Back to Journals » Clinical Ophthalmology » Volume 9
Scotoma analysis of 10–2 visual field testing with a red target in screening for hydroxychloroquine retinopathy
Authors Browning D , Lee C
Received 3 May 2015
Accepted for publication 10 June 2015
Published 20 August 2015 Volume 2015:9 Pages 1499—1509
DOI https://doi.org/10.2147/OPTH.S87850
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
David J Browning, Chong Lee
Department of Ophthalmology, Charlotte Eye, Ear, Nose and Throat Associates, Charlotte, NC, USA
Objective: To quantify the variability of scotomas detected by 10–2 visual field (VF) testing with a red target in patients taking hydroxychloroquine without and with retinopathy.
Design: Retrospective review of clinical charts and VFs.
Methods: Twenty-four patients taking hydroxychloroquine without retinopathy, and eight patients taking hydroxychloroquine with retinopathy were tested in this study. Retinopathy was defined by annular scotomas on 10–2 VF testing with corroborative spectral domain optical coherence tomographic outer retinal changes and multifocal electroretinographic changes leading to cessation of hydroxychloroquine or chloroquine. Location and depth of scotoma points on 10–2 VF testing were recorded and their fates followed in serial, reliable 10–2 VFs performed with a red target over time. The main outcome measures for this study were the number of scotoma points and locations, percentage of persistent scotoma points, size of scotomas, location of scotomas, and percentage of scotomas deepening.
Results: A median of 3, interquartile range (IQR) (2, 5), scotoma points per VF occurred in patients without retinopathy. A median of 86%, IQR (77, 100), of these resolved on the subsequent field. For patients with retinopathy, a median of 50%, IQR (46, 79), resolved, a difference compared to patients without retinopathy that was significant (P=0.0158). The median percentage of scotoma points in the zone from 2° to 8° from fixation in eyes with retinopathy was 72%, IQR (54, 100), compared to 49%, IQR (40, 54), in eyes without retinopathy (P=0.0069). The number of persistent scotoma locations at the last visit was higher in eyes with retinopathy: 3, IQR (1, 3), versus 0, IQR (0, 1), in patients without retinopathy, P=0.0156.
Conclusion: Point scotomas are common and variable in 10–2 VF testing with a red target for hydroxychloroquine retinopathy in subjects without retinopathy. Scotoma points in eyes with retinopathy are less variable. The annular zone 2°–8° from fixation was useful for distinguishing the significance of scotoma points. Discriminating eyes with retinopathy from eyes without retinopathy is probably easier using the 10–2 VF with a white target than a red target.
Keywords: ideal body weight, toxicity, red test object, ancillary testing
Introduction
Screening for hydroxychloroquine retinopathy is dependent on ancillary testing as the clinical examination is insensitive and nonspecific for retinopathy.1 Since the early 1990s, the 10–2 visual field (VF) has been the most commonly used ancillary test.2–4 Multifocal electroretinography (mfERG), spectral domain optical coherence tomography (SD-OCT), and fundus autofluorescence (FAF) have been introduced more recently, and gained wider acceptance when advocated in the revised guidelines of the American Academy of Ophthalmology, but they are still not used as often as 10–2 VF, and add additional cost to screening with unproven added benefit.5–10
A reservation regarding VF testing has been its variability. Discriminating a change representing development or progression of retinopathy rather than test variability has been difficult.11 Part of the variability stems from the subjective nature of the test, dependent on the cooperation of the patient and on the expertise and consistency of the test operator.1 This variability has been a force driving the adoption of the other tests, which have been called objective tests.
Given the widespread use of 10–2 VF testing, it is remarkable that only one study has quantified the variability of the scotomas detected by the test, and this study focused on the 10–2 VF with a white target.12 Many clinicians use the 10–2 VF with a red target instead. This study was designed to assess and report on the variability of scotomas detected by 10–2 VF testing using a red target in patients taking hydroxychloroquine who do and do not have retinopathy. The aim is to assist practitioners who use these tests in their interpretations.
Methods
This was a retrospective study of 24 patients taking hydroxychloroquine without retinopathy who took the drug throughout the study, and eight patients who took hydroxychloroquine, developed retinopathy, and had their drugs stopped. All patients received the medications for autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis. Retinopathy was defined by annular scotomas on 10–2 VF testing with corroborative spectral domain optical coherence tomographic outer retinal changes and multifocal electroretinographic changes leading to cessation of hydroxychloroquine. All patients had VF testing using the Humphrey Field Analyzer (Carl Zeiss Meditec AG, Jena, Germany) using the10–2 program, which tests 68 points within 10° of fixation. The points tested occur at eccentricities of 1°, 3°, 5°, 7°, and 9° from fixation. It can be performed with a size III (4 mm2 in area) red or white target. The background color is white with luminance of 10 cd/m2. The target is presented for 0.2 seconds. The 10–2 VF test with a red target is the subject of this study.
