Back to Journals » Clinical Pharmacology: Advances and Applications » Volume 8
Skin models for the testing of transdermal drugs
Authors Abd E, Yousuf SA, Pastore MN, Telaprolu K, Mohammed YH, Namjoshi S, Grice JE, Roberts MS
Received 6 May 2016
Accepted for publication 14 July 2016
Published 19 October 2016 Volume 2016:8 Pages 163—176
DOI https://doi.org/10.2147/CPAA.S64788
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Arthur E. Frankel
Eman Abd,1 Shereen A Yousef,1 Michael N Pastore,2 Krishna Telaprolu,1 Yousuf H Mohammed,1 Sarika Namjoshi,1 Jeffrey E Grice,1 Michael S Roberts1,2
1Translational Research Institute, School of Medicine, University of Queensland, Brisbane, 2School of Pharmacy and Medical Sciences, University of South Australia, Adelaide, Australia
Abstract: The assessment of percutaneous permeation of molecules is a key step in the evaluation of dermal or transdermal delivery systems. If the drugs are intended for delivery to humans, the most appropriate setting in which to do the assessment is the in vivo human. However, this may not be possible for ethical, practical, or economic reasons, particularly in the early phases of development. It is thus necessary to find alternative methods using accessible and reproducible surrogates for in vivo human skin. A range of models has been developed, including ex vivo human skin, usually obtained from cadavers or plastic surgery patients, ex vivo animal skin, and artificial or reconstructed skin models. Increasingly, largely driven by regulatory authorities and industry, there is a focus on developing standardized techniques and protocols. With this comes the need to demonstrate that the surrogate models produce results that correlate with those from in vivo human studies and that they can be used to show bioequivalence of different topical products. This review discusses the alternative skin models that have been developed as surrogates for normal and diseased skin and examines the concepts of using model systems for in vitro–in vivo correlation and the demonstration of bioequivalence.
Keywords: percutaneous permeation, dermal delivery, transdermal, bioequivalence, ex vivo skin models, reconstructed skin
Introduction
The skin is a major physical, immunological, and sensory barrier to our environment. While it has long been used as a portal for drug delivery, it is a formidable barrier that requires appropriate technology for successful delivery. It is particularly effective in preventing large (ie, molecular weight >500) or polar molecules from entering the body. It is also a heterogeneous organ, with several delivery routes and sites that could be targeted for desirable pharmacological and immune responses. A key challenge is to deliver to the target site sufficient quantities of the drugs, peptides, vaccines, and dyes that are mainly larger and polar to achieve these responses. This may require the design of a specific chemical or physical delivery system to enhance the permeation of the active substance.
The assessment of percutaneous permeation is key to the successful development of new formulations intended for human use. It is also an important quality-control measure to ensure batch-to-batch uniformity in the pharmaceutical industry.1 Clinical end-point bioequivalence studies have generally been used for bioequivalence assessments of locally acting products. However, this is not the most feasible approach, due to the high costs involved, as well as the lack of sensitivity in highlighting formulation differences. Alternative methods for evaluating product performance include a range of models. More commonly used models to conduct skin-permeation studies are ex vivo human or animal skin. Through the standardization of protocols and techniques, the available skin models can be useful as surrogate models for in vivo human skin to evaluate the bioequivalence of topical products.
This review discusses the alternative skin models that have been developed as surrogates for normal and diseased skin, and examines the concepts of using model systems for in vitro–in vivo correlation (IVIVC) and the demonstration of bioequivalence. Table 1 lists a range of appropriate skin models.
  | Table 1 Skin models |
Human skin structure and function
A comprehensive review of the structure and function of skin can be found in Monteiro-Riviere.2 The skin accounts for approximately 16% of human body weight, with a surface area of approximately 2 m2 in adults.3,4 It provides a physical barrier to the environment, maintains homeostasis by limiting the loss of water, electrolytes, and heat, and protects against microorganisms, toxic agents, and ultraviolet radiation.5 There are three basic layers: the epidermis, the dermis, and the subcutaneous layer. Hair, nails, sebaceous glands, and sweat glands (apocrine and eccrine) are considered to be skin derivatives or appendages. Even though it is structurally continuous throughout the body, skin varies in thickness according to the age of the individual and the anatomical site.
The epidermal layer is formed from squamous epithelium and is subdivided into separate layers, according to the degree of keratinization of the cells. The layers of the epidermis from the bottom to the surface are stratum basale (basal cell layer), the stratum spinosum (spinous or prickle-cell layer), the stratum granulosum (granular cell layer), and the stratum corneum (SC; horny layer) (Figure 1).6
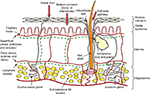  | Figure 1 Structure of the skin. |
The outermost layer of the epidermis, the SC, consists of denucleated, nonliving, flattened cells called corneocytes. There are ten to 25 layers of stacked corneocytes, which are nonhydrated cells lying parallel to the skin surface.5 The SC layers are united by SC lipid bilayers assembled into a “brick and mortar” arrangement.7
Below the SC, the remainder of the epidermis is viable tissue, called viable epidermis, containing nucleated cells called keratinocytes. The viable epidermis is a region for drug binding, metabolism, active transport, and surveillance. In addition to keratinocytes, it contains melanocytes (dendritic cells found on the basement membrane and in the basal layer), Merkel cells (functioning as mechanoreceptors involved in mediation of touch responses, found in the basal region), and Langerhans cells (dendritic cells playing a key role in protective immune function, present mainly in the stratum spinosum).2
The viable epidermis is separated from the dermis at the dermal–epidermal junction. The dermis is rich in collagen. The subcutaneous (hypodermis) layer is the deepest layer of the skin and is formed from loose connective tissue and fat (50% of the body fat), which may be more than 3 cm thick on the abdomen. The dermis and subcutaneous layers contain blood vessels, lymphatics, and nerve cells, in addition to skin appendages.8
The SC acts as the primary skin barrier, with the essential functions of protecting the body from the surrounding environment, providing an efficient obstacle to the permeation of exogenous molecules8 and microorganisms9 and maintaining homeostasis by preventing excessive loss of water.10 The surveillance, metabolic, and transport processes located in the deeper skin layers11,12 also contribute to the protective functions of the skin.
Models used to evaluate dermal absorption
Ex vivo human skin models
Measurement of dermal absorption for the purpose of targeted skin delivery, systemic delivery, or toxicological assessment should be done under the correct conditions, ideally using the gold-standard experimental model: in vivo human skin.13 However, this is not always possible, due to the high cost of human trials and concerns over applying substances or materials with potentially toxic effects. As well, in vivo responses may be difficult to measure and interpret and subject to significant variability. Alternative methods are needed to derive data that are reproducible and reliable and which provide a meaningful prediction of the in vivo human situation.14 There is a large body of work based on ex vivo human skin, and as we shall discuss in more detail later, some success has been achieved in correlating in vitro and in vivo dermal absorption, often driven by regulators seeking a standard, robust assessment method.15,16
Excised human skin is most commonly obtained from plastic surgery or cadavers, and in both cases, appropriate ethical approval is required to use the tissue. Abdominal, breast, or back skin is most convenient, due to the large areas that may be available. There are considerable differences in skin absorption across different body sites,17 attributed to such factors as differences in SC thickness,18 hydration,19,20 and lipid composition.21 Clearly, this needs to be recognized when designing studies.
