activities of daily living (ADL) and independent activities of daily living (IADL) such as how to eat safely,6,22,44,45 everyday multi-step activities, such as using the oven, preparing tea, setting an alarm clock,46 using mobile devices and managing voicemail,47 putting things back where they belong, and following a sequence of instructions to guide behaviours.47,48
In addition to semantic and procedural memory, SR has been used to support prospective memory in order to decrease problematic behaviours,49,50,51 manage medications, prevent wandering and support wayfinding, use mobility aids,52,53 and increase social interaction and participation in recreational activities in residential care settings ).50,7,83,51
While there is a growing body of evidence that SR training can be used to compensate for deficits in semantic, procedural, and prospective memory, there is still limited research that investigates the effect of SR training on episodic memory, particularly recent memory in AD. Small18 conducted a pilot study using SR to support recall of information from a recent episodic event. Findings showed that all AD participants benefitted from the training and were able to respond to the prompt question and recall core details from the event. In addition, cues dramatically increased participants’ response accuracy, providing evidence that they encoded the information to some extent during training. These pilot study findings indicate that SR training has the potential to effectively enhance episodic recent memory for persons with AD. However, given the small sample size, limited number of training sessions, and the need for revisions to the study protocol, the current study was carried out to replicate and extend the findings from Small.18 More specifically, whereas in our pilot study most participants showed substantial gains following episodic training, these gains were still not close to maximum performance (ie, recall of all core details), and the greatest gains were observed after providing participants with cues. Therefore, in the present study, we increased the number of training sessions (from 2 to 4) and opportunities for rehearsal in order to optimize deeper encoding of event details and spontaneous recall. Some support for the benefits of an increase in training duration comes from Cherry and Simmons-D’Gerolamo,54 who reported better training outcomes following 6 (than 3) training sessions [cp.55].
The focus of the SR training in this study was on improving recall of recent events. Therefore, our primary outcome measures assessed event recall, measured by scoring trainee recall of event core details from training and maintenance sessions. In addition, previous research has reported that there may also be a positive indirect impact of SR training on care partners. For example, Camp, Foss, O’Hanlon, & Stevens84 reported “caregivers described perceptions of reduced stress, increased optimism and increased perceived control after implementation of the [SR] intervention.” It is also possible that participants with AD may increase their perceived self-confidence and memory abilities, and that this may also be observed by their caregivers. Thus, the following two secondary outcome measures of various aspects of caregiver’s and trainee’s quality of life were included: 1) Revised Memory and Behaviour Problems Checklist (RMBPC)56 and 2) Quality of Life-Alzheimer’s Disease (QoL-AD).57
It was hypothesized that persons with AD would retain episodic information over time by using salient prompts (ie, video clips) to support SR training immediately following a recent event. In particular, we predicted that there would be significantly greater recall of trained event information compared to control event information. In addition, we hypothesized that the training effect would persist over time for up to 3 months and that it would be correlated with positive changes in the quality of life measures.
Methods
Participants
Inclusion Criteria
Fifteen participants were recruited who had been diagnosed by their attending physician with probable AD or possible AD according to currently acceptable research criteria and clinical practice.58,59,60,4,61–63,85 This convenience sample was recruited through the Clinic for Alzheimer’s Disease and Related Disorders (CARD) at the University of British Columbia as well as through advertisements posted in the Alzheimer Society of British Columbia newsletter.
Prior to beginning SR training, participants with AD were administered the Mini-Mental State Examination (MMSE) and the Modified Mini-Mental State (3MS),64 which are screening measures of cognitive performance. MMSE scores of 19 to 26 out of 30 were used to identify mild stage AD participants. MMSE scores of 10 to 18 out of 30 were used to identify moderate stage AD participants. As shown in Table 1, 13 of the AD participants were mildly impaired, and 2 moderately impaired. Participants mean MMSE was 21 (SD = 3.56) (see Table 2). We also administered the following neuropsychological assessments of verbal and visual episodic memory and executive functions: Trail Making Test—A and B [executive function, mental flexibility;65] (see Table 2); Doors and People Test [verbal and visual recognition and recall;66] (see Table 2). Participants were not taking medications that interfere with speech, language, hearing, or cognitive performances (eg, anti-depressants), other than medications specific for AD (eg, cholinesterase inhibitors). They were not clinically depressed in scoring 14 or less out of 30 on the Geriatric Depression Scale.67 All baseline assessments were administered prior to training.
 |
Table 1 Participant Demographics |
 |
Table 2 Cognitive Assessment Baseline |
Eight of the participants were male, and seven were female. Participants were between 57 and 91 years of age and resided in the community with a full-time care partner, who also consented to participate in the study. Participants had sufficient functional vision to read newspaper-size fonts and hearing to perceive the material in test and training activities. No participants in this study were involved in concurrent memory training research.
Materials
Each participant participated in a training event and a control event (see Table 3). The target duration of each event was approximately 5 mins in its edited format (ie, capturing only the core details of the event; see below). Events were contextualized for each participant by adapting the events to each participant. In so doing, we increased the personal meaningfulness of the events and the potential effectiveness of the training.55 Each event included three core details to be recalled and assessed throughout the period of training and follow-up maintenance. Core details were identified and ranked (in terms of meaningfulness, significance) by the first author and trainer before an event took place. In the final process of selecting three core details for an event, spontaneous content that arose during the actual event was considered as well. Whenever possible, training and control events were held on different days. Events were video-recorded with a JVC digital camcorder and an iPad.
 |
Table 3 Sample Training and Control Activities |
The primary outcome measures assessed event recall, measured by scoring trainee recall of event core details from training and maintenance sessions. The two secondary outcome measures of various aspects of caregiver’s and trainee’s quality of life were 1) Revised Memory and Behaviour Problems Checklist (RMBPC)56 and 2) Quality of Life-Alzheimer’s Disease (QoL-AD).57 The RMBPC is a standardized caregiver-completed 24-item measure that addresses the frequency of memory-related behavioural problems in persons with dementia, and caregivers’ reactions to them. The RMBPC has strong reliability (r’s ≥ 0.70) and good construct validity (r’s ≥ 0.36); The QoL-AD is a measure of the quality of life in people at different stages of dementia severity. It has two components and is administered and scored separately for the persons with AD (QoL-AD) and their caregivers (QoL-CG). The QoL-AD consists of 13 items, covering the domains of physical health, energy, mood, living situation, memory, family, marriage, friends, chores, fun, money, self, and life as a whole. The QoL-AD has strong reliability (r’s ≥ 0.60) and validity (r’s ≥ 0.50)69 (see also68). The RMBPC and QoL-AD post-training assessments were conducted one-week and 3 months following training.