All patients had at least two consecutive reliable VFs. Unreliable fields for which fixation losses were 20% or greater were excluded from analysis. Clinical information was extracted from the patients’ charts, including height, weight, renal and liver functional status, presence of preexisting maculopathy, daily dose of drug, duration of drug use, cumulative dose of drug, and use of tamoxifen. Ideal body weights were calculated from the algorithm of the National Heart Lung and Blood Institute.13–15 The adjusted daily dose was calculated as the daily dose divided by the lesser of the actual weight and ideal weight expressed in kilograms.10 Information extracted from the VFs included foveal sensitivity, and location, depth, and persistence of scotomas. The patients were managed in a private, multispecialty practice having 32 ophthalmologists and four optometrists. Waiver of informed consent and waiver of Health Insurance Portability and Accountability Act authorization were approved by the Presbyterian Hospital Institutional Review Board.
Several terms used repeatedly in the text are defined:
- Location: one of the 68 points in the 10–2 VF, which can be specified by an (x,y) coordinate; for example, (1,1) refers to the location in the northeast quadrant of the 10–2 VF that is 1 degree to the right and 1 degree superior to the origin.
- Scotoma point: for a 10–2 VF with a red target, a scotoma point is any point for which the retinal sensitivity is decreased more than 4 dB compared to a normal value for that location. The gradations of abnormality are continuous as given by the decrease in sensitivity in decibels compared to the normal value for that location.
- Scotoma location: a scotoma location refers to a position in the grid of 68 points for a 10–2 VF where a scotoma point occurred in at least one VF. It differs from a scotoma point in that a scotoma point can come and go from VF to VF in a series of VFs taken over time. Thus, it is possible to have two separate scotoma points at a single scotoma location if the point was a scotoma during one test, resolved at the next testing, and reappeared at a third testing. It follows that the number of scotoma locations will be less than or equal to the number of scotoma points.
- Scotoma: one or more contiguous scotoma points. Scotoma points that are adjacent to each other belong to the same scotoma. It follows that the number of scotomas will always be less than the number of scotoma points.
- High-risk scotoma point loci: locations from 2° to 8° from fixation in the 10–2 VF. Locations from 2° to 8° from fixation are those where toxicity typically first appears.1
- Low-risk scotoma point loci: locations <2° and >8° from fixation in the 10–2 VF. Locations in these zones may be involved in more advanced toxicity, but are typically not the locations involved early.1
- Low-reliability VF: a field with greater than or equal to 20% fixation losses is considered to have low reliability.16,17 For this study, only reliable VFs were included in the analysis. We calculated parallel analyses with all VFs (ie, including the ones labeled low reliability) because in clinical practice many physicians are not meticulous about excluding the low-reliability VFs from consideration. No important differences were found between analyses with only reliable VFs and all VFs.
- VF quadrants: SN is the superonasal quadrant, ST the superotemporal quadrant, IN the inferonasal quadrant, and IT the inferotemporal quadrant in the 10–2 VF.
- Total number of points tested: the overall number of reliable VFs multiplied by 68 location points for each VF (ie, including all normal and abnormal points).
- Total number of scotoma locations: the sum of abnormal locations found in all reliable VFs whether the scotoma point resolved or not.
- Total number of scotoma points: the sum of abnormal tested points found in all reliable VFs. This consists of scotoma points that resolved after one appearance and those that persisted for multiple VFs.
- Average number of scotoma points per VF: a ratio equal to the total number of scotoma points divided by the total number of reliable 10–2 VFs.
- Number of (percentage of) scotoma points that resolved: the number of (percentage of) points that were scotomatous in one testing and normal in the next testing. If a scotoma point appeared at one testing, resolved in the next testing, and reappeared in the third testing without any further persistence in later VFs, it is considered a scotoma location associated with two resolving scotoma points. The percentage is taken with the denominator equal to the total number of scotoma points.
- Number of (percentage of) persistent scotoma locations: the number of (percentage of) of all locations associated with a scotoma point that persisted on more than one VF test. The percentage is taken with the denominator equal to the total number of scotoma locations.
- Number of (percentage of) persistent scotoma points: the number of scotoma points that remained scotomatous for at least two consecutive VFs. The percentage is taken with the denominator equal to the total number of scotoma points.