As noted earlier, the SC represents the main barrier to penetration of exogenous substances into the skin,8 as well as controlling the loss of water from inside the body.10 It is believed that skin may be stored for up to 6 months without loss of SC barrier function,22 particularly if 10% glycerol is used as a preservative.23 Nielsen et al also found little effect of freezing at –20°C for 3 weeks, with or without polyethylene glycol as a preservative, but significant damage with storage at –80°C.24 On the contrary, other evidence suggests significant loss of barrier function, causing increased skin permeation, with frozen storage of animal skin.25,26 Barbero and Frasch concluded that carefully handled frozen human skin was suitable for testing the passive permeation of chemicals, when skin viability and metabolic activity were not being investigated.23 Cellular viability and metabolic activity within the human epidermis are likely to be reduced by frozen storage or heat.27,28 Our unpublished results from an MTT assay showed a complete elimination of epidermal viability following heat separation by the method of Kligman and Christophers.29 For studies requiring the presence of viable epidermal tissue, such as imaging of endogenous skin autofluorescence by multiphoton tomography,30 or investigations of skin metabolism,28 fresh tissue is required.
Several different membrane types may be prepared from ex vivo human skin for use in permeation experiments. “Full-thickness skin” is prepared by removal of connective tissue and subcutaneous fat and consists of all layers down to and including the dermis.31 To reduce variability while retaining significant dermal thickness, full-thickness skin may be cut to approximately 500–750 μm with a dermatome.32 However, the presence of the hydrated dermis may introduce an additional, artificial barrier to permeation, particularly for more lipophilic molecules.
In contrast to the in vivo situation, where capillary circulation rapidly clears penetrated molecules, full-thickness or dermatomed skin mounted in a diffusion cell represents a situation analogous to vasoconstriction.33 Consequently, the use of a membrane consisting of only the SC and the viable epidermis may be preferred. According to Cross and Roberts, this membrane represents a situation of “infinite dilatation”, since all material making its way past the SC barrier is immediately available to the receptor solution.33 Atrux-Tallau et al found that dermatomed human skin and heat-separated epidermal membranes gave the same flux for caffeine, a hydrophilic compound.34 Results from our group where steroids were applied to epidermal membranes, full-thickness skin, and isolated dermis showed that there was a minimal effect of increasing lipophilicity on epidermal maximum flux and a trend toward decreased dermal penetration rates.35
Epidermal membranes are most commonly prepared by immersing full-thickness skin in hot water (~60°C) for approximately 1 minute.29 Other techniques designed to separate the membrane at the dermal–epidermal junction include chemical treatments with ethylenediaminetetraacetic acid, ammonia, and enzymes.32 Some researchers have used the isolated SC for permeation experiments36 and for desorption studies designed to study SC heterogeneity,37 and the SC reservoir for water and other substances.38 The SC is prepared by enzymatic methods, usually by incubation with aqueous trypsin solution, after which the digested epidermal material is rinsed and wiped off.39
Ex vivo human skin: use in bioequivalence studies
For the majority of topical drug products, comparative clinical end-point studies are used to demonstrate bioequivalence to a reference drug. While this provides a direct in vivo assessment, it is also associated with a number of challenges. Clinical end points are associated with high variability (intrasubject) and low sensitivity (drug-related), which makes such studies less reliable and less efficient. The other clinical alternative is to use pharmacokinetic studies to demonstrate bioequivalence for topical products, but this is limited to particular cases where significant systemic absorption of the drug occurs. Recently, the use of techniques including in vitro permeation testing (IVPT), in vivo tape stripping, or dermatopharmacokinetics, and in vivo microdialysis or microperfusion, has been advocated for testing bioequivalence.40
IVPT using human dermatomed skin mounted in diffusion cells is increasingly seen as a suitable tool for demonstrating bioequivalence of topical dosage forms.40 Indeed, the generation of such data has been encouraged by regulatory agencies, such as the European Medicines Agency, the US Environmental Protection Agency, and the US Food and Drug Administration (FDA). Their utility is grounded on substantial evidence that 1) there is a good correlation between the in vitro and in vivo rates and extents of human skin absorption of a number of different substances and 2) there is good agreement on the bioequivalence of topical products seen with IVPT and in vivo clinical studies.
Currently, however, there are no approved protocols for carrying out IVPT studies. Franz et al pointed out that the demonstration of valid IVIVC is greatly dependent upon the protocols used, and they recommended that the in vitro and in vivo protocols followed should be as closely harmonized as possible to maximize the chance of achieving a good correlation.15,41 The work on which this conclusion was based was an analysis of historical literature data that was available to the researchers, and despite the wide variation in the way in which it was collected, their conclusion was that there was compelling evidence that it was possible to correlate IVPT data with human in vivo skin-absorption data. Others who have demonstrated good IVIVC include Hadgraft et al, who compared in vitro and in vivo delivery from nitroglycerin patches,42 and more recently Yang et al, who compared their own IVPT data with literature reports of in vivo estradiol delivery from patches.16
While the use of IVPT for bioequivalence has only recently been formalized, the design of in vitro permeation tests has been subject to consideration and validation for many years. In 1987, the FDA published a report on the important factors to be considered, which included the membrane type (dermatomed skin or heat-separated epidermis?), the receptor fluid, the cell design (static or flow-through?), application (finite or infinite dose?), and temperature.43
The in vivo dermatopharmacokinetic (DPK) method uses tape stripping to remove SC layers. The FDA has investigated the possibility of introducing a DPK method for evaluating bioavailability and/or bioequivalence of topical dermatological drug products.44,45 In the DPK method, it is assumed that 1) in normal circumstances, the SC is the rate-determining barrier to percutaneous absorption, 2) the SC concentration of the drug is related to the amount that diffuses into the underlying viable epidermis, and 3) SC drug levels are more useful and relevant for assessing local, dermatological efficacy than plasma concentrations.46 It is also possible to deduce partitioning and diffusion parameters that characterize the absorption process and which can subsequently be used to predict an entire absorption profile from a single short-contact-duration experiment.44 The technique is very operator-dependent, and care needs to be taken to apply and remove the tapes reproducibly. The success of the method is equally dependent on the development of sensitive analytical methods to quantify the amount of drug in the tapes.
Microdialysis involves the insertion of an ultrathin hollow fiber as a probe into the dermis. The probe is semipermeable and perfused with sterile buffer using a microdialysis pump. This involves the exchange of the small diffusible molecules from the extracellular fluid into the probe and vice versa. This method is used to determine the concentration of the unbound drug or biomarkers at the site to establish the concentration-versus-time profile of the applied compound. There are several issues associated with microdialysis. Probe insertion in the skin can lead to inflammatory responses, as may interactions of the perfusing buffer with the tissue.47,48 Recovery is low for highly lipophilic molecules, which may be resolved to some extent by using albumin, cyclodextrins, and cosolvents such as ethanol and dimethyl sulfoxide in the buffer,49 while highly protein-bound molecules may be difficult to detect, due to binding to the probe material. A major disadvantage of the method is the intrasubject variability.50 The newer technique of dermal open-flow microperfusion (dOFM) differs from microdialysis, in that it gives continuous, membrane-free (ie, unfiltered) access to dermal fluid.51 Like microdialysis, dOFM provides a direct estimate of the time course of delivery of the permeant near its site of application. Because of the lack of interaction with a membrane in dOFM, it can be used for a wider range of compounds than microdialysis.40 The technical difficulty of microdialysis and dOFM means that significant operator expertise is required, and as such they are generally only available in a research setting.