Procedures
Trainers
The first author trained three graduate research assistants who had previous experience working with persons with AD. Training on the SR technique consisted of using a training manual (adapted from70) in role play and in actual demonstration by the author, as well as return demonstration by the trainer when working with a participant in the study. Adherence to the training protocol was measured and ensured through a) use of the training manual that sets forth what each training trial consists of, b) the author’s review of the audio-recorded training sessions, and c) post-session reports completed by the trainer. By having more than one trainer deliver the SR training, the application and results of the therapy were validated.71
Introductory Screening Session
In the first session, the trainer got acquainted with both the trainee and his/her care partner in their home. The trainer discussed the objective of the study and asked the participants if they had any questions, prior to their signing informed consent. Following this, two secondary outcome measures (the Revised Memory and Behaviour Problems Checklist (RMBPC) and the Quality of Life-Alzheimer’s Disease (QoL-AD) were administered (see below). Lastly, the trainer administered the SR screening test to determine the suitability of SR for each participant. The screening test consisted of a photo and name of a boy previously unknown to the participant. The screening was conducted using the procedures outlined in Brush and Camp.22 The trainee was given three attempts to produce the correct response to the prompt at each of 0-, 15-, and 30-sec intervals. None of the participants failed the screening test.
Training Sessions
Once an event was discussed and planned by the trainer, participating trainee, and the family member(s), a day and time for holding the event was scheduled. Each person involved in the event provided informed consent, and was informed that the event will be video-recorded. Immediately after a target (but not control) event was completed, the training for that event began by the trainer stating to the trainee the target activity to be trained (eg, wife’s birthday party), followed by the prompt question and the expected target response of the trainee to the prompt, exemplified here:
Trainer Prompt Question: “What can you do to help you remember your wife’s birthday party?”
Trainee Target Response: “I can watch the video/movie” … participant points at the iPad device.
The trainee was then immediately provided with the target prompt question and was asked to provide the correct target response (0 min interval). If the trainee succeeded, he/she was provided affirmation, followed by elicited recall and review of the video, after which the next scheduled interval (1 min) was initiated for issuing the target prompt again. If the trainee was unsuccessful in providing the correct target response at any interval, the trainer immediately provided the correct response and reiterated the target prompt-response sequence to ensure encoding by the trainee. The trainer then reverted to the last successful interval and resumed training (eg, if it had been the 2-min interval when the trainee failed, the trainer would go back to the 1-min interval).
Following the prompt-response sequence at each interval, but before viewing the video of the event with the participant, the trainer asked the participant what he/she could spontaneously recall from the event (eg, “Can you tell me anything you remember about what happened at the party?”). If the participant could not spontaneously generate all of the core details, a cue was provided for core details not recalled (eg, “Something happened at the beginning, when I was serving coffee, and we jumped?”). The trainer logged the participant’s spontaneous and cued recall of the three core details and reminded the participant of any missing core details following spontaneous and cued recall at each interval. For the purpose of reinforcing memory of episodic emotions, when appropriate the trainer inquired about the participant’s feelings during the event (eg, “What did you think about singing happy birthday in three languages?”). For each event, the spontaneous and cued recall of the selected core details by the trainee served as the key primary dependent measures.
In this study, we utilized 4 training sessions over a 2-week period (two 90-min sessions per week). All training sessions used the same inter-trial interval schedule. Although much previous research on semantic and prospective training has employed training intervals up to a maximum of 4 or 8 mins24, and for some of our pilot study results an 8-min interval seemed to represent a plateau in performance in the semantic and prospective conditions, we hypothesized that including an interval of 16 mins would provide an important extra opportunity for rehearsing the target event details and encoding these into long-term storage. This rationale is supported by Hopper et al,36 who found that it took participants with AD longer to learn novel associations (ie, establish new knowledge) than previously familiar associations. In addition, previous studies have found that after 16 mins of SR training, target information is consolidated into long-term memory and considered successfully learned.16,29 In the author’s pilot study, there was very little change in performance in the episodic condition from the 16-min interval to the 32-min interval. Having a maximum 16-min interval also enabled the session to fit within a 90-min period (which some pilot study participants would have preferred to the 2-hr session that was necessary to accommodate a 32-min interval). Thus, training intervals were spaced according to the following schedule: 0, 1, 2, 4, 8, and 16 mins. Intervals were filled with activities unrelated to the training targets (eg, conversation on a variety of topics), which is supported by Hopper et al’s36 results showing there was no benefit of engaging the trainee in target-related conversation during intervals (see also,72). As mentioned above, in cases involving an incorrect prompt response at one interval, the usual SR procedure was followed by providing the trainee with the correct response and returning to the previous successful interval.70
Control Events
For the control (untrained) event, since there could be no procedural component to the control condition (ie, no watching the video), the trainer used a verbal prompt that did not identify the event itself (eg, “Can you tell me what activity you and your wife did in our previous session?”). If the participant was not able to recall core details, they were provided with a cue for each core detail (eg, “I brought you a present”). Regardless of participants’ success in recalling details after being cued, we did not provide the correct responses or probe further because we did not want to engage in reinforcing the participants’ immediate memory of the control activity. Recall of control events was assessed at the beginning and end of every session subsequent to the event.
Maintenance Sessions
To examine long-term outcomes of the SR training, the 2 weeks of training were followed by 1 post-training assessment (at 1 week), and 2 long-term maintenance assessments at 1 and 3 months. Thus, in total there were 4 training sessions and 3 follow-up maintenance assessments over a 5-month period (allowing 1 week for the introductory session). Each maintenance session consisted of both an SR target and a control assessment, one at the beginning of the session and one at the end. Within a maintenance session, in between the two assessments, the trainer and trainee engaged in conversation or other mutually agreeable activities, and/or completed outcome assessments. The SR assessments employed similar procedures to those used during training in that the trainer elicited the target/control response by asking the prompt question for that event, followed by elicitation of core details of the event (spontaneous and cued), as appropriate. There was no review of the video-recorded event during maintenance sessions since that would be considered “booster” training. However, at their request, trainees were given a personal DVD copy of the video-recorded event only at the conclusion of the study so as to control for inter-session exposure to the event.
Overview of Design
This study employed a quasi-experimental within-subject group design.73 Training was administered in participants’ homes for a target event, with the concurrent assessment of an untrained control event. Maintenance sessions for both target and control events were conducted to assess long-term memory of events.
All sessions were digitally audio and video recorded for purposes of checking adherence to the training protocol and the accuracy of real-time scoring during the session. Scoring of participants’ responses of core details was conducted in two ways: spontaneous recall, and cued recall. While it would have been advantageous to conduct maintenance assessments and scoring in a blinded fashion, blinding was not possible in this design because different probes must be used in the experimental and control conditions, and thus the assessor would have known which condition was being assessed. Participant responses that were ambiguous were discussed by the author and trainer until consensus was reached. In addition, all of the audio-video data and raw data scoring sheets were re-reviewed by a third research assistant for accuracy.