- Persistent scotomas present at last visit: a number of scotoma locations on the last (ie, most current) 10–2 VF that were also scotomatous at the previous VF.
- Total number of scotomas: the sum of all scotomas, regardless of size, found in all of the tested VFs. In every 10–2 VF, the scotoma points were grouped into 1-point, 2-point, 3-point, 4-point, or larger than 4-point scotomas. All the 1-point scotomas from each VF test were added. This was done for the 2-point, 3-point, 4-point, and larger than 4-point scotomas as well. From this, the different size scotomas were added to get the total number of scotomas.
- 1-point scotoma: a scotoma consisting of one isolated scotoma point on a particular VF.
- 2-point scotoma: a scotoma consisting of two neighboring scotoma points.
- 3-point scotoma: a scotoma consisting of three neighboring scotoma points.
- 4-point scotoma: a scotoma consisting of four neighboring scotoma points.
- Larger than 4-point scotomas: a scotoma consisting of more than four neighboring scotoma points.
- Number of scotoma points in the SN VF: the number of scotoma points, ie, both resolved and persisting, found in the SN of the 10–2 VF. Definitions of scotoma points in ST, IN, and IT are analogous.
- Number of persistent scotoma points deepening by ≤5 dB: the number of persistent scotoma points for which the retinal sensitivity decreased by ≤5 dB from one test to the next. Analogous definitions apply for the following phrases: number of persistent scotoma points deepening by 6–10 dB; number of persistent scotomas shallowing by ≤5 dB; and number of persistent scotoma points shallowing by 6–10 dB.
To avoid problems of correlated results between eyes, only one eye was included per patient.18 When only one of two eyes had non-confounded testing (eg, other pathology such as old retinal detachment interfered with testing the fellow eye), that eye was chosen. When two eyes had non-confounded testing, a random number generator was used to pick which of the two was included. Statistical analysis was performed with JMP 4.0 software (SAS Institute Inc., Cary, NC, USA). The Kruskal–Wallis test was used for nonparametric comparisons of distributions of values between retinopathy and no-retinopathy groups. Fisher’s exact test was used to compare proportions. Alpha was chosen to be 5% in statistical testing with no adjustment for number of tests as the primary function of a retrospective study is hypothesis generation.
Results
All patients were female (Table 1). Patients with retinopathy tended to be older (Table 1). The median daily dose of hydroxychloroquine was 400 mg for both groups (Table 1). The median duration of therapy and median cumulative dose were greater for patients with retinopathy (Table 1). The adjusted daily dose was higher in patients with retinopathy (Table 1). The percentage of patients receiving toxic dosing was higher in patients with retinopathy (Table 1). The median numbers of VFs and reliable VFs were similar between no-retinopathy and retinopathy patients in the two groups (Table 1). No patient was taking tamoxifen.
The percentage of scotoma points that persisted was higher for eyes with retinopathy. The median percentages of persistent scotoma points were 14%, interquartile range (IQR) (0, 23), and 50%, IQR (21, 54), for the no-retinopathy and retinopathy groups, respectively, which was statistically significant (P=0.0158). Likewise, the percentage of scotoma points that fell in the high-risk zone was greater for eyes with retinopathy. The median percentages of scotoma points in the high-risk zone were 49%, IQR (40, 54), and 72%, IQR (54, 100), for the no-retinopathy and retinopathy groups, respectively, which was statistically significant (P=0.0069).
In addition, the number of persistent scotoma locations at the last visit was greater for eyes with retinopathy than for eyes without. The median numbers of persistent scotoma locations at the last visit were 0, IQR (0, 1), and 3, IQR (1, 3), for the no-retinopathy and retinopathy groups, respectively, which was statistically significant (P=0.0156).
No differences were seen between the number of scotoma locations and points in eyes without or with retinopathy, nor in the average number of scotoma points per VF (Table 2). There were also no differences in the ratio of number of scotoma locations to number of scotoma points in eyes without and with retinopathy (Table 2). The percentage of larger scotomas was not different between eyes without and with retinopathy (Table 2). The percentage of persistent scotomas deepening over consecutive visits was not different between eyes with and without retinopathy (Table 2).
  | Table 2 10–2 VF outcomes |
The percentage of scotoma points was higher in the SN and lower in the IN for eyes with retinopathy compared to eyes without retinopathy, but no significant differences between groups were noted for the other quadrants (Table 2).
Clinical examples
Two representative case studies illustrate the results found in the study.