Ex vivo animal skin models
The assessment of percutaneous absorption of molecules is an important step in the evaluation of any topical drug-delivery system or formulation. As we have already noted, if the dosage form is to be used in humans, the most relevant skin-absorption data should come from in vivo human studies. However, such studies are generally not feasible during the initial development of a novel pharmaceutical dosage form. Moreover, ex vivo human skin may not be readily available, and so researchers have relied on animal studies for much of the experimental data. This creates a major challenge in correlating results from ex vivo animal experiments with ex vivo and in vivo human studies for prediction of human percutaneous absorption.
A wide range of animal models has been used as alternatives to human skin to evaluate percutaneous permeation of substances. These include pig, mouse, rat, guinea pig, and snake models. Porcine (pig) skin is histologically similar to human skin,52,53 with a comparable SC thickness of 21–26 μm.54,55 In addition, the average hair-follicle density in porcine ear skin is 20/cm2 compared to 14–32/cm2 in human forehead skin.54 As well as being similar to human skin, porcine ear skin is also convenient to obtain and has been widely used in skin-permeation studies.56
The SC lipids are known to be important regulators of skin permeability. With this in mind, the conformational disordering and lateral packing of lipids in isolated porcine and human SC were compared using Fourier-transform infrared spectroscopy. The SC of both species differ markedly, with porcine SC lipids being arranged predominantly in a hexagonal lattice, while lipids in human SC were predominantly packed in the denser orthorhombic lattice.57 In human as well as porcine SC, the main lipid classes are ceramide, cholesterol, and free fatty acid, and these lipid classes are present in an approximately equimolar ratio.58 However, the compositions of free fatty acid and ceramide in the two species are different.
In a range of studies using both lipophilic59,60 and hydrophilic59,61 permeants, the permeability of pig skin was found to be similar to that of human skin, but to differ to a greater extent from dog61 or rodent skin.59,61 Sato et al attributed the similarity in permeability to the similar SC lipids, barrier thickness, and morphological aspects of pig and human skin.61 Nicoli et al further investigated the differences between pig skin and rabbit ear skin, finding that although they had similar SC thicknesses, pig skin was four to seven times more permeable to hydrophilic compounds than rabbit ear skin, most likely due to its different SC lipid composition.62 The relationship between permeability and SC lipids is analogous to early findings by Lampe et al,63 who showed the total lipid-weight percentage at various human body sites (face > abdomen > leg > plantar SC) was inversely proportional to the relative permeability of skin reported for those sites by Scheuplein and Blank.64 Caussin et al also reported the similarity in SC lipid composition, as well as in lamellar organization, between pigs and humans.63,65 Interestingly, however, they also saw a substantial difference in lateral packing between the two species. As with human skin, permeation behavior was found to correlate with barrier function, as measured by transepidermal water loss in a study by Sekkat et al, who applied caffeine, lidocaine, and phenobarbital to tape-stripped pig skin.66
Skin of rodents (mice, rat, and guinea pigs) is the most commonly used in in vitro percutaneous permeation studies, due to its availability, their small size, and relatively low cost. There are different hairless strains of each species that are reported to mimic the permeation properties of human skin better than the hairy variety.67 Among rodents, rat skin is most structurally similar to human skin and it is the most frequently used rodent model. A large number of studies comparing permeation through human and rat skin have been carried out, showing that rat skin is generally more permeable than human skin across a range of permeants of different physicochemical properties,68–72 in some cases with differences of more than an order of magnitude. For example, for compounds with log-P-values ranging from 0.7 to 4.5, van Ravenzwaay and Leibold72 found that mean in vitro permeation flux through rat skin was around elevenfold greater than through human skin, while a similar comparison by Schmook et al71 found flux increase of 50-fold for the relatively lipophilic molecules hydrocortisone and terbinafine.
Shed snake skin is another interesting membrane that was suggested as a suitable alternative to human skin.73 This membrane, which can be obtained without killing the animal, has some similarity to human skin, in that it consists of thin, flat squamate cells surrounded by intercellular phospholipids, although it does lack hair follicles. Rigg and Barry compared permeation of fluorouracil (5-FU) through dermatomed human abdominal skin and shed skin from two snake species.74 The permeability coefficients were similar between human and dorsal and ventral skin of one snake species, whereas there was a 30-fold increase in dorsal skin from Elaphe (Pantherophis) obsoleta. These authors found no changes in 5-FU permeability in human or snake skin after acetone pretreatment, whereas Megrab et al75 found differential responses in human and dorsal snake skin with vehicles containing different ethanol concentrations. Apart from possible interspecies differences, it is likely that solvent effects in snake skin are influenced by both the lower water content75 and the nature of the intercellular lipids. While snake skin may be a reasonable model for human skin, it is not readily available, and doubts must exist over the quality and consistency. As Rigg and Barry noted, “[. . .] if at all possible, investigative problems should not be made more complex by selection of an animal tissue to represent human skin”.74
It may be useful, particularly in the interpretation of dermal absorption for human risk assessment, to predict human in vivo dermal absorption from known in vitro human, in vivo animal, and in vitro animal data, the so-called triple-pack approach.76 The animal in question is normally considered to be the rat. Human in vivo dermal absorption may be derived by the equation:
in vivo human absorption = in vivo rat absorption × (in vitro human absorption / in vitro rat absorption) (1)
Here, it is assumed that 1) the factor between in vitro and in vivo dermal absorption is the same for rats and humans and 2) the factor between rat and human skin absorption is the same in vitro and in vivo, despite the morphological species differences.