Descriptive statistics and graphical summaries were generated to evaluate performance on prompt responses and recall of core details (spontaneous and cued) for the trained and untrained/control events over time. To examine the overall effects of SR training, repeated measures ANOVA (Training Condition: Training/Control Events; Response Type: Spontaneous/Cued; Session: Training/Maintenance) were conducted, along with follow-up paired t-tests of percent recall of trained vs control events (ie, post-training target maintenance average vs control event average). The post-training maintenance average represented mean performance across all maintenance sessions; however, we also examined separately the short-term (1–4 weeks), mid-term (1 month), and long-term (3 months) outcomes. To estimate the importance of the training outcomes, calculation of effect sizes was done for both participant and group average difference scores.74
All study protocols were approved by the Behavioural Research Ethics Board at the University of British Columbia, and all participants provided written informed consent.
Results
The findings are presented below across all participants, conditions, and over time. A total of 15 participants participated in the SR training sessions, SR maintenance sessions, and the control condition. However, due to time constraints, some participants were unable to complete all of the SR scheduled time intervals.
Comparing Training, Maintenance and Control
The mean recall proportions for prompt response, spontaneous, and cued recall during the SR training and SR maintenance conditions (separately and combined) and the control condition were calculated across all participants (see Table 4 and Figure 1). Participants were able to respond successfully to prompt responses less than 50% of the time, and this was observed for both the training and maintenance conditions. A repeated measures ANOVA indicated that the overall mean recall proportions for spontaneous recall differed significantly between the SR training, SR maintenance, and control conditions (F(1, 19)=12.74, p=0.001). Post hoc pairwise comparison t-tests revealed that there were no significant differences between SR training and SR maintenance (M=0.43 vs M=0.47) (t(14)=−1.13, p = 0.28). However, there were significant differences with large effect sizes when comparing SR training to control (M=0.43 vs M=0.18) (t(14)=3.65, p= 0.003, d=0.83) and SR maintenance to control conditions (M=0.47 vs M=0.18) (t(14)=3.87, p= 0.002, d=0.85). When participants used SRT (collapsing training and maintenance) they were able to spontaneously recall on average more than twice as much event information as in the control condition. A repeated measures ANOVA also revealed that the overall mean recall proportions for cued recall differed significantly between the SR training, SR maintenance, and control conditions (F(2, 24)=36.80, p=0.000). Post hoc pairwise comparison t-tests comparing SR training and SR maintenance revealed no significant differences (M=0.71 vs M=0.71) t(12)=0.06, p = 0.95. However, there were significant differences between both SR training and control (M=0.75 vs M=0.18) (t(14)=9.19, p= 0.000, d=2.60), and SR maintenance and control (M=0.71 vs M=0.11) (t(12)=6.70, p= 0.000, d=2.85) conditions with large effect sizes. A post hoc pairwise comparison t-test revealed that participants performed better overall under the spaced retrieval condition than under the control condition for cued recall (M=0.75 vs M=0.18) (t(14)=9.20, p=0.000; d=2.59). When participants were provided with cueing during SRT (collapsing training and maintenance) they were able on average to recall more than four times as much event information as in the control condition.
 |
Table 4 Grand Total Means Spaced Retrieval Training |
 |
Figure 1 Spaced retrieval training grand total means. |
Finally, a paired samples t-test revealed that when comparing overall performance in the spontaneous versus the cued conditions (collapsing across training and maintenance), there was a significant difference between the two (SR Spontaneous M= 0.44 and SR Cued M= 0.75) t(14)=5.05, p=0.000, d=1.15.
Comparing Training, Maintenance, and Control Across Time
The mean recall proportions for prompt response, spontaneous, and cued recall across the seven spaced retrieval condition time points (four training and three maintenance) and six control condition time points were calculated across all participants (see Figure 2). A repeated measures ANOVA revealed that there were no significant differences over time for prompt response. Although participants were able to increase their correct responses to prompt response over the four SR training sessions by nearly 30%, the differences were not statistically significant (see Figure 2A), and those gains were lost over the three maintenance sessions. A repeated measures ANOVA indicated that the differences between the overall mean recall proportions for spontaneous recall were statistically significant across the four SR training and three SR maintenance time points (F(3,35)=7.55, p=0.001). When participants used SR training, their spontaneous recall rates improved by nearly 30%; however, these gains were lost by the final maintenance session. A repeated measures ANOVA revealed that there were no significant differences for overall mean proportions over time in the control condition for spontaneous recall (F(5,25)=1.38, p=0.266). As shown in Figure 2B, t-tests indicated that participants had significantly higher spontaneous recall rates during each of the SR training and maintenance condition time points when compared to the control condition time points, with the exception of the early training sessions (1 and 2) where there was a reduced sample size of control data. A repeated measures ANOVA showed that the overall differences between mean recall proportions for cued recall across the four SR training and three SR maintenance time points (F(6,42)=1.97, p=0.092), as well as across the control condition time points (F(5,25)=2.53, p=0.055) were not statistically significant (see Figure 2C). However, similar to the spontaneous recall condition, t-tests revealed that participants had higher cued recall rates during each of the SR training and maintenance condition time points when compared to the control condition time points.
 |
Figure 2 Training session means. (A) Prompt response. (B) Spontaneous recall. (C) Cued recall. |
Secondary Outcome Measures
Regarding our secondary outcome measures, paired samples t-tests revealed that there were no significant mean differences in pre-post QOL-AD measures between baseline, M1, or M3 data collection points; QOL-AD Baseline and M1 (M=39.87 vs M=39.47) t(14)0.34, p=0.74; QOL-AD Baseline and M3 (M=39.68 vs 39.82) t(10)-0.11, p=0.91; QOL-AD M1 and M3 (M=39.23 vs 39.82) t(10)-0.67, p=0.52. However, the QOL-CG revealed significant mean differences between Baseline and M3 (M=35.33 vs M=32.38) t(11)4.49, p=0.001, d=0.76; and between M1 and M3 (M=35.86 vs 34.57) t(6)2.79, p=0.032; however, no there was no significant difference between Baseline and M1 (M=33.90 vs M=33.50) t(9)0.34, p=0.74 (see Table 5). Paired samples t-tests revealed that there were no significant differences between the pre-post RMBPC sections or total test scores: all p’s >0.10 (see Table 6).
 |
Table 5 Secondary Analysis: QOL Pre-Post Test |
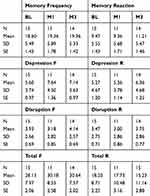 |
Table 6 Secondary Analysis: RMBPC Pre-Post Test |
Discussion
Overview
The primary objective of this study was to explore the use of spaced retrieval (SR) training immediately following a recent event to facilitate recall from episodic memory by persons with AD. We hypothesized that SR training would benefit participants’ spontaneous and cued recall of core details of recent episodic events and that this training effect would persist for up to 3 months. The findings showed that participants had significantly better spontaneous and cued recall of the target event when compared to their recall of control event details, thereby supporting our first hypothesis that SR training can be used to compensate for deficits in episodic memory. While performance varied across participants and training sessions, SR training was associated with consistently large improvements compared to the control condition. Moreover, introducing a cue to facilitate recall increased participant’s response accuracy significantly compared to spontaneous recall. These findings provide strong evidence that participants were able to encode event information during training. On the other hand, the gains in spontaneous and cued recall across the four training sessions were not sustained by the third maintenance session. Thus, our second hypothesis was not confirmed insofar as information from the recent event was not reliably retrieved 3 months after SR training had ended (though recall was still significantly better at this point than for control event details). In what follows, we discuss the implications of the findings in relation to participants’ performance on each component of our training protocol.
