Back to Journals » Clinical Interventions in Aging » Volume 15
Some Nursing Screening Tools Can Be Used to Assess High-Risk Older Adults Who Undergo Colorectal Surgery for Cancer
Authors Cooper L, Siam B, Sagee A, Orgad R, Levi Y, Wasserberg N, Beloosesky Y, Kashtan H
Received 8 May 2020
Accepted for publication 3 July 2020
Published 25 August 2020 Volume 2020:15 Pages 1505—1511
DOI https://doi.org/10.2147/CIA.S258992
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Lisa Cooper,1,2 Baha Siam,2,3 Aviv Sagee,2,4 Ran Orgad,2,3 Yochai Levi,1,2 Nir Wasserberg,2,3 Yichayaou Beloosesky,1,2 Hanoch Kashtan2,3
1Department of Geriatric Medicine, Rabin Medical Center, Campus Beilinson, Petah Tiqva, Israel; 2The Sackler School of Medicine, Tel-Aviv University, Tel Aviv, Israel; 3Department of Surgery, Rabin Medical Center, Campus Beilinson, Petah Tiqva, Israel; 4Department of Internal Medicine C, Rabin Medical Center, Campus Beilinson, Petah Tiqva, Israel
Correspondence: Hanoch Kashtan
Department of Surgery, Rabin Medical Center, Campus Beilinson, Petah Tikva 49100, Israel
Tel +972-3-9376201
Fax +972-3-9376251
Email [email protected]
Aim: Life expectancy and incidence of cancer among older adults are increasing. The aim of this study was to assess whether routinely used nursing screening tools can predict surgical outcomes in older adults with colorectal cancer.
Methods: Data of patients who underwent elective colorectal cancer surgery at Rabin Medical Center during the years 2014– 2016 were collected retrospectively. Patients were divided into study group (age 80– 89 y), and control group (age 60– 69 y) for comparing surgical outcomes and six-month mortality. In the study group, screening tool scores were evaluated as potential predictors of surgical outcomes. These included Malnutrition Universal Screening Tool (MUST), Admission Norton Scale Scores (ANSS), Morse Fall Scale (MFS), and Charlson Co-morbidity Index (CCI).
Results: The study group consisted of 77 patients, and the control group consisted of 129 patients. Postoperative mortality and morbidity were similar in both groups. Nursing screening tools did not predict immediate postoperative outcomes in the study group. MUST and CCI were predictors for six-month mortality. CCI score was 9.43± 2.44 in those who died within six months from surgery compared to 7.07 ± 1.61 in those who were alive after six months (p< 0.05). Post-operative complications were not associated with increased 30-day mortality. Advanced grade complications were associated with an increased six-month mortality (RR=1.37, 95% CI 0.95– 1.98, p=0.013).
Conclusion: Different screening tools for high-risk older adults who are candidates for surgery have been developed, with the caveat of necessitating skilled physicians and resources such as time. Routinely used nursing screening tools may be helpful in better patient selection and informed decision making. These tools, specifically MUST and CCI who were found to predict six-month survival, can be used to additionally identify high-risk patients by the nursing staff and promote further evaluation. This can be a valuable tool in multidisciplinary and patient-centered care.