Artificial and reconstructed skin models
Artificial and reconstructed skin models are useful tools in specific circumstances, driven by the need to find convenient, reproducible alternatives to in vivo and ex vivo tests with human and animal skin. The artificial skin models range from simple homogeneous polymer materials, such as poly(dimethoxysilane) or silicone membranes through to lipid-based parallel artificial membrane-permeability assay (PAMPA) or phospholipid vesicle-based permeation-assay membranes,77 with the latter material designed to mimic the SC. By eliminating the complexity of human or animal skin, the simple homogeneous materials are particularly useful for studying the basic mechanisms controlling passive transport though a membrane.78–80 The main advantage they have in this regard is their relative reproducibility due to their simple standardized construction. However, they are not intended to represent, nor are they capable of, representing the multitude of in vivo skin properties.81
The PAMPA can be used for rapid screening of passive transport.82 The PAMPA assay is conducted in a 96-well filter plate coated with a liquid artificial membrane to separate two compartments: one containing a buffer solution of compounds to be tested (donor compartment) and the other containing an initial fresh buffer solution (acceptor compartment). Significant correlations with gastrointestinal absorption in humans were seen with PAMPA using filters impregnated with a solution of phospholipids or hexadecane.82 To develop a new artificial membrane to be used in PAMPA for prediction of skin permeation, Ottaviani et al investigated the permeability coefficients of a number of compounds through human skin and the PAMPA-skin artificial membrane comprised of dimethylpolysiloxane (silicone) membranes. They reported a good correlation between the two skin models.83
The FDA has encouraged the use of porous synthetic membranes for evaluating the performance of topical products, as they act as a support without posing a rate-limiting barrier.84 Shah et al from the FDA used different microporous membranes, such as pure cellulose acetate, cellulose, and polysulfone, of similar pore sizes and thicknesses to examine the permeation of hydrocortisone from two commercial creams. They found that the hydrocortisone flux was consistent irrespective of the types of synthetic membrane.85 Nitroglycerin drug release from commercial ointments was investigated by Wu et al86 using ten types of commercial synthetic membranes, such as polysulfone, cellulose mixed esters, polytetrafluoroethylene, and polypropylene, with different pore size and thickness. From the results obtained in this study, the synthetic membranes may be classified into two groups: group 1, consisting of polysulfone, acrylic polymer, glass fiber, silicone, and mixed cellulose ester, showed higher drug permeation compared to group 2, which included polytetrafluoroethylene–polyethylene, mixed cellulose ester (of greater thickness), and polypropylene. The effect of membrane types upon ketoprofen drug release from a gel has also been studied. It was found that nylon exhibited the least rate-limiting effects, although it is a thicker synthetic membrane compared to others.87
Reconstructed skin models are culture-based, with layers of human cells in culture laid down over a polymeric matrix. This allows different cell types to be incorporated to achieve a structure of the desired composition and complexity. Reconstructed models are generally designed to simulate the epidermis (reconstructed human epidermis [RHE] models) or the full human skin (living skin equivalents [LSEs]).22,77
Some reconstructed skin models are produced in-house for particular research purposes, such as drug-candidate or toxicological screening88 or the assessment of photodamage and photoprotection.89 In one particular reconstructed model, consisting of layers of human dermal fibroblasts and human epidermal HaCaT cells, there was no change in the permeability coefficients of ibuprofen after freezing the membrane over liquid nitrogen for 24 hours or 6 months. Such a property would make reconstructed membranes attractive for general screening uses. In addition, there are commercially available RHEs (eg, EpiSkin®, SkinEthic®, and EpiDerm®) and LSEs (eg, GraftSkin®, EpiDermFT®, and Pheninon®) that have been suggested as suitable candidates for in vivo and ex vivo skin models in evaluating skin absorption, testing of cosmetic products, and for the toxicological screening of topically applied compounds. A number of studies have compared LSE and HRE models with animal and human skin.71,90,91 Schmook et al studied salicylic acid, hydrocortisone, clotrimazole, and terbinafine permeation through ex vivo human (dermatomed), porcine, and rat skin, GraftSkin LSE, and SkinEthic RHE.71 The fluxes and skin accumulation were generally in the order human ≤ porcine < rat < GraftSkin << SkinEthic. Comparing human and pig skin with two RHE models, Schreiber et al91 found permeation coefficients of caffeine and testosterone were both in the order human < pig < EpiDerm << SkinEthic. Schäfer-Korting et al published an extensive comparison of human epidermal membranes, porcine skin, and three RHE models – EpiDerm, EpiSkin, and SkinEthic – with a series of hydrophilic and lipophilic permeants.90 Their general conclusions were that the RHE models, particularly SkinEthic, were significantly more permeable than the ex vivo skins, although the ranking of the permeation of the compounds through pig skin and the RHEs mirrored that through human epidermis. Interestingly, they did not observe the expected improvement in reproducibility with the RHEs compared to the ex vivo skin.
As of 2013, reconstructed skin models had received Organization for Economic Co-operation and Development approval for testing of skin corrosion, acute skin irritation, and phototoxicity.22 None is currently approved for testing of skin absorption. Further work is needed to validate the various models, particularly the LSEs, for this purpose, although they may be useful for in vitro screening. Interestingly, Schäfer-Korting et al concluded that the tested RHEs were applicable to both finite- and infinite-dose studies.90
Models for skin diseases
The skin is not only a convenient portal to the systemic circulation but also a logical site of application for treatment of various localized skin disorders, such as skin cancers, inflammatory illnesses, and damaged skin. As the investigation of disease mechanisms and new therapies is usually difficult or impossible in humans, it is necessary to use alternative methods. Models representing normal or healthy skin are appropriate to test the delivery and targeting of topically applied drugs or other substances, often for the purpose of evaluating the delivery system used. However, models that are designed to mimic the effects of disease states can be used to study the delivery and effects of topical therapies or to gain insight into the molecular mechanisms responsible for particular diseases. In the following sections, we review some of the various animal and artificial models that have been applied to studies of skin diseases. Some recently published studies using animal and reconstructed human skin models for the study of skin diseases, including their use in therapeutic screening, are summarized in Tables 2 and 3.
Ex vivo animal models for skin diseases
A plethora of in vivo animal models employing fish, guinea pigs, mice, rats, rabbits, and pigs have been developed to mimic human skin diseases. Some recent reviews have focused on the most widely studied areas of melanoma,92 atopic dermatitis,93 and psoriasis.94 Other applications include skin infections (eg, acne, viral infections), damaged skin (eg, wounding, photo-damaged skin), hair disorders (eg, different types of alopecia), and skin cancers, such as basal and squamous cell carcinomas. A significant number of models use genetically engineered mice, due to the fact that many human skin diseases are cause by gene mutations.95 These animal models have been extensively used for the understanding of disease mechanisms and to a lesser extent for the clinical evaluation of drug candidates. For example, epidermal VEGF-knockout mice were used to identify a specific role for epidermal VEGF in the maintenance of epidermal permeability-barrier homeostasis and pointed to the disruption of VEGF pathways in the development of psoriasis.96 In very recent work by Rossbach et al,97 histamine H4 receptor (H4R)-knockout mice showed significant reductions in ovalbumin-induced skin lesions analogous to those caused by atopic dermatitis. Their findings suggested that H4R could be a new therapeutic target in allergic skin diseases like atopic dermatitis.
In addition to melanoma, mouse models have been used particularly for the other common skin cancers, squamous cell carcinoma,98–103 and basal cell carcinoma.104–106 An overview of animal models for a wide range of skin conditions, with an emphasis on their application to drug discovery, has been published by Avci et al.107
Of particular interest are the chimeric models, in which living human skin is grafted on to the skin of severe combined immunodeficient (SCID) mice. In this way, responses or treatments can be studied in living human skin. For example, targeted Kv1.3-cell immunotherapy was shown to be effective in reducing human epidermal thickness and the number of CD3+ lymphocytes in an SCID mouse–human psoriatic skin xenograft model, leading the authors to propose the investigated therapy for treatment of psoriasis and possibly other inflammatory skin conditions.108 Similar investigative work in psoriasis used the SCID mouse–human psoriatic skin xenograft model to identify a role for Hsp90 in signaling pathways that are upregulated in psoriasis. Mice treated orally with the Hsp90 inhibitor Debio 0932 showed a reduction in xenograft epidermal thickness.
The xenograft model has also been used for investigation of cancer targets and therapies. Targeted oral109 or intravenous110 treatments in SCID mouse–human melanoma xenografts caused significant reductions in tumor proliferation and size in BRAF- and ALDH+-specific melanomas.109,110
Reconstructed skin models for diseases
Today, there are increasing regulatory restrictions on the use of animals, and the availability of excised human diseased skin is limited. For these reasons and following the advances in tissue engineering, the development of artificial in vitro human skin models to mimic both healthy and diseased skin has intensified. Another important benefit of using artificial skin models is that they allow the incorporation of specific disease characteristics in a controlled and relatively reproducible manner. In vitro models have been developed for a wide range of skin diseases, such as inflammatory disorders, fungal infections, skin cancer, photodamaged skin, and wounding. A general review has recently been published by Küchler et al.22 The models are generally developed in-house by researchers, with the goals of understanding disease mechanisms and progression, or less commonly to use as screening tools for the assessment of therapeutic modalities. A major challenge in the use of these models is to assess whether they are relevant to and predictable of the in vivo situation.