Case 1
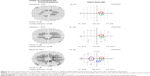
A 40-year-old woman with undifferentiated connective tissue disease had been taking hydroxychloroquine for 7.7 years. She was originally on 400 mg/day but more recently was taking 300 mg/day. Her height was 162.6 cm, her ideal body weight was 63.5 kg, her actual body weight was 65.8 kg, and her cumulative dose was 1,124 g. Her adjusted daily dose was 6.29 mg/kg/d based on her ideal body weight, which is in a range recognized as nontoxic. She had no renal or liver disease and no preexisting maculopathy. Figure 1 shows four consecutive 10–2 VFs performed with a red target. The first VF (January 25, 2007) had a single scotoma point on the defect depth display (circled in red) that resolved on the second field (February 12, 2008), which in turn had four new scotoma points (circled in green). Three of the four green-circled scotoma points occurred in the low-risk zone outside the 2°–8° annulus encircling the fixation point. In the third field (February 17, 2009), all four scotoma points circled in green had resolved, and remained so in the fourth field (March 22, 2010). As the patient had a low pretest probability of retinopathy (nontoxic daily dosing, no renal disease, and no liver disease), no intensified testing was done. Figure 2 shows that in 2014, she had normal mfERG ancillary testing, corroborating the lack of evidence of retinopathy seen in all of the VFs.
Case 2
A 69-year-old woman had been taking hydroxychloroquine for rheumatoid arthritis for 17 years. She had been taking 400 mg/day the entire time. She was 160.0 cm, had an ideal body weight of 61.2 kg, but an actual body weight of 64.9 kg. Therefore, her adjusted daily dose was in a toxic range of 6.52 mg/kg/d for the duration of therapy. Her cumulative dose was 2,450 g. She had no renal or liver disease, nor any preexisting maculopathy. Figure 3 shows three consecutive 10–2 VFs with a red target. The first field on January 28, 2009 shows an annular ring scotoma on the gray scale display. The defect depth display shows a single scotoma point (green circle). The red-circled point was not scotomatous in this display. The second VF on February 1, 2010 shows that the green-circled scotoma point persisted and the adjacent red-circled point had now become scotomatous (ie, the scotoma was reproducible and has enlarged). By the third VF of March 28, 2011, the red-circled scotoma point had deepened by 4 dB, the scotoma had enlarged to include the adjacent blue-circled scotoma point, and a separate scotoma (purple-circled) in the high-risk zone from 2° to 8° from fixation had developed. At this point, the patient’s ophthalmologist recognized retinopathy and her hydroxychloroquine was stopped. Scotoma points associated with hydroxychloroquine retinopathy do not resolve, unlike the situation with patients taking hydroxychloroquine who do not have retinopathy. Moreover, the scotomas tend to broaden over time. Figure 4 shows that on October 25, 2012, there was marked diminution of the mfERG amplitudes in rings R1–R2.
Discussion
The characteristics of the patients studied resemble those of other series of patients taking hydroxychloroquine without and with retinopathy. All of the patients were female.1,19 The predominant risk factors manifest in patients with retinopathy were toxic adjusted daily dosing and high cumulative dosing.3,13,20,21
The variability of scotoma detection by standard automated perimetry has been documented in glaucoma22 and, by analogy and anecdotal observation, inferred in patients taking hydroxychloroquine without or with retinopathy. This variability is the source of the lower specificity reported for perimetry than for optical coherence tomography in hydroxychloroquine retinopathy.23
In the interpretation of 10–2 VFs, it has been written that any scotoma deserves to be taken seriously with follow-up actions triggered to determine if early retinopathy is being signaled.20 The results of this study indicate that this may be going beyond what the evidence justifies. Evanescent, isolated scotoma points are common among patients taking hydroxychloroquine with no evidence of retinopathy. On average, three such points are found in every 10–2 VF performed with a red target among patients taking hydroxychloroquine without retinopathy. If the patient has a low pretest probability of retinopathy based on correct dosing for ideal body weight, absence of renal and liver disease, and low-risk cumulative dose of drug, there is no reason to respond to isolated scotoma points on the 10–2 VF with actions such as having the patient return for earlier retesting. Since the revised American Academy of Ophthalmology guidelines of 2011, adding mfERG, SD-OCT, or FAF imaging is already accepted practice, though unproven to result in improved outcomes. The presence of a scotoma point in a patient with a low pretest probability of disease is not necessarily a reason to add other ancillary tests, which also add cost.10
Previous work has suggested that 10–2 VF testing with the red target is more sensitive but less specific than 10–2 VF testing with the white target.24 Other work has emphasized the value of the pattern standard deviation (PSD), which is only displayed with the 10–2 VF using the white target.20 There is consistent agreement that the 10–2 VF protocol is preferable to the 24–2 or 30–2 protocols, which minimize the display of the VF in the high-risk zone 2°–8° from fixation.1,25
Certain characteristics of a 10–2 VF printout raise suspicion. For example, a cluster of scotoma points in the high-risk zone 2°–8° from fixation, a scotoma that persists and grows in breadth or depth, and the appearance of new scotomas should lead to further investigation. In most cases, the next advisable test would be SD-OCT, which has the highest reproducibility of any of the ancillary tests used for screening hydroxychloroquine retinopathy.1 mfERG has a high test–retest variability, making it less useful for independent risk assessment.26 Likewise, FAF is highly subjective in interpretation, and is less useful.1 However, in a difficult case, both mfERG and FAF can be useful adjunctive ancillary tests.