Keywords: colorectal cancer, nursing screening tools, octogenarians, post-operative outcomes, pre-operative assessment
Introduction
Life expectancy in the Western world is increasing, and as a result, older patients represent a rapidly growing percentage of the population in industrialized countries.1 The incidence of cancer among older people is also increasing. This increase can be attributed the common pathophysiology of cancer and ageing. Older adults may differ from their younger counterparts by higher incidence of comorbidities, for some, poorer functional status, and by some reports, increased likelihood to initially present with more advanced disease or as an emergency case.2
Colorectal cancer (CRC) is primarily a disease of older adults. Colorectal cancer is most frequently diagnosed among people aged 65–74 with a median age at diagnosis of 67 years.3 It is the fourth most common type of cancer in men and women and represents 8.1% of all new cancer cases in the US.3 CRC can be lethal and represents the second leading cause of cancer death worldwide.4 Nowadays, surgery remains the mainstay of CRC treatment.5–7 Older adults and their treating physicians often face complex decisions regarding the most appropriate treatment plan. Many studies have shown that age alone is not an independent risk factor for CRC.8–10 In fact, the functional status as well as comorbidities, have been repeatedly evaluated as an important prognostic factor for survival.11 Indeed, patients with severe comorbidities often receive adjusted treatment and at times are even excluded from surgical management to prevent excessive morbidity and mortality.12
There is an ethical as well as a practical dilemma regarding how aggressive one should be when it comes to treating cancer in the older population. Different tools have been developed in order to address the question of selecting the best approach for an older patient and ASCO and ESMO guidelines13,14 have recommended the use of Comprehensive Geriatric Assessment (CGA) for older adults diagnosed with cancer. CGA can identify vulnerabilities associated with older adults (functional level, cognitive status, frailty, social support, etc.) that have not been identified by routine screening in up to 50% of adults with cancer.15 On the other hand, CGA is time consuming, and takes approximately 90 minutes to perform16 while requiring an expert in geriatric medicine to be part of the multidisciplinary team. Up to recently in our institution, an expert in Geriatric medicine was not part of the multidisciplinary team and therefore there was no standard method for assessing older adults with cancer. We aimed to assess whether routine nursing screening tools can be used to evaluate older patient and predict whether this is a high-risk patient who will benefit from further, detailed evaluation. There are currently 4 main screening tools to assess different domains in hospitalized patients: The Malnutrition Universal Screening Tool; the Admission Norton Scale Scores; the Morse Fall Scale; and the Charlson Comorbidity Index. These are more accessible than CGA since they are routinely carried out by nursing staff. Therefore, the aim of this study was to evaluate the surgical outcomes of elective colectomy for cancer in older patients as compared to younger counterparts, in a tertiary referral center in Israel, and to examine whether pre-operative scores from routine nursing screening tools and a comorbidity index can serve to evaluate high-risk surgical patients.
Patients and Methods
Data of patients in the study were collected retrospectively. This included patients who underwent elective surgery for primary colorectal malignancy at the Department of Surgery, Rabin Medical Center, a tertiary referral hospital in the center of Israel, between January 2014 and December 2016. These patients were then divided into 2 groups: the study group consisting of patients aged 80–89 years, and a younger control group of patients aged 60–69 years. Inclusion criteria for the study included patients who underwent any curative or palliative, elective surgery for primary colorectal cancer with either an open or minimally-invasive procedure. Patients were excluded from the study if they had any non-malignant colon resection or presented as a surgical emergency for treatment of colorectal cancer. The study was approved by the Institutional Review Board (IRB) of Rabin Medical Center (0441–17). The study met the guidelines outlined in the Declaration of Helsinki. Due to the minimal risk nature of this study, the need for informed consent was waived by the IRB. Patients’ confidentiality was kept through data collection and analysis by replacing protected personally identifiable information with research identification codes (ID codes).
All patients in the study were admitted to the surgical ward 24 hours prior to their elective surgery for colorectal cancer. They underwent routine screening by the surgery department’s nursing staff with the assistance of a family member if needed. The following four screening tools were utilized and data collected from the patients’ electronic medical files:
- The Malnutrition Universal Screening Tool (MUST) is employed to identify patients who are at risk for malnutrition. It is used to evaluate the nutritional status of the patient and includes: body mass index (BMI), involuntary weight loss in the previous 2–3 months, and other parameters. The score is between zero (a low risk for malnutrition) and six. We used a cutoff point of two to identify patients at high risk for malnutrition.17
- The Norton score was originally developed in 1962 to evaluate the risk of developing pressure ulcers.18 Admission Norton Scale Scores (ANSS) are used for predicting hospitalization length, complications during hospitalization other than pressure ulcers, and in-hospital mortality in older patients admitted to an internal medicine department.19 It was also found useful in predicting postoperative complications in older patients undergoing elective hip replacement.20 The score is between 5 and 20. A score of 14 and below was used to identify patients at high risk.