Inflammatory and autoimmune diseases for which artificial skin models have been developed include psoriasis,111,112 atopic dermatitis,113,114 and eczematous dermatitis.115 In most cases, the specific pathway leading to expression of the disease state was induced by suitable interventions, such as stimulation by psoriasis-associated cytokines,112 or in the generation of atopic dermatitis by downregulation of filaggrin113 or treatment with an inflammatory cocktail.114 Some of the models were developed as potential screening tools for drugs to treat the expressed disease states.112,114
Skin-cancer models were constructed by incorporating various tumor entities within the three-dimensional (3-D) matrix, including cultured melanoma116 cells, an A375 metastatic melanoma cell line,117 and melanoma-tumor spheroids,118 as well as various cutaneous squamous cell carcinoma cell lines.119 Like the inflammatory models, these were used to study disease progression and targeted therapeutic interventions. Mohapatra et al117 used the cyclin-dependent kinase inhibitor roscovitine to inhibit growth of the A375 cells within the dermal layer of the 3-D matrix. Using combination therapies, Vörsmann et al118 showed significant advantages in using their 3-D melanoma model to deliver in vivo-like responses compared to a standard 2-D monolayer culture.
A novel chimeric model consisting of a human artificial 3-D skin construct grafted onto the back of SCID mice has also been reported.120 The bioengineered skin, containing human keratinocytes and fibroblasts isolated from skin biopsies of healthy donors or scleroderma patients, was generated ex vivo and then grafted onto the back of SCID mice. Results implicated the involvement of a PDGF receptor-mediated pathway in the disease and confirmed the suitability for testing in vivo the disease progression-screening antifibrotic drugs.
Summary and conclusion
Despite ethical concerns, the use of animals or isolated animal skin models to assess percutaneous absorption of molecules is frequently reported. These models are generally more widely available than human skin, and prove important in basic research to improve our understanding of the processes, pathways, and driving forces of various agents across the skin barrier. However, because of a large number of animal skin models described in the literature, it may be difficult to compare the results obtained across various species, in addition to the variations in experimental methodology used with a specific skin model, such as type of diffusion cells, body site, skin temperature, receiver media, application dose, and diffusion area. Therefore, it is important to emphasize that in vitro and animal models provide important tools for screening a series of drug formulations, evaluation of skin permeation-enhancing properties and mechanism of action of the carrier systems, and estimation of rank of skin transport for a series of drug molecules. Also, the majority of the work on synthetic membranes for transdermal and topical delivery studies has been focused on the use of polymeric materials, usually silicone based. Such membranes are ideal for replacing ex vivo skin, as they can be prepared with a defined thickness, are easy to handle and store, are comparatively cheap, inert, and provide reproducible results. Despite all of these advantages, they cannot completely replace human or animal skin for prediction of skin absorption in vivo. These membranes generally lack the type of barrier normally provided by the SC in ex vivo or in vivo skin, and this may lead to some false-positive results in toxicity studies and permeation studies. Therefore, we recommend that where possible, human skin should be used in skin-permeation studies.
A wide range of skin models for testing skin absorption for cutaneous and transdermal delivery has been developed. There is an increasing need, largely driven by regulatory authorities and industry, to ensure that the models and testing protocols are standardized and reproducible, and are validated to show that they accurately reflect the in vivo situation.
Disclosure
The authors report no conflicts of interest in this work.
References
Clowes HM, Scott RC, Heylings JR. Skin absorption: flow-through or static diffusion cells. Toxicol In Vitro. 1994;8(4):827–830. | ||
Monteiro-Riviere NA. Structure and function of skin. In: Monteiro-Riviere NA, editor. Toxicology of the Skin. New York: Informa Healthcare; 2010:1–18. | ||
Bensouilah J, Buck P. Aromadermatology: Aromatherapy in the Treatment and Care of Common Skin Conditions. London: Radcliffe; 2006. | ||
Hadgraft J. Skin, the final frontier. Int J Pharm 2001;224(1–2):1–18. | ||
Barry BW. Dermatological Formulations: Percutaneous Absorption. Vol 18. New York: Marcel Dekker; 1983. | ||
Montagna W, Parakkal PF. The Structure and Function of Skin. 3rd ed. New York: Academic Press; 2012. | ||
Roberts MS, Cross SE, Pellett MA, Walters KA. Skin transport. In: Walters KA, editor. Dermatological and Transdermal Formulations. New York: Marcel Dekker; 2002:89–196. | ||
Jepps OG, Dancik Y, Anissimov YG, Roberts MS. Modeling the human skin barrier: towards a better understanding of dermal absorption. Adv Drug Deliv Rev. 2013;65(2):152–168. | ||
Duckney P, Wong HK, Serrano J, Yaradou D, Oddos T, Stamatas GN. The role of the skin barrier in modulating the effects of common skin microbial species on the inflammation, differentiation and proliferation status of epidermal keratinocytes. BMC Res Notes. 2013;6:474. | ||
Boer M, Duchnik E, Maleszka R, Marchlewicz M. Structural and biophysical characteristics of human skin in maintaining proper epidermal barrier function. Postepy Dermatol Alergol. 2016;33(1):1–5. | ||
Dancik Y, Anissimov YG, Jepps OG, Roberts MS. Convective transport of highly plasma protein bound drugs facilitates direct penetration into deep tissues after topical application. Br J Clin Pharmacol. 2012;73(4):564–578. | ||
Oesch F, Fabian E, Guth K, Landsiedel R. Xenobiotic-metabolizing enzymes in the skin of rat, mouse, pig, guinea pig, man, and in human skin models. Arch Toxicol. 2014;88(12):2135–2190. | ||
Lai J, Maibach HI. Experimental models in predicting topical antifungal efficacy: practical aspects and challenges. Skin Pharmacol Physiol. 2009;22(5):231–239. | ||
Walters KA, Watkinson AC, Brain KR. In vitro skin permeation evaluation: the only realistic option. Int J Cosmet Sci. 1998;20(5):307–316. | ||
Franz TJ, Lehman PA, Raney SG. Use of excised human skin to assess the bioequivalence of topical products. Skin Pharmacol Physiol. 2009;22(5):276–286. | ||
Yang Y, Manda P, Pavurala N, Khan MA, Krishnaiah YS. Development and validation of in vitro-in vivo correlation (IVIVC) for estradiol transdermal drug delivery systems. J Control Release. 2015;210:58–66. | ||