Prompt Response
Participants responded correctly to the target prompt question nearly half of the time. Although participants generally improved their responses to the target prompt question across the four training sessions, the response rate was low compared with previous research,18 where participants were able to respond correctly to a target prompt question nearly 100% of the time. Typically, the SR training protocol has supported new learning through utilizing a verbal prompt-response with high success.75,22,45,76 More recently, trainers have combined the use of external aids (ie, calendar, instruction card, phone directory) and other compensatory strategies (ie, write in a notebook) with the verbal prompt-response to accommodate memory deficits and further reinforce the learning effects of SR.6 The current study aligns with similar prompt-response strategies that were used in previous SR studies. Specifically, we selected an iPad as an external aid to support the prompt-response association. We were optimistic that the prompt question, “What can you do to remember the event?” would elicit the prompt response “I could watch the video on the iPad”. Furthermore, we had expected that having the iPad device visible in the testing context would facilitate retrieval of the prompt response.
One of the possible explanations for the poor prompt-response rates was that our participant sample was unfamiliar with the iPad device itself. Although we did not document participant’s previous experience, frequency of use, technology literacy, or confidence around using an iPad device, research suggests that both seniors and people with disabilities are slower to adopt technology than the general population.77,78,79 Despite iPads being introduced in 2010, surveys conducted by the Pew Research Centre80,81 report that roughly only 36% of people with disabilities and 32% of seniors actually owned a tablet computer. One could deduce that seniors with disabilities, and more specifically people with AD, are an especially vulnerable population caught on the wrong side of the digital divide. As time goes on, and future seniors adopt technology at younger ages, tablet usage may become more routine, thereby activating the implicit memory system and enhancing encoding of the prompt-response in SR. Other studies producing more consistently successful prompt-response results have used a prompt response that was more familiar or natural to participants, or part of a previously followed routine.7 For instance, referring to a calendar or checking a notebook for information are activities that the majority of older adults have used over the course of their lives.
Introducing an unfamiliar piece of technology to participants, and expecting participants to recall the specific name or function of the device may present a challenge. The current study took into consideration new learning and word-finding difficulties associated with AD and accepted alternate responses to the prompt question such as “I could watch the … video … movie … screen” while at the same time pointing to the device. Interestingly, in some cases, participants quickly responded to the prompt question by saying “I could use my brain” or “I could ask someone.” However, when the participant was provided with the correct response they sometimes responded with a chuckle and commented “Oh right, I knew that!” suggesting that the prompt-association was encoded but not automatically retrieved. Feeling vulnerable and driven by an awareness that the question requires a response, perhaps these quick responses were the most automatic or readily available to recall. An alternative explanation is that the participants wanted to demonstrate agency, creativity or the ability to think outside the box and come up with a response on their own.
Clearly, further investigation into what factors facilitate or limit establishing associations between the prompt-response sequences in episodic recent SR training is warranted. Particular attention should be directed at the different phases of memory (encoding, consolidation, retrieval) and their interactions.
Spontaneous Recall
The effect size for spontaneous recall was consistently large across participants and across the four SR training sessions. Although the gains in spontaneous recall extended beyond the four SR training sessions into the first maintenance session, which was 1 week following completion of the training, the diminished recall in subsequent maintenance sessions suggests that the encoding and consolidating of the target information was not maximised. Adjusting the spaced retrieval training, for example, by extending training intervals or training sessions, may lead to more durable representations of the core details.54
Different SR training and interval schedules have been applied in previous SR studies. Additional training sessions may provide participants with more opportunities to encode the target core details to the point where their spontaneous recall performance would plateau, reaching a state of little or no change following a steep increase. This would indicate the maximum training capacity for the participant’s ability level under the training condition. For example, Benigas and Bourgeois’6 intensive SR training program consisted of 30–45 min sessions five times per week. SR training continued until the participant had mastered all three targets, which participants were trained on one at a time. The number of training sessions required to meet mastery varied across participants and across behaviours, with the mean number of sessions to meet mastery criterion for each of the three responses being 6.73 sessions, ranging from 4 to 25 sessions.6 Similarly, Brush and Camp’s22 SR training consisted of 30–60 min sessions, three times per week, and ceased once participants accomplished mastery criterion (7.94 sessions, with a range of 3–20 sessions). Thus, it appears that tailoring the number of SR training sessions to each participant’s ability and achievement level may be worth considering as people learn at different rates.
It is interesting to note that the two aforementioned studies trained participants on three targets consecutively until mastery was achieved. In the current study, we trained participants on all three core details simultaneously. By the end of the first maintenance session, participants were able to achieve successful recall of approximately two out of three core details. This suggests that people with AD may be able to encode multiple core details/target items at once. An alternative explanation is that people with AD are able to encode and consolidate memory of several core details but in a cumulative fashion. This is evident through their score increase from 0.31 at T1 to 0.60 at M1. Many of the participants would remember the same core detail consistently over the training sessions, and with practice, and the provision of cues, participants were able to recall one or two additional core details. Training people with AD on one target item/core detail at a time, or exposing them to all three at once, yielded comparable successful recall scores. However, exposing participants to all three at once may allow participants to naturally gravitate to the most meaningful core detail, and build on it. This makes the process of learning less restrictive, and allows the participant a greater amount of control of their experience, increasing enjoyment. It also may promote a sense of accomplishment of being able to remember spontaneously.
Cued Recall
Although participants were able to spontaneously recall core details of recent events with considerable success (M =0.45), the provision of cues dramatically increased participants’ response accuracy (M =0.76). This provides compelling evidence that participants had encoded the target information to some extent during training even though it was not readily retrieved during spontaneous recall. Providing a cue supports retrieval by minimizing cognitive effort for persons with AD, which is thought to be one of the main reasons for the success of SR in this population.
Kinsella et al86 recommended providing support to persons with AD to help with encoding episodic information into long-term memory. The encoding specificity principle is a framework that accounts for how contextual information affects memory.5 Namely, memory is improved when the same contextual information is available at both encoding and retrieval stages. Once information has been encoded into long-term memory, context-based retrieval cues can be used to recall that information. Retrieval cues are most useful if they are also present at encoding, explicitly encoded with the target, and similar to the original cue available at encoding. Some examples of contextual information at encoding and contextual cues used to assist retrieval are spatio-temporal (physical surroundings, objects, and time), mood (emotional state), physiological (pharmacological/physical state), and cognitive (a person’s thoughts about the event). Regarding the latter, providing semantic cues to support encoding as well as retrieval has been found to produce similar memorial benefits for persons with AD and healthy older adults.54,87, 88
Because memory is enhanced when relevant information is presented at both the encoding and retrieval stages, not having the iPad available during the encoding stage in the present study may have had a negative effect on the participants’ response outcomes. If instead we had introduced and drew attention to the iPad before and during the event, it may have supported encoding of the iPad as an integral part of the event. For example, immediately prior to the event, the trainer could have shown the participant the iPad and said,
This object is called an iPad. We are going to use the iPad to video record the event today. We will watch the video recording of the event on this iPad many times during the SR training.