- The Morse Fall Scale (MFS) was originally developed across acute wards, rehabilitation wards and nursing homes in Canada. Six variables (history of falling, secondary diagnosis, ambulatory aid, intravenous infusion, gait/transferring and mental status) were assigned scores ranging from zero to 125 points. We used a cutoff of 50 and above to identify patients at high risk.21 Though widely used in general hospitals, the clinical significance of this score is controversial.22
- The Charlson Comorbidity Index (CCI) is a method of categorizing comorbidities of patients based on the International Classification of Diseases (ICD) diagnosis codes. The original Index was developed with 19 categories,23 but has been modified to 17 categories.24 Each comorbidity category has an associated weight (from 1 to 6), based on the adjusted risk of mortality or resource use, and the sum of all the weights results in a single comorbidity score for a patient. A score of zero indicates that no comorbidities were found. The higher the score, the more likely the predicted outcome will result in mortality or higher resource use.
In order to determine the perioperative outcomes of elective surgery for colorectal cancer in the study group as compared to the control group, we collected the following baseline characteristics: patients’ demographics, co-morbidities, stage of disease by TNM staging,25 type of operation, postoperative morbidity and mortality. Surgical outcomes included perioperative mortality which was defined as any in-hospital death or death occurring within 30 days after surgery; postoperative complications which were classified according to the Clavien-Dindo Classification;26 and six-month overall survival.
Statistical Analysis
The statistical analysis for this paper was generated using SAS Software, Version 9.4. Continuous variables were presented by Mean±Std, categorical variables were presented by (N, %).
T-Test was used to compare the value of continuous variables between study groups and Fisher’s exact test (for two groups) or Chi-square (for more than two groups) were used to compare the value of categorical variables between study groups. Two-sided p values less than 0.05 were considered statistically significant.
Results
The study group consisted of 77 patients, while the control group consisted of 129 patients. The patients’ characteristics are summarized in Table 1. The only significant difference between the two groups was a lower incidence of rectal cancer in the older patients in the study group compared to their younger counterparts in the control group (14.3% versus 34.1%, respectively; p<0.002). Furthermore, in the study group, there were quantifiably fewer patients who were operated for stage 0 disease (polyps with high-grade dysplasia), though this did not reach statistical significance. Most of the patients in both groups (96.1% in the study group and 94.6% in the control group) were operated with a curative intent.
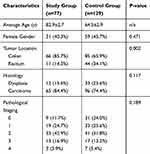 |
Table 1 Characteristics of Patients Who Underwent Elective Surgery for Colorectal Cancer |
For surgical outcomes, there were no differences in the surgical approach, duration of surgery, and perioperative morbidity and mortality between the two groups (Table 2). Post-operative mortality was low and similar in both groups (1.3% and 0.8% in the study and control groups, respectively). In addition, there were no differences between the two groups in overall post-operative or severe complications according to the Clavien-Dindo classification system. There were also no differences in either surgical or medical complications (Table 3). One exception to this was acute renal failure which occurred in 4 study group patients compared to none in the younger control group (p=0.019). The mean postoperative hospitalization period was longer in the study group as compared to the control group (11.7 versus 9.0 days, p=0.052). Furthermore, seven (9.1%) patients in the study group were admitted to the ICU in the postoperative period, while nine (11.7%) patients in the study group could not return home after surgery and were referred to a rehabilitation facility.
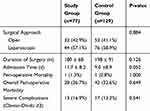 |
Table 2 Post-Operative Outcomes of Patients Who Underwent Elective Surgery for Colorectal Cancer |
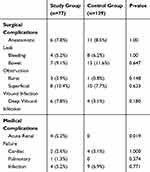 |
Table 3 Complications Arising from Elective Surgery for Colorectal Cancer |
As to post-operative complications, they were not associated with increased 30-day mortality in both the study and control groups. However, when considering six-month mortality, although overall postoperative complications in the study group were not associated with this parameter (RR 1.09, 95% CI 0.92–1.29, p=0.248), advanced grade complications (Clavien Dindo≥3) were associated with an increased six-month mortality, RR=1.37 (95% CI 0.95–1.98, p=0.013).
In the study group, the use of three different screening tools to assess different domains in hospitalized patients was evaluated (Table 4). Results showed no significant differences in perioperative morbidity between high and low-risk patients according to MUST, ANSS and MFS scores. However, the MUST score was a predictor for post-operative six-month mortality (21.9% and 0% for high and low-risk MUST scorers, respectively; p<0.001). Conversely, there was no significant difference in six-month mortality between high and low-risk patients according to the ANSS score, and it only approached significance with the MFS score (25% and 6.25%, respectively; p=0.074).