Rougier A, Lotte C, Maibach HI. In vivo percutaneous penetration of some organic compounds related to anatomic site in humans: predictive assessment by the stripping method. J Pharm Sci. 1987;76(6):451–454. | ||
Sandby-Møller J, Poulsen T, Wulf HC. Epidermal thickness at different body sites: relationship to age, gender, pigmentation, blood content, skin type and smoking habits. Acta Derm Venereol. 2003;83(6):410–413. | ||
Marrakchi S, Maibach HI. Biophysical parameters of skin: map of human face, regional, and age-related differences. Contact Dermatitis. 2007;57(1):28–34. | ||
Shriner DL, Maibach HI. Regional variation of nonimmunologic contact urticaria: functional map of the human face. Skin Pharmacol. 1996;9(5):312–321. | ||
Lampe MA, Burlingame AL, Whitney J, et al. Human stratum corneum lipids: characterization and regional variations. J Lipid Res. 1983;24(2):120–130. | ||
Küchler S, Strüver K, Friess W. Reconstructed skin models as emerging tools for drug absorption studies. Expert Opin Drug Metab Toxicol 2013;9(10):1255–1263. | ||
Barbero AM, Frasch HF. Effect of Frozen human epidermis storage duration and cryoprotectant on barrier function using two model compounds. Skin Pharmacol Physiol. 2016;29(1):31–40. | ||
Nielsen JB, Plasencia I, Sørensen JA, Bagatolli LA. Storage conditions of skin affect tissue structure and subsequent in vitro percutaneous penetration. Skin Pharmacol Physiol. 2011;24(2):93–102. | ||
Abdayem R, Roussel L, Zaman N, Pirot F, Gilbert E, Haftek M. Deleterious effects of skin freezing contribute to variable outcomes of the predictive drug permeation studies using hydrophilic molecules. Exp Dermatol. 2015;24(12):972–974. | ||
Ahlstrom LA, Cross SE, Mills PC. The effects of freezing skin on transdermal drug penetration kinetics. J Vet Pharmacol Ther. 2007;30(5):456–463. | ||
Messager S, Hann AC, Goddard PA, Dettmar PW, Maillard JY. Assessment of skin viability: is it necessary to use different methodologies? Skin Res Technol. 2003;9(4):321–330. | ||
Wester RC, Christoffel J, Hartway T, Poblete N, Maibach HI, Forsell J. Human cadaver skin viability for in vitro percutaneous absorption: storage and detrimental effects of heat-separation and freezing. Pharm Res. 1998;15(1):82–84. | ||
Kligman AM, Christophers E. Preparation of isolated sheets of human stratum corneum. Arch Dermatol. 1963;88(6):702–705. | ||
Sanchez WY, Prow TW, Sanchez WH, Grice JE, Roberts MS. Analysis of the metabolic deterioration of ex vivo skin from ischemic necrosis through the imaging of intracellular NAD(P)H by multiphoton tomography and fluorescence lifetime imaging microscopy. J Biomed Opt. 2010;15(4):046008. | ||
Cross SE, Magnusson BM, Winckle G, Anissimov Y, Roberts MS. Determination of the effect of lipophilicity on the in vitro permeability and tissue reservoir characteristics of topically applied solutes in human skin layers. J Invest Dermatol. 2003;120(5):759–764. | ||
Haigh JM, Smith EW. The selection and use of natural and synthetic membranes for in-vitro diffusion experiments. Eur J Pharm Sci. 1994;2(5–6):311–330. | ||
Cross SE, Roberts MS. Use of in vitro human skin membranes to model and predict the effect of changing blood flow on the flux and retention of topically applied solutes. J Pharm Sci. 2008;97(8):3442–3450. | ||
Atrux-Tallau N, Pirot F, Falson F, Roberts MS, Maibach HI. Qualitative and quantitative comparison of heat separated epidermis and dermatomed skin in percutaneous absorption studies. Arch Dermatol Res. 2007;299(10):507–511. | ||
Magnusson BM, Cross SE, Winckle G, Roberts MS. Percutaneous absorption of steroids: determination of in vitro permeability and tissue reservoir characteristics in human skin layers. Skin Pharmacol Physiol. 2006;19(6):336–342. | ||
Täuber A, Müller-Goymann CC. In vitro model of infected stratum corneum for the efficacy evaluation of poloxamer 407-based formulations of ciclopirox olamine against Trichophyton rubrum as well as differential scanning calorimetry and stability studies. Int J Pharm. 2015;494(1):304–311. | ||
Anissimov YG, Roberts MS. Diffusion modeling of percutaneous absorption kinetics: 3. Variable diffusion and partition coefficients, consequences for stratum corneum depth profiles and desorption kinetics. J Pharm Sci. 2004;93(2):470–487. | ||
Anissimov YG, Roberts MS. Diffusion modelling of percutaneous absorption kinetics: 4. Effects of a slow equilibration process within stratum corneum on absorption and desorption kinetics. J Pharm Sci. 2009;98(2):772–781. | ||
Zhang Q, Li P, Liu D, Roberts MS. Effect of vehicles on the maximum transepidermal flux of similar size phenolic compounds. Pharm Res. 2013;30(1):32–40. | ||
Raney SG, Franz TJ, Lehman PA, Lionberger R, Chen ML. Pharmacokinetics-based approaches for bioequivalence evaluation of topical dermatological drug products. Clin Pharmacokinet. 2015;54(11):1095–1106. | ||
Lehman PA, Franz TJ. Assessing topical bioavailability and bioequivalence: a comparison of the in vitro permeation test and the vasoconstrictor assay. Pharm Res. 2014;31(12):3529–3537. | ||
Hadgraft J, Beutner D, Wolff HM. In vivo-in vitro comparisons in the transdermal delivery of nitroglycerin. Int J Pharm 1993;89(1):R1–R4. | ||
Skelly JP, Shah VP, Maibach HI, et al. FDA and AAPS report of the workshop on principles and practices of in vitro percutaneous penetration studies: relevance to bioavailability and bioequivalence. Pharm Res. 1987;4(3):265–267. | ||
Shah V. Topical Dermatological Drug Product NDAs and ANDAs: In Vivo Bioavailability, Bioequivalence, In Vitro Release and Associated Studies. Rockville (MD): US Dept of Health and Human Services; 1998:1–19. | ||
Alberti I, Kalia YN, Naik A, Bonny JD, Guy RH. In vivo assessment of enhanced topical delivery of terbinafine to human stratum corneum. J Control Release. 2001;71(3):319–327. | ||
Nicoli S, Bunge AL, Delgado-Charro MB, Guy RH. Dermatopharmacokinetics: factors influencing drug clearance from the stratum corneum. Pharm Res. 2009;26(4):865–871. | ||
Stenken JA, Church MK, Gill CA, Clough GF. How minimally invasive is microdialysis sampling? A cautionary note for cytokine collection in human skin and other clinical studies. AAPS J. 2010;12(1):73–78. | ||
Narkar Y. Bioequivalence for topical products: an update. Pharm Res. 2010;27(12):2590–2601. | ||
Sun L, Stenken JA. Improving microdialysis extraction efficiency of lipophilic eicosanoids. J Pharm Biomed Anal. 2003;33(5):1059–1071. | ||
Benfeldt E, Hansen SH, Vølund A, Menné T, Shah VP. Bioequivalence of topical formulations in humans: evaluation by dermal microdialysis sampling and the dermatopharmacokinetic method. J Invest Dermatol. 2007;127(1):170–178. | ||
Bodenlenz M, Aigner B, Dragatin C, et al. Clinical applicability of dOFM devices for dermal sampling. Skin Res Technol. 2013;19(4):474–483. | ||