Similarly, when using the iPad to record the event, the trainer could have directed the attention of the participant to the use of the iPad for capturing the event so that it could be reviewed to reinforce memory.
The video recordings used in this study served as a context-dependent cue, capturing much of the visual and auditory content of each event. Reinstating some of the external contextual information available during encoding allowed the scene to be replicated and presented to the participant over each training trial. Given that the video recordings provided a rich multi-modality context, there may have been other contextual factors that constrained the recall gains by participants.
One possibility is that the core details were not represented strongly enough in the video due to compromised sound quality (ie, volume, distractors), visual details (ie, colours, contrast, brightness) or angle and dimensions. Perhaps if participants had interacted with actual objects used in the event it would have reinforced the encoding of the video content into memory. Using multimodal cueing (visual, olfactory, kinesthetic, auditory, gustatory) has been shown to strengthen autobiographical memory retrieval. For example, Miles et al89 reported that multi-sensory environmental cues matching early memories provided additional retrieval support, thereby reducing the demands of retrieval. This suggests that the multimodal nature of the object along with specific cue characteristics, such as time reference, texture, and shape, may constrain the retrieval search, potentially minimizing executive function demands, and hence strategic processing requirements, thus facilitating access to autobiographical memories in AD.
Another factor may have been that the core details were masked by other event information that was more personally relevant to the participant. In some instances, the participant more readily reported idiosyncratic details during recall than the three core details that the trainer had selected. In future research, trainers could collaborate with the participants not only in selecting the event but also in choosing which details from the event are most meaningful to them. One approach that lends itself to more personal control over to-be-remembered material is the use of lifelogging devices by persons with dementia during their everyday activities (e.g.,17). By targeting such personally relevant information participants should have a heightened motivation to remember thereby enhancing their performance as suggested by Wigfield and Eccles’90 Expectancy–Value Theory of Achievement Motivation [as cited in91]. Alternatively, the protocol could have been reversed such that immediately prior to the target activity we explicitly state, and perhaps have on a note card, the three core details that the participant would attend to as the activity occurs. Introducing the three core details to the participant prior to the target event could help direct their attention and encoding process and maximize establishing memory associations to that information.
Long-Term Maintenance
General Results
The training in the current study consisted of four training sessions over a 2-week period. In addition to the training itself, participants were provided with opportunities to review the trained targets in three maintenance sessions. These maintenance sessions were held at 1 week, 1 month, and 3 months following SR training. Participants had higher spontaneous and cued recall rates during all of the SR maintenance condition time points compared to their control condition time points. These recall differences between the SR training and control conditions were greatest immediately following training at maintenance session 1, but by maintenance session three, the differences between conditions had narrowed indicating that participants were unable to maintain the prompt response or spontaneous and cued recall gains that were made during the spaced retrieval training. Thus, the findings did not support our hypothesis that the SR training effects would persist well beyond the training period.
Providing participants with booster sessions as supplemental training in the weeks following an SR training program has the potential to strengthen memory of recent events. Previous research has been shown that booster sessions can promote long-term maintenance for up to 6 months.92 An alternative to booster sessions that are conducted by a clinician is use of the trained information by the person with AD in their everyday life. Clare et al11 reported that use of the trained names in a real-life setting (ie, no formalized practice) leads to relatively stable recall (compared to control stimuli) nearly 3 years post training. Introducing more training sessions or spacing them closer together has also improved long-term recall of target information.6,22 In addition to changes in training duration, spacing, and/or frequency, another factor that could enhance training outcomes is maximizing participants’ motivation. While some studies have provided little evidence of the long-term effectiveness of SR training for making name-face or name-object associations,54,82 other research reports long-term effectiveness of SR training to maintain behavioural changes for up to several years post-intervention,49,7,11,52,51 including remembering personally meaningful information, such as a clinician’s name.22 This suggests that it may be easier to encode information into long-term memory if the participant believes that it will be personally beneficial (eg, making a behavioural change in order to be more independent vs remembering to choose a specific coloured coupon). If trainers took time to explain why SR memory training is important, identified potential benefits, and elicited personally significant information, the participant may be more motivated and invested in the training program.
Maintenance of training effects is also dependent on support from the participant’s caregiver. Evidence from expert-driven training approaches such as the train the trainer (TTT) model could be applied to transfer the skills and knowledge of SR to the caregiver. In this training model, the SR trainer, who is the subject-matter expert, would train caregivers to administer SR training with the participant. The TTT strategy has been used in clinical settings, where experts teach clinicians how to care for and communicate effectively with patients.93,94 There are many workshops available through community organizations to support family caregivers. However, these workshops are often limited to provide general information and resources to caregivers through a facilitator. Having a trained clinician provide evidence-based practical skills such as SRT to family caregivers through a TTT program may increase the transfer and efficacy of SR training. Offering a TTT SR program that combines the face-to-face training and support from a clinician, along with a Spaced Retrieval Therapy App (eg, Tactus Therapy©) to support its implementation, would provide persons with AD and their caregivers with the skills and flexibility in adapting the training to best meet their needs.
Conclusion and Future Directions
Applying SR memory training to support episodic recent memory for persons with AD is a relatively new domain of research. Our findings replicated previous research that demonstrated strong effect sizes for SR facilitating both spontaneous and cued recall of recent episodic memories during training sessions. Furthermore, the provision of cues allowed participants to access memories that were otherwise inaccessible spontaneously. Although participants were able to increase their recall rates during training, the limited long-term maintenance of memory requires further exploration. Future research should investigate different ways to support deeper levels of encoding of the core details so that cues are no longer necessary and memory retention is enhanced (eg, contextual support at encoding/retrieval phases, multimodal cueing, training until mastery, booster sessions, shorter intervals between training sessions). These findings expand the scope of using SR to support people with AD beyond the preserved implicit and non-declarative memory system to include some aspects of the declarative and explicit memory systems. Future research is warranted to examine the generality of these findings.
Acknowledgments
The authors are grateful to the participants who contributed their time and energy to this study, and to the Clinic for Alzheimer Disease and Related Disorders at the University of British Columbia Hospital, and the Alzheimer Society of B.C., for their assistance in the participant recruitment process. We would also like to thank the many research personnel who contributed to materials preparation, data collection, and/or data analysis.
Funding
This research was funded by an operating grant from the Canadian Institutes of Health Research.
Disclosure
Dr. Small was a consultant for the SRT app developed by Tactus Therapy©. The authors have no other relationships with commercial/financial interests or potential for associated benefits, nor any other conflict(s) of interest related to this research study. All study protocols were developed and conducted in alignment with the Declaration of Helsinki.