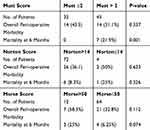 |
Table 4 Scores Evaluation of the Study Group (n=77) |
The Charlson Comorbidity Index (CCI) of the study group prior to surgery was assessed in correlation to perioperative outcomes. The findings showed no differences in CCI scores between patients who experienced postoperative complications and those who had an uneventful postoperative course (7.29±1.84 and 7.30±1.83, respectively). Similarly, the CCI score did not predict the occurrence of severe complications (Clavien-Dindo 3–4, data not shown). However, the CCI score was a significant predictor of 6 months mortality. The CCI score was 9.43±2.44 in those who died within 6 months from their colectomy compared to 7.07 ±1.61 in those who were alive after 6 months (p<0.05).
Discussion
In this retrospective study, we evaluated the surgical outcomes of elective surgery for colorectal cancer in 77 octogenarian patients and in 127 patients aged 60–69. There were more operations for colon cancer in the octogenarian group compared to their younger counterparts (85.7% vs 65.9%, respectively, p<0.01). Hence, there were fewer operations for rectal cancer in the octogenarians (14.3% vs 34.1%, respectively, p<0.01). There are two possible explanations for this observation. The first, surgery for rectal cancer has more potential for complications compared to surgery for colon cancer27 and therefore selecting patients for surgery could be more biased. The second explanation could be that treatment for rectal cancer is often based on pre-operative neo-adjuvant therapy and thus results of this therapy (ie, complete response) could affect the decision to undergo surgery in older adults, even though this approach has been evaluated only in recent years.28,29 Furthermore, there was no difference in disease stage between the two age groups which implies that in our cohort, older adults are not diagnosed with a more advanced stage of disease as previously shown.30
Thirty-day mortality was low in the octogenarian study group as well as in the younger group, similar to recent literature.31 The overall and severe complications rate was similar in both groups and similar to the literature.31 Acute renal failure occurred in 4 patients (5.2%) of the study group and none of the control group (p<0.01). As the sample size is small, it is difficult to draw any conclusion out of this observation even though it has been shown that advanced age is an independent risk factor for postoperative kidney injury.32
Screening scores are routinely used by nurses for pre-operative evaluation of surgical patients and are not specific for older adults. The main finding of the study showed that MUST, ANSS and MFS scores of patients in the study group were not significant predictors for postoperative complications. Furthermore, CCI which represents the morbidity burden of patients failed to predict postoperative complications. Our study supports similar results that have been reported by others.10,33 For example, Weerink and colleagues evaluated long-term outcomes of colorectal surgery in older adults with cancer. They demonstrated that post-operative complications rather than the patients’ comorbidities were associated with early mortality.10
Although nursing screening scores and CCI failed to predict early postoperative outcomes, the MUST score and CCI were predictors of six-month mortality in older adults, as supported by other studies.10,31 It should be emphasized that the outcome of six-month mortality is multifactorial, in which the operation and immediate post-operative complications are part of the factors involved. Huisman et al carried out a systemic review of preoperative geriatric assessment domains and screening tools.34 They found that all domains were, to a varying degree, associated with different adverse postoperative outcomes. Functional status, comorbidity and frailty were assessed most frequently and were most often significant. They acknowledged that it is unlikely that one universal geriatric assessment tool will fit all. Nevertheless, they recommend that medical teams should tailor an optimal geriatric assessment tool based on the time, expertise, and resources available in each institution.
There are several other studies that address the issue of pre-operative assessment of older population. Fagard35 reported 1.6% and 41% peri-operative mortality and morbidity, respectively, in patients older than 70 years who underwent surgery for colorectal cancer. Age and functional status were predictors for these outcomes. On the other hand, Suhool et al36 did not find a statistical correlation between age and the risk score from a geriatric assessment in patients over 75 years old with rectal cancer.
This study has several limitations. First, it is a retrospective study and therefore, the data available was limited to that which was collected and saved in the hospital’s electronic files. This means there might have been a bias while selecting older patients for surgery, based on the surgeon’s decision alone. Second, the screening tools and CCI are not specific tools for the older population and were designed to assess specific risks in the inpatient population. Third, although various demographic and clinical variables were recorded, adjuvant oncological treatment and its possible effect on six-months survival was not recorded. Forth, sample size of the study may have affected the results. Finally, the study analyzed the association with overall six-month survival rates, and not cancer-specific survival rates.