Gray G, Yardley H. Lipid compositions of cells isolated from pig, human, and rat epidermis. J Lipid Res. 1975;16(6):434–440. | ||
Wester RC, Melendres J, Sedik L, Maibach H, Riviere JE. Percutaneous absorption of salicylic acid, theophylline, 2, 4-dimethylamine, diethyl hexyl phthalic acid, and p-aminobenzoic acid in the isolated perfused porcine skin flap compared to man in vivo. Toxicol Appl Pharmacol. 1998;151(1):159–165. | ||
Jacobi U, Kaiser M, Toll R, et al. Porcine ear skin: an in vitro model for human skin. Skin Res Technol. 2007;13(1):19–24. | ||
Wester RC, Maibach HI. In vivo methods for percutaneous absorption measurements. J Toxicol Cutaneous Ocul Toxicol. 2001;20(4):411–422. | ||
Lademann J, Richter H, Meinke M, Sterry W, Patzelt A. Which skin model is the most appropriate for the investigation of topically applied substances into the hair follicles? Skin Pharmacol Physiol. 2010;23(1):47–52. | ||
Caussin J, Gooris GS, Janssens M, Bouwstra JA. Lipid organization in human and porcine stratum corneum differs widely, while lipid mixtures with porcine ceramides model human stratum corneum lipid organization very closely. Biochim Biophys Acta. 2008;1778(6):1472–1482. | ||
Wertz P, Downing D, Goldsmith L. Physiology, Biochemistry, and Molecular Biology of the Skin. Oxford: Oxford University Press; 1991. | ||
Dick IP, Scott RC. Pig ear skin as an in-vitro model for human skin permeability. J Pharm Pharmacol. 1992;44(8):640–645. | ||
Singh S, Zhao K, Singh J. In vitro permeability and binding of hydrocarbons in pig ear and human abdominal skin. Drug Chem Toxicol. 2002;25(1):83–92. | ||
Sato K, Sugibayashi K, Morimoto Y. Species differences in percutaneous absorption of nicorandil. J Pharm Sci. 1991;80(2):104–107. | ||
Nicoli S, Padula C, Aversa V, et al. Characterization of rabbit ear skin as a skin model for in vitro transdermal permeation experiments: histology, lipid composition and permeability. Skin Pharmacol Physiol. 2008;21(4):218–226. | ||
Lampe MA, Williams ML, Elias PM. Human epidermal lipids: characterization and modulations during differentiation. J Lipid Res. 1983;24(2):131–140. | ||
Scheuplein RJ, Blank IH. Permeability of the skin. Physiol Rev. 1971;51(4):702–747. | ||
Caussin J, Gooris GS, Janssens M, Bouwstra JA. Lipid organization in human and porcine stratum corneum differs widely, while lipid mixtures with porcine ceramides model human stratum corneum lipid organization very closely. Biochim Biophys Acta. 2008;1778(6):1472–1482. | ||
Sekkat N, Kalia YN, Guy RH. Porcine ear skin as a model for the assessment of transdermal drug delivery to premature neonates. Pharm Res. 2004;21(8):1390–1397. | ||
Simon GA, Maibach HI. Relevance of hairless mouse as an experimental model of percutaneous penetration in man. Skin Pharmacol Physiol. 1998;11(2):80–86. | ||
Barber E, Teetsel NM, Kolberg KF, Guest D. A comparative study of the rates of in vitro percutaneous absorption of eight chemicals using rat and human skin. Fundam Appl Toxicol. 1992;19(4):493–497. | ||
Chowhan ZT, Pritchard R. Effect of surfactants on percutaneous absorption of naproxen I: comparisons of rabbit, rat, and human excised skin. J Pharm Sci. 1978;67(9):1272–1274. | ||
Hughes MF, Edwards BC. In vitro dermal absorption of pyrethroid pesticides in human and rat skin. Toxicol Appl Pharmacol. 2010;246(1):29–37. | ||
Schmook FP, Meingassner JG, Billich A. Comparison of human skin or epidermis models with human and animal skin in in-vitro percutaneous absorption. Int J Pharm. 2001;215(1–2):51–56. | ||
van Ravenzwaay B, Leibold E. A comparison between in vitro rat and human and in vivo rat skin absorption studies. Hum Exp Toxicol. 2004;23(9):421–430. | ||
Roberts JB. Use of squamate epidermis in percutaneous absorption studies: a review. J Toxicol Cutaneous Ocul Toxicol. 1986;5(4):319–324. | ||
Rigg PC, Barry BW. Shed snake skin and hairless mouse skin as model membranes for human skin during permeation studies. J Invest Dermatol. 1990;94(2):235–240. | ||
Megrab NA, Williams A, Barry B. Oestradiol permeation through human skin and silastic membrane: effects of propylene glycol and supersaturation. J Control Release. 1995;36(3):277–294. | ||
Organization for Economic Co-operation and Development. Guidance Notes on Dermal Absorption. 2011. Available from: https://www.oecd.org/chemicalsafety/testing/48532204.pdf. Accessed April 25, 2016. | ||
Flaten GE, Palac Z, Engesland A, Filipović-Grčić J, Vanić Ž, Škalko-Basnet N. In vitro skin models as a tool in optimization of drug formulation. Eur J Pharm Sci. 2015;75:10–24. | ||
Zhang J, Sun M, Fan A, Wang Z, Zhao Y. The effect of solute-membrane interaction on solute permeation under supersaturated conditions. Int J Pharm. 2013;441(1–2):389–394. | ||
Oliveira G, Hadgraft J, Lane ME. The role of vehicle interactions on permeation of an active through model membranes and human skin. Int J Cosmet Sci. 2012;34(6):536–545. | ||
Ng SF, Rouse JJ, Sanderson FD, Eccleston GM. The relevance of polymeric synthetic membranes in topical formulation assessment and drug diffusion study. Arch Pharm Res. 2012;35(4):579–593. | ||
Dabrowska AK, Rotaru GM, Derler S, et al. Materials used to simulate physical properties of human skin. Skin Res Technol. 2016;22(1):3–14. | ||
Kansy M, Senner F, Gubernator K. Physicochemical high throughput screening: parallel artificial membrane permeation assay in the description of passive absorption processes. J Med Chem. 1998;41(7):1007–1010. | ||
Ottaviani G, Martel S, Carrupt PA. Parallel artificial membrane permeability assay: a new membrane for the fast prediction of passive human skin permeability. J Med Chem. 2006;49(13):3948–3954. | ||
US Food and Drug Administration. Guidance for Industry: Nonsterile Semisolid Dosage Forms Scale-Up and Postapproval Changes – Chemistry, Manufacturing, and Controls: In Vitro Release Testing and In Vivo Bioequivalence Documentation. Rockville (MD): FDA; 1997. | ||
Shah VP, Elkins J, Lam SY, Skelly JP. Determination of in vitro drug release from hydrocortisone creams. Int J Pharm. 1989;53(1):53–59. | ||
Wu ST, Shiu GK, Simmons JE, Bronaugh RL, Skelly JP. In vitro release of nitroglycerin from topical products by use of artificial membranes. J Pharm Sci. 1992;81(12):1153–1156. | ||
Gallagher SJ, Trottet L, Carter TP, Heard CM. Effects of membrane type and liquid/liquid phase boundary on in vitro release of ketoprofen from gel formulations. J Drug Target. 2003;11(6):373–379. | ||
van Drongelen V, Danso MO, Mulder A, et al. Barrier properties of an N/TERT-based human skin equivalent. Tissue Eng Part A. 2014;20(21–22):3041–3049. | ||
Bernerd F, Asselineau D. An organotypic model of skin to study photodamage and photoprotection in vitro. J Am Acad Dermatol. 2008;58(5 Suppl 2):S155–S159. | ||