References
1. Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Demen 2018. 2018;14(3):367–429.
2. Kopelman MD. Rates of forgetting in Alzheimer-type dementia and Korsakoff’syndrome. Neuropsychologia. 1985;23(5):623–638. doi:10.1016/0028-3932(85)90064-8
3. Kopelman MD. Storage, forgetting and retrieval in the anterograde and retrograde amnesia of Alzheimer dementia. In: Bäckman L, editor. Memory Functioning in Dementia. Amsterdam: Elsevier Science; 1992.
4. Minati L, Edginton T, Bruzzone MG, Giaccone G. Current concepts in Alzheimer’s disease: a multidisciplinary review. Am J Alzheimers Dis Other Demen. 2009;24(2):95–121. doi:10.1177/1533317508328602
5. Tulving E, Thomson DM. Encoding specificity and retrieval processes in episodic memory. Psychol Rev. 1973;80(5):352–373. doi:10.1037/h0020071
6. Benigas JE, Bourgeois M. Using spaced retrieval with external aids to improve use of compensatory strategies during eating for persons with dementia. Am J Speech Lang Pathol. 2016;25(3):321–334. doi:10.1044/2015_AJSLP-14-0176
7. Bourgeois MS, Camp C, Rose M, et al. A comparison of training strategies to enhance use of external aids by persons with dementia. J Commun Disord. 2003;36(5):361–378. doi:10.1016/S0021-9924(03)00051-0
8. Edvardsson D, Winblad B, Sandman P. Person-centred care of people with severe Alzheimer’s disease: current status and ways forward. Lancet Neurol. 2008;7(4):362–367. doi:10.1016/S1474-4422(08)70063-2
9. Fleming VB. The preliminary evidence supports the use of spaced retrieval paired with a visual aid for teaching individuals with mild to moderate dementia to use compensatory strategies during oral intake in a controlled environment1. Evid Based Commun Assess Interv. 2017;11(3–4):130–134. doi:10.1080/17489539.2017.1393895
10. Savundranayagam MY. Missed opportunities for person-centered communication: implications for staff-resident interactions in long-term care. Int Psychogeriatr. 2014;26(4):645–654. doi:10.1017/S1041610213002093
11. Clare L, Wilson BA, Carter G, Hodges JR, Adams M. Long-term maintenance of treatment gains following a cognitive rehabilitation intervention in early dementia of Alzheimer type: a single case study. Neuropsychol Rehabil. 2001;11(3–4):477–494. doi:10.1080/09602010042000213
12. Clare L, Woods RT, Moniz Cook ED, Orrell M, Spector A. Cognitive rehabilitation and cognitive training for early-stage Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev. 2003;(4):CD003260.
13. Grandmaison E, Simard M. A critical review of memory stimulation programs in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2003;15:130–144. doi:10.1176/jnp.15.2.130
14. Hopper T, Cleary S, Oddson B, Donnelly MJ, Elgar S. Service delivery for older Canadians with dementia: a survey of speech-language pathologists. Can J Speech Lang Pathol Audiology. 2007;31(3):114–126.
15. Silva AR, Pinho MS, Macedo L, Moulin CJA. A critical review of the effects of wearable cameras on memory. Neuropsychol Rehabil. 2018;28(1):117–141. doi:10.1080/09602011.2015.1128450
16. Vance DE, Struzick T, Farr K. Spaced retrieval technique-a cognitive tool for social workers and their clients. J Gerontol Soc Work. 2010;53(2):148–158. doi:10.1080/0163437090340974
17. Silva A, Pinho M, Macedo L, Moulin C, Caldeira S, Firmino H. It is not only memory: effects of sensecam on improving well-being in patients with mild Alzheimer disease. Int Psychogeriatr. 2017;29(5):741–754. doi:10.1017/S104161021600243X
18. Small JA. A new frontier in spaced retrieval memory training for persons with Alzheimer’s disease. Neuropsychol Rehabil. 2012;22(3):329–361. doi:10.1080/09602011.2011.640468
19. Haslam C, Hodder K, Yates P. Errorless learning and spaced retrieval: how do these methods fare in healthy and clinical populations? J Clin Exp Neuropsychol. 2011;33(4):432–447. doi:10.1080/13803395.2010.533155
20. Bier N, Provencher V, Gagnon L, Van Der Linden M, Adam S, Desrosiers J. New learning in dementia: transfer and spontaneous use of learning in everyday life functioning. two case studies. Neuropsychol Rehabil. 2008;18(2):204–235. doi:10.1080/09602010701406581
21. Bier N, Van Der Linden M, Gagnon L, et al. Face-name association learning in early alzheimer’s disease: a comparison of learning methods and their underlying mechanisms. Neuropsychol Rehabil. 2008;18(3):343–371. doi:10.1080/09602010701694723
22. Brush JA, Camp CJ. Using spaced retrieval as an intervention during speech-language therapy. Clin Gerontol. 1998a;19(1):51–64. doi:10.1300/J018v19n01_05
23. Logan JM, Balota DA. Expanded vs. equal interval spaced retrieval practice: exploring different schedules of spacing and retention interval in younger and older adults. Aging Neuropsychol Cognition. 2008;15(3):257–280. doi:10.1080/13825580701322171
24. Camp CJ, Bird MJ, Cherry KE. Retrieval strategies as a rehabilitation aid for cognitive loss in pathological aging. In: Hill RD, Backman L, Stigsdotter Neely A, editors. Cognitive Rehabilitation in Old Age. New York: Oxford University Press; 2000:224–248.
25. Camp CJ, McKitrick LA. Memory interventions in Alzheimer’s-type dementia populations: methodological and theoretical issues. In: Sinnott JD, editor. Everyday Memory and Aging: Current Research and Methodology. New York, NY: Springer-Verlag; 1992:155–172.
26. Clare L, Wilson BA, Carter G, Breen K, Gosses A, Hodges JR. Intervening with everyday memory problems in dementia of the Alzheimer type: an errorless learning approach. J Clin Exp Neuropsychol. 2000;22(1):132–146. doi:10.1076/1380-3395(200002)22:1;1-8;FT132
27. Terrace HS. Errorless transfer of a discrimination across two continua. J Exp Anal Behav. 1963;6(2):223. doi:10.1901/jeab.1963.6-223
28. Hawley KS, Cherry KE. Memory interventions and quality of life for older adults with dementia. Act Adapt Aging. 2008;32:89–102.