In conclusion, this study further shows that age alone is not a sufficient predictor of surgical outcomes. As so, different screening tools for high-risk older adults who are candidates for surgery have been developed, with the caveat of necessitating skilled physicians and resources such as time. Nursing screening tools that are being used routinely may be helpful in better patient selection and informed decision making. These tools, specifically MUST and CCI who were found to predict 6 months survival, can be used to additionally recognize high-risk patients by the nursing staff and promote further evaluation. This can be a valuable tool in multidisciplinary and patient-centered care.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The study was approved by the Rabin Medical Center Institutional Review Board (IRB 0441-17). Due to minimal risk nature of the study, the need for informed consent was waived by the IRB.
Acknowledgments
Tzippy Shochat, MSc. for statistical consultation. Dr Ruth Moont for reviewing and editing the manuscript.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
There was no funding for this study.
Disclosure
The authors report no conflicts of interest for this work.
References
1. GBD. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. doi:10.1016/S0140-6736(16)31012-1
2. Yancik R. Population aging and cancer: a cross-national concern. Cancer J. 2005;11(6):437–441. doi:10.1097/00130404-200511000-00002
3. Colorectal Cancer - Cancer Stat Facts [Internet]. Available from: https://seer.cancer.gov/statfacts/html/colorect.html.
4. Cancer [Internet]. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer.
5. Costas-Chavarri A, Nandakumar G, Temin S, et al. Treatment of patients with early-stage colorectal cancer: ASCO resource-stratified guideline. J Glob Oncol. 2019;5:1–19.
6. Luzietti E, Pellino G, Nikolaou S, et al. Comparison of guidelines for the management of rectal cancer. BJS Open. 2018;2(6):433–451. doi:10.1002/bjs5.88
7. Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv22–iv40. doi:10.1093/annonc/mdx224
8. Gurevitch AJ, Davidovitch B, Kashtan H. Outcome of right colectomy for cancer in octogenarians. J Gastrointest Surg. 2009;13(1):100–104. doi:10.1007/s11605-008-0643-3
9. Oh BY, Huh JW, Kim HC, et al. Oncologic outcome of colorectal cancer patients over age 80: a propensity score-matched analysis. Int J Colorectal Dis. 2018;33(8):1011–1018. doi:10.1007/s00384-018-3028-4
10. Weerink LBM, Gant CM, van Leeuwen BL, de Bock GH, Kouwenhoven EA, Faneyte IF. Long-term survival in octogenarians after surgical treatment for colorectal cancer: prevention of postoperative complications is key. Ann Surg Oncol. 2018;25(13):3874–3882. doi:10.1245/s10434-018-6766-1
11. Biondi A, Vacante M, Ambrosino I, Cristaldi E, Pietrapertosa G, Basile F. Role of surgery for colorectal cancer in the elderly. World J Gastrointest Surg. 2016;8(9):606–613. doi:10.4240/wjgs.v8.i9.606
12. Vacante M, Cristaldi E, Basile F, Borzì AM, Biondi A. Surgical approach and geriatric evaluation for elderly patients with colorectal cancer. Updates Surg. 2019;71(3):411–417. doi:10.1007/s13304-019-00650-3
13. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326–2347. doi:10.1200/JCO.2018.78.8687
14. ESMO Handbook of Cancer in the Senior Patient | oncologyPRO [Internet]. Available from: https://oncologypro.esmo.org/Education-Library/Handbooks/Cancer-in-the-Senior-Patient.
15. Caillet P, Canoui-Poitrine F, Vouriot J, et al. Comprehensive geriatric assessment in the decision-making process in elderly patients with cancer: ELCAPA study. J Clin Oncol. 2011;29(27):3636–3642. doi:10.1200/JCO.2010.31.0664
16. McIsaac DI, Huang A, Wong CA, Wijeysundera DN, Bryson GL, van Walraven C. Effect of preoperative geriatric evaluation on outcomes after elective surgery: a population-based study. J Am Geriatr Soc. 2017;65(12):2665–2672. doi:10.1111/jgs.15100
17. Weekes CE, Elia M, Emery PW. The development, validation and reliability of a nutrition screening tool based on the recommendations of the British Association for Parenteral and Enteral Nutrition (BAPEN). Clin Nutr. 2004;23(5):1104–1112. doi:10.1016/j.clnu.2004.02.003
18. An investigation of geriatric nursing problems in hospital/Doreen Norton, Rhoda McLaren, A. N. Exton-Smith. - Version details - Trove [Internet]. Available from: https://trove.nla.gov.au/work/16860709?q&versionId=26132113.