Schäfer-Korting M, Bock U, Diembeck W, et al. The use of reconstructed human epidermis for skin absorption testing: results of the validation study. Altern Lab Anim. 2008;36(2):161–187. | ||
Schreiber S, Mahmoud A, Vuia A, et al. Reconstructed epidermis versus human and animal skin in skin absorption studies. Toxicol In Vitro. 2005;19(6):813–822. | ||
van der Weyden L, Patton EE, Wood GA, et al. Cross-species models of human melanoma. J Pathol. 2016;238(2):152–165. | ||
Jin H, He R, Oyoshi M, Geha RS. Animal models of atopic dermatitis. J Invest Dermatol. 2009;129(1):31–40. | ||
Schön MP. Animal models of psoriasis: a critical appraisal. Exp Dermatol. 2008;17(8):703–712. | ||
Scharfenberger L, Hennerici T, Király G, Kitzmüller S, Vernooij M, Zielinski JG. Transgenic mouse technology in skin biology: generation of complete or tissue-specific knockout mice. J Invest Dermatol. 2014;134(1):1–5. | ||
Elias PM, Arbiser J, Brown BE, et al. Epidermal vascular endothelial growth factor production is required for permeability barrier homeostasis, dermal angiogenesis, and the development of epidermal hyperplasia: implications for the pathogenesis of psoriasis. Am J Pathol. 2008;173(3):689–699. | ||
Rossbach K, Schaper K, Kloth C, et al. Histamine H4 receptor knockout mice display reduced inflammation in a chronic model of atopic dermatitis. Allergy. 2016;71(2):189–197. | ||
Burns EM, Tober KL, Riggenbach JA, et al. Preventative topical diclofenac treatment differentially decreases tumor burden in male and female Skh-1 mice in a model of UVB-induced cutaneous squamous cell carcinoma. Carcinogenesis. 2013;34(2):370–377. | ||
Cozzi SJ, Le TT, Ogbourne SM, James C, Suhrbier A. Effective treatment of squamous cell carcinomas with ingenol mebutate gel in immunologically intact SKH1 mice. Arch Dermatol Res. 2013;305(1):79–83. | ||
Singh A, Singh A, Sand JM, et al. Topically applied Hsp90 inhibitor 17AAG inhibits UVR-induced cutaneous squamous cell carcinomas. J Invest Dermatol. 2015;135(4):1098–1107. | ||
Sonavane K, Phillips J, Ekshyyan O, et al. Topical curcumin-based cream is equivalent to dietary curcumin in a skin cancer model. J Skin Cancer 2012;2012:147863. | ||
Viros A, Hayward R, Martin M, et al. Topical 5-fluorouracil elicits regressions of BRAF inhibitor-induced cutaneous squamous cell carcinoma. J Invest Dermatol. 2013;133(1):274–276. | ||
Wang H, Li J, Lv T, Tu Q, Huang Z, Wang X. Therapeutic and immune effects of 5-aminolevulinic acid photodynamic therapy on UVB-induced squamous cell carcinomas in hairless mice. Exp Dermatol. 2013;22(5):362–363. | ||
Filocamo G, Brunetti M, Colaceci F, et al. MK-4101 – a potent inhibitor of the Hedgehog pathway – is highly active against medulloblastoma and basal cell carcinoma. Mol Cancer Ther. 2016;15(6):1177–1189. | ||
Larsimont JC, Youssef KK, Sanchez-Danes A, et al. Sox9 controls self-renewal of oncogene targeted cells and links tumor initiation and invasion. Cell Stem Cell. 2015;17(1):60–73. | ||
Tang T, Tang JY, Li D, et al. Targeting superficial or nodular basal cell carcinoma with topically formulated small molecule inhibitor of Smoothened. Clin Cancer Res. 2011;17(10):3378–3387. | ||
Avci P, Sadasivam M, Gupta A, et al. Animal models of skin disease for drug discovery. Expert Opin Drug Discov. 2013;8(3):331–355. | ||
Kundu-Raychaudhuri S, Chen YJ, Wulff H, Raychaudhuri SP. Kv1.3 in psoriatic disease: PAP-1, a small molecule inhibitor of Kv1.3 is effective in the SCID mouse psoriasis – xenograft model. J Autoimmun. 2014;55:63–72. | ||
Salton M, Kasprzak WK, Voss T, Shapiro BA, Poulikakos PI, Misteli T. Inhibition of vemurafenib-resistant melanoma by interference with pre-mRNA splicing. Nat Commun. 2015;6:7103. | ||
Yue L, Huang ZM, Fong S, et al. Targeting ALDH1 to decrease tumorigenicity, growth and metastasis of human melanoma. Melanoma Res. 2015;25(2):138–148. | ||
Chiricozzi A, Nograles KE, Johnson-Huang LM, et al. IL-17 induces an expanded range of downstream genes in reconstituted human epidermis model. PLoS One. 2014;9(2):e90284. | ||
Tjabringa G, Bergers M, van Rens D, de Boer R, Lamme E, Schalkwijk J. Development and validation of human psoriatic skin equivalents. Am J Pathol. 2008;173(3):815–823. | ||
Pendaries V, Malaisse J, Pellerin L, et al. Knockdown of filaggrin in a three-dimensional reconstructed human epidermis impairs keratinocyte differentiation. J Invest Dermatol. 2014;134(12):2938–2946. | ||
Rouaud-Tinguely P, Boudier D, Marchand L, et al. From the morphological to the transcriptomic characterization of a compromised three-dimensional in vitro model mimicking atopic dermatitis. Br J Dermatol. 2015;173(4):1006–1014. | ||
Engelhart K, El Hindi T, Biesalski HK, Pfitzner I. In vitro reproduction of clinical hallmarks of eczematous dermatitis in organotypic skin models. Arch Dermatol Res. 2005;297(1):1–9. | ||
Li L, Fukunaga-Kalabis M, Herlyn M. The three-dimensional human skin reconstruct model: a tool to study normal skin and melanoma progression. J Vis Exp. 2011;(54):2937. | ||
Mohapatra S, Coppola D, Riker AI, Pledger WJ. Roscovitine inhibits differentiation and invasion in a three-dimensional skin reconstruction model of metastatic melanoma. Mol Cancer Res. 2007;5(2):145–151. | ||
Vörsmann H, Groeber F, Walles H, et al. Development of a human three-dimensional organotypic skin-melanoma spheroid model for in vitro drug testing. Cell Death Dis. 2013;4(7):e719. | ||
Commandeur S, Drongelen V, Gruijl FR, El Ghalbzouri A. Epidermal growth factor receptor activation and inhibition in 3D in vitro models of normal skin and human cutaneous squamous cell carcinoma. Cancer Sci. 2012;103(12):2120–2126. | ||
Luchetti MM, Moroncini G, Escamez MJ, et al. Induction of scleroderma fibrosis in skin-humanized mice by anti-platelet-derived growth factor receptor agonistic autoantibodies. Arthritis Rheumatol. Epub 2016 Apr 25. | ||
Koga H, Nanjoh Y, Kaneda H, Yamaguchi H, Tsuboi R. Short-term therapy with luliconazole, a novel topical antifungal imidazole, in guinea pig models of tinea corporis and tinea pedis. Antimicrob Agents Chemother. 2012;56(6):3138–3143. | ||
Andersen F, Hedegaard K, Petersen TK, Bindslev-Jensen C, Fullerton A, Andersen KE. The hairless guinea-pig as a model for treatment of cumulative irritation in humans. Skin Res Technol. 2006;12(1):60–67. | ||
Schröder H, Komljenovic D, Hecker M, Korff T. Transdermal drug targeting and functional imaging of tumor blood vessels in the mouse auricle. FASEB J. 2016;30(2):923–932. | ||
Chen Y, Wu Q, Zhang Z, Yuan L, Liu X, Zhou L. Preparation of curcumin-loaded liposomes and evaluation of their skin permeation and pharmacodynamics. Molecules. 2012;17(5):5972–5987. | ||
Yu X, Du L, Zhu L, et al. Melanoma therapy with transdermal mitoxantrone cubic phases. Drug Deliv. 2016;23(5):1565–1570. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