29. Vance DE, Farr KF. Spaced retrieval for enhancing memory: implications for nursing practice and research. J Gerontol Nurs. 2007;33(9):46–52. doi:10.3928/00989134-20070901-08
30. Haslam C, Gilroy D, Black S, Beesley T. How successful is errorless learning in supporting memory for high and low-level knowledge in dementia? Neuropsychol Rehabil. 2006;16(5):505–536. doi:10.1080/09602010500231867
31. Crowe J, Gabriel L. Errorless learning and spaced retrieval training for clients with Alzheimer’s dementia. Phys Occup Ther Geriatr. 2013;31(3):254–267. doi:10.3109/02703181.2013.796037
32. Cherry KE, Walvoord AAG, Hawley KS. Spaced retrieval enhances memory for a name-face-occupation association in older adults with probable Alzheimer’s disease. J Genet Psychol. 2010;171(2):168–181. doi:10.1080/00221320903548118
33. Clare L, Wilson BA, Carter G, Roth I, Hodges JR. Relearning face-name associations in early Alzheimer’s disease. Neuropsychology. 2002;16(4):538–547. doi:10.1037/0894-4105.16.4.538
34. Hawley KS, Cherry KE, Boudreaux EO, Jackson EM. A comparison of adjusted spaced retrieval versus a uniform expanded retrieval schedule for learning a name-face association in older adults with probable Alzheimer’s disease. J Clin Exp Neuropsychol. 2008;30(6):639–649. doi:10.1080/13803390701595495
35. Hawley KS, Cherry KE. Spaced- retrieval effects on name-face recognition in older adults with probable Alzheimer’s disease. Behav Modif. 2004;28(2):276–296. doi:10.1177/0145445503259283
36. Hopper T, Drefs SJ, Bayles KA, Tomoeda CK, Dinu I. The effects of modified spaced-retrieval training on learning and retention of face-name associations by individuals with dementia. Neuropsychol Rehabil. 2010;20(1):81–102. doi:10.1080/09602010902937590
37. Joltin A, Camp CJ, McMahon CM. Spaced retrieval over the telephone: an intervention for persons with dementia. Clin Psychol. 2003;7(1):50–55. doi:10.1080/13284200410001707483
38. Cherry KE, Simmons-D’Gerolamo SS. Long-term effectiveness of spaced retrieval memory training for older adults with probable Alzheimer’s disease. Exp Aging Res. 2005;31(3):261–289. doi:10.1080/03610730590948186
39. Cherry KE, Simmons-D’Gerolamo SS, Camp CJ. Spaced retrieval enhances memory in older adults with probable Alzheimer’s disease. J Clin Geropsychology. 1999;5(3):159–175. doi:10.1023/A:1022983131186
40. McKitrick LA, Camp CJ. Relearning the names of things: the spaced-retrieval intervention implemented by a caregiver. Clin Gerontol. 1993;14(2):60–62.
41. Bier N, Macoir J, Gagnon L, Van der Linden M, Louveaux S, Desrosiers J. Known, lost, and recovered: efficacy of formal-semantic therapy and spaced retrieval method in a case of semantic dementia. Aphasiology. 2009;23(2):210–235. doi:10.1080/00207590801942906
42. Hochhalter AK, Bakke BL, Holub RJ, Overmier JB. Adjusted spaced retrieval training: a demonstration and initial test of why it is effective. Clin Gerontol. 2004;27(1–2):159–168. doi:10.1300/J018v27n01_12
43. Lee SB, Park CS, Jeong JW, et al. Effects of spaced retrieval training (SRT) on cognitive function in Alzheimer’s disease (AD) patients. Arch Gerontol Geriatr. 2009;49(2):289–293. doi:10.1016/j.archger.2008.10.005
44. Hsu C, Lin L, Wu S. The effects of spaced retrieval training in improving hyperphagia of people living with dementia in residential settings. J Clin Nurs. 2017;26(19–20):3224–3231. doi:10.1111/jocn.13671
45. Lin L-C, Huang Y-J, Su S-G, Watson R, Tsai B, Wu S-C. Using spaced retrieval and Montessori-based activities in improving eating ability for residents with dementia. Int J Geriatr Psychiatry. 2010;25(10):953–959. doi:10.1002/gps.v25:10
46. Bourgeois J, Laye M, Lemaire J, et al. Relearning of activities of daily living: a comparison of the effectiveness of three learning methods in patients with dementia of the Alzheimer type. J Nutr Health Aging. 2016;20(1):48–55. doi:10.1007/s12603-016-0675-4
47. Thivierge S, Simard M, Jean L, Grandmaison E. Errorless learning and spaced retrieval techniques to relearn instrumental activities of daily living in mild Alzheimer’s disease: a case report study. Neuropsychiatr Dis Treat. 2008;4(5):987. doi:10.2147/NDT.S3684
48. Bird M, Kinsella G. Long-term cued recall of tasks in senile dementia. Psychol Aging. 1996;11(1):45–56. doi:10.1037/0882-7974.11.1.45
49. Alexopoulos P. Management of sexually disinhibited behaviour by a dementia patient. Aust J Ageing. 1994;13(3):119. doi:10.1111/j.1741-6612.1994.tb01083.x
50. Bird M, Alexopoulos P, Adamowicz J. Success and failure in five case studies: use of cued recall to ameliorate behaviour problems in senile dementia. Int J Geriatr Psychiatry. 1995;10(4):305–311. doi:10.1002/gps.930100407
51. Hunter CEA, Ward L, Camp CJ. Transitioning spaced retrieval training to care staff in an Australian residential aged care setting for older adults with dementia: a case study approach. Clin Gerontol. 2012;35(1):1–14. doi:10.1080/07317115.2011.626513
52. Creighton AS, Davison TE, van der Ploeg ES, Camp CJ, O’Connor DW. Using spaced retrieval training to teach people with dementia to independently use their walking aids: two case studies. Clin Gerontol. 2015;38(2):170–178. doi:10.1080/07317115.2014.988899
53. Creighton AS, van der Ploeg ES, O’Connor DW. A literature review of spaced-retrieval interventions: a direct memory intervention for people with dementia. Int Psychogeriatr. 2013;25(11):1743–1763. doi:10.1017/S104161021300123
54. Cherry KE, Simmons-D’Gerolamo SS. Spaced-retrieval with probable alzheimer’s. Clin Gerontol. 2004;27(1–2):139–157. doi:10.1300/J018v27n01_11
55. Mateer CA. Neurobehavioral interventions for memory impairment and the role of single-case design methodologies. J Int Neuropsychol Soc. 2009;15(4):623–628. doi:10.1017/S1355617709090924
56. Teri L, Truax P, Logsdon R, Uomoto J, Zarit S, Vitaliano PP. Assessment of behavioral problems in dementia: the revised memory and behavior problems checklist. Psychol Aging. 1992;7(4):622–631. doi:10.1037/0882-7974.7.4.622
57. Logsdon RG, Gibbons LE, McCurry SM, Teri L. Quality of life in Alzheimer’s disease: patient and caregiver reports. J Ment Health Aging. 1999;5(1):21–32.
58. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders.
59. Mckhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of the department of health and human service task force on Alzheimer’s disease. Neurology. 1984;34(7):939–944. doi:10.1212/WNL.34.7.939
60. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dementia. 2011;7(3):263. doi:10.1016/j.jalz.2011.03.005
61. Nasreddine ZS, Phillips NA, Be ́dirian V, et al. The Montreal cognitive assessment MOCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi:10.1111/j.1532-5415.2005.53221.x
62. Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–1992. doi:10.1001/archneur.58.12.1985
63. Peterson RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi:10.1001/archneur.56.3.303
64. Teng E, Chang Chui H. The modified mini-mental state (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318.
65. Tombaugh TN. Trail making test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–214. doi:10.1016/S0887-6177(03)00039-8
66. Davis C, Bradshaw CM, Szabadi E. The doors and people memory test: validation of norms and some new correction formulae. Br J Health Psychol. 1999;38(3):305–314. doi:10.1348/014466599162881
67. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982–1983;17(1):37–49. doi:10.1016/0022-3956(82)90033-4
68. Naglie G, Hogan DB, Krahn M, et al. Predictors of family caregiver ratings of patient quality of life in alzheimer disease: cross-sectional results from the Canadian Alzheimer’s disease quality of life study. Am J Geriatr Psychiatry. 2011;19(10):891–901. doi:10.1097/JGP.0b013e3182006a7f
69. Thorgrimsen L, Selwood A, Spector A, et al. Whose quality of life is it anyway? The validity and reliability of the quality of life- Alzheimer’s disease (QoL-AD) scale. Alzheimer Dis Assoc Disord. 2003;17(4):201–208. doi:10.1097/00002093-200310000-00002
70. Brush JA, Camp CJ. A Therapy Technique for Improving Memory: Spaced Retrieval. Beachwood, OH: Menorah Park Center for Senior Living; 1998b.
71. Tate R, McDonald S, Perdices M, Togher L, Schultz R, Savage S. Rating the methodological quality of single-subject designs and n-of-1 trials: introducing the single- case experimental design (SCED) scale. Neuropsychol Rehabil. 2008;18(4):385–401. doi:10.1080/09602010802009201
72. Foss JW, Camp CJ. Effortless learning in Alzheimer’s disease: evidence that spaced-retrieval training engages implicit memory.
73. Robey RR, Schultz MC. A model for conducting clinical-outcome research: adaptation of the standard protocol for use in aphasiology. Aphasiology. 1998;12(9):787–810. doi:10.1080/02687039808249573
74. Beeson PM, Robey RR. Evaluating single-subject treatment research: lessons learned from the aphasia literature. Neuropsychol Rev. 2006;16(4):161–169. doi:10.1007/s11065-006-9013-7
75. Abrahams JP, Camp CJ. Spaced-retrieval training intervention for anomia in patients with progressive dementia. Clin Gerontol. 1993;12(3):58–72.
76. Schacter DL, Rich SA, Stampp MS. Remediation of memory disorders: experimental evaluation of the spaced-retrieval technique. J Clin Exp Neuropsychol. 1985;7(1):79. doi:10.1080/01688638508401243
77. Czaja SJ, Charness N, Fisk AD, et al. Factors predicting the use of technology: findings from the center for research and education on aging and technology enhancement (CREATE). Psychol Aging. 2006;21(2):333–352. doi:10.1037/0882-7974.21.2.333
78. Duplaga M. Digital divide among people with disabilities: analysis of data from a nationwide study for determinants of internet use and activities performed online. PLoS One. 2017;12(6):e0179825. doi:10.1371/journal.pone.0179825
79. Vaportzis E, Clausen MG, Gow AJ. Older adults perceptions of technology and barriers to interacting with tablet computers: a focus group study. Front Psychol. 2017;8:1687. doi:10.3389/fpsyg.2017.01687
80. Pew Research Centre. Disabled Americans are less likely to use technology; 2017. Available from: https://www.pewresearch.org/fact-tank/2017/04/07/disabled-americans-are-less-likely-to-use-technology/.
81. Pew Research Centre. Technology use among seniors; 2017. Available from: https://www.pewinternet.org/2017/05/17/technology-use-among-seniors/.
82. Hochhalter AK, Overmier JB, Gasper SM, Bakke BL, Holub RJ. A comparison of spaced retrieval to other schedules of practice for people with dementia. Exp Aging Res. 2005;31(2):101–118. doi:10.1080/03610730590914976
83. Camp CJ, Foss JW, Stevens AB, O’Hanlon AM. Improving prospective memory task performance in persons with Alzheimer’s disease. In: McDaniel MA, editor. Prospective Memory: Theory and Applications. Mahwah, NJ: Lawrence Erlbaum Associates; 1996:351–367.
84. Camp CJ, Foss JW, O’Hanlon AM, Stevens AB. Memory interventions for persons with dementia. Appl Cogn Psychol. 1996;10(3):193–210. doi:10.1002/(ISSN)1099-0720
85. Mckhann G. Changing concepts of Alzheimer disease. J Am Med Assoc. 2011;305(23):2458–2459. doi:10.1001/jama.2011.810
86. Kinsella GJ, Ong B, Storey E, Wallace J, Hester R. Elaborated spaced-retrieval and prospective memory in mild Alzheimer’s disease. Neuropsychol Rehabil. 2007;17(6):688–706. doi:10.1080/09602010600892824
87. Bird M, Luszcz M. Encoding specificity, depth of processing, and cued recall in Alzheimer’s disease. J Clin Exp Neuropsychol. 1991;13(4):508. doi:10.1080/01688639108401067
88. Herlitz A, Adolfsson R, Bäckman L, Nilsson L. Cue utilization following different forms of encoding in mildly, moderately, and severely demented patients with Alzheimer’s disease. Brain Cogn. 1991;15(1):119–130. doi:10.1016/0278-2626(91)90020-9
89. Miles AN, Fischer-Mogensen L, Nielsen NH, Hermansen S, Berntsen D. Turning back the hands of time: autobiographical memories in dementia cued by a museum setting. Conscious Cogn. 2013;22(3):1074–1081. doi:10.1016/j.concog.2013.07.008
90. Wigfield A, Eccles JS. Expectancy–value theory of achievement motivation. Contemp Educ Psychol. 2000;25(1):68–81. doi:10.1006/ceps.1999.1015.
91. Choi J, Twamley EW. Cognitive rehabilitation therapies for Alzheimer’s disease: a review of methods to improve treatment engagement and self-efficacy. Neuropsychol Rev. 2013;23(1):48–62. doi:10.1007/s11065-013-9227-4
92. Cherry KE, Hawley KS, Jackson EM, Boudreaux EO. Booster sessions enhance the long-term effectiveness of spaced retrieval in older adults with probable Alzheimer’s disease. Behav Modif. 2009;33(3):295–313. doi:10.1177/0145445509333432
93. Sampson EL, Vickerstaf FV, Lietz S, Orrell M. Improving the care of people with dementia in general hospitals: evaluation of a whole-system train-the-trainer model. Int Psychogeriatr. 2017;29(4):605–614. doi:10.1017/S1041610216002222
94. Franzmann J, Haberstroh J, Pantel J. Train the trainer in dementia care: a program to foster communication skills in nursing home staff caring for dementia patients. Z Gerontol Geriatr. 2016;49(3):209–215. doi:10.1007/s00391-016-1041-1
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