19. Leshem-Rubinow E, Vaknin A, Sherman S, Justo D. Norton scale, hospitalization length, complications, and mortality in elderly patients admitted to internal medicine departments. Gerontology. 2013;59(6):507–513. doi:10.1159/000353710
20. Asleh K, Sever R, Hilu S, et al. Association between low admission Norton scale scores and postoperative complications after elective THA in elderly patients. Orthopedics. 2012;35(9):e1302–6. doi:10.3928/01477447-20120822-13
21. Morse JM, Black C, Oberle K, Donahue P. A prospective study to identify the fall-prone patient. Soc Sci Med. 1989;28(1):81–86. doi:10.1016/0277-9536(89)90309-2
22. Healey F, Haines TP. A pragmatic study of the predictive values of the Morse falls score. Age Ageing. 2013;42(4):462–468. doi:10.1093/ageing/aft049
23. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
24. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi:10.1016/0895-4356(92)90133-8
25. Colorectal Cancer Stages [Internet]. Available from: https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/staged.html.
26. Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi:10.1097/SLA.0b013e3181b13ca2
27. Park EJ, Baik SH, Kang J, et al. The impact of postoperative complications on long-term oncologic outcomes after laparoscopic low anterior resection for rectal cancer. Medicine. 2016;95(14):e3271. doi:10.1097/MD.0000000000003271
28. Smith JJ, Strombom P, Chow OS, et al. Assessment of a watch-and-wait strategy for rectal cancer in patients with a complete response after neoadjuvant therapy. JAMA Oncol. 2019;5(4):e185896. doi:10.1001/jamaoncol.2018.5896
29. Van der Valk MJM, Hilling DE, Bastiaannet E, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391(10139):2537–2545. doi:10.1016/S0140-6736(18)31078-X
30. Green SL, Dawe DE, Nugent Z, Cheung WY, Czaykowski PM. The use of chemotherapy in older patients with stage II and III colon cancer: variation by age and era of diagnosis. J Geriatr Oncol. 2019;10(1):132–137. doi:10.1016/j.jgo.2018.07.012
31. Fagard K, Casaer J, Wolthuis A, et al. Postoperative complications in individuals aged 70 and over undergoing elective surgery for colorectal cancer. Colorectal Dis. 2017;19(9):O329–O338. doi:10.1111/codi.13821
32. Gumbert SD, Felix Kork ML, Jackson NV, Ghebremichael SJ, Wang CY, Holger K. Eltzschig; perioperative acute kidney injury. Anesthesiology. 2020;132(1):180–204. doi:10.1097/ALN.0000000000002968
33. Devon KM, Vergara-Fernandez O, Victor JC, McLeod RS. Colorectal cancer surgery in elderly patients: presentation, treatment, and outcomes. Dis Colon Rectum. 2009;52(7):1272–1277. doi:10.1007/DCR.0b013e3181a74d2e
34. Huisman MG, Kok M, de Bock GH, van Leeuwen BL. Delivering tailored surgery to older cancer patients: preoperative geriatric assessment domains and screening tools - a systematic review of systematic reviews. Eur J Surg Oncol. 2017;43(1):1–14. doi:10.1016/j.ejso.2016.06.003
35. Fagard K, Casaer J, Wolthuis A, et al. Value of geriatric screening and assessment in predicting postoperative complications in patients older than 70 years undergoing surgery for colorectal cancer. J Geriatr Oncol. 2017;8(5):320–327. doi:10.1016/j.jgo.2017.07.008
36. Suhool A, Moszkowicz D, Cudennec T, et al. Optimal oncologic treatment of rectal cancer in patients over 75 years old: results of a strategy based on oncogeriatric evaluation. J Visc Surg. 2018;155(1):17–25. doi:10.1016/j.jviscsurg.2017.06.005
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