The results of 10–2 VF testing with a red target are not the same as with a white target.12 With a white target, the numbers of scotoma locations and scotoma points are consistently higher in eyes with than in eyes without hydroxychloroquine retinopathy.12 The ratio of scotoma locations to scotoma points was consistently higher in eyes without retinopathy when the 10–2 VF with white target was used.12 Such was not the case when VFs were done with a red target as in this study. The two studies involved different samples of patients, but there is no reason to believe that the results obtained were dependent on the peculiarities of the particular samples.
Certain indices present in the 10–2 VF with white target printout are not given for the 10–2 VF with red target, including the mean defect and pattern standard deviation.3,4,27,28 These are useful indices that have been shown to differ, on average, between eyes with and without retinopathy.12 Other evidence suggests that the 10–2 VF with a white target better separates eyes with and without retinopathy compared to the 10–2 VF with a red target. The number of scotoma locations, number of scotoma points, ratio of number of scotoma locations to scotoma points, average number of scotoma points per VF, percentage of 1-point scotomas, percentage of larger than 4-point scotomas, and percentage of persistent scotomas that deepen are all indices that separate toxic from nontoxic eyes with the white target but not the red target.12 For this reason, we recommend the 10–2 VF with a white target as the preferred static automated perimetric test for hydroxychloroquine retinopathy screening and staging.
This work has limitations including its retrospective methodology and relatively small number of subjects. Comparisons of performance of the 10–2 VF with red and white targets have been made across studies with different samples. Nevertheless, it examines the issue of variability of the 10–2 VF test in this setting for which only anecdotal observations have been provided previously. Larger, prospective studies would be welcome to explore the topic with greater depth and reliability.
Conclusion
In conclusion, isolated scotoma points are common in patients taking hydroxychloroquine without retinopathy, and usually resolve from one test to the next. There is little need to react to their presence with additional testing and shortened follow-up intervals as long as the pretest probability of retinopathy is low based on consideration of retinopathy risk factors such as adjusted daily dose and cumulative dose. However, selective use of ancillary tests other than the 10–2 VF is worthwhile for detecting retinopathy in patients with clinical profiles indicating higher risk.
Disclosure
The authors report no conflicts of interest in this work.
References
Browning DJ. Hydroxychloroquine and Chloroquine Retinopathy. New York: Springer; 2014. | ||
Johnson MW, Vine AK. Hydroxychloroquine therapy in massive total doses without retinal toxicity. Am J Ophthalmol. 1987;104:139–144. | ||
Mititelu M, Wong BJ, Brenner M, Bryar PJ, Jampol LM, Fawzi AA. Progression of hydroxychloroquine toxic effects after drug therapy cessation. new evidence from multimodal imaging. JAMA Ophthalmol. 2013;131:1187–1197. | ||
Xiaoyun MA, Dongyi HE, Linping HE. Assessing chloroquine toxicity in RA patients using retinal nerve fiber layer thickness, multifocal electroretinography and visual field test. Br J Ophthalmol. 2010;94:1632–1636. | ||
Lai TY, Chan WM, Li H, Lai RY, Lam DS. Multifocal electroretinographic changes in patients receiving hydroxychloroquine therapy. Am J Ophthalmol. 2005;140:794–807. | ||
Lyons JS, Severns ML. Detection of early hydroxychloroquine retinal toxicity enhanced by ring ratio analysis of multifocal electroretinography. Am J Ophthalmol. 2007;143:801–809. | ||
Chen E, Brown DM, Benz MS, et al. Spectral domain optical coherence tomography as an effective screening test for hydroxychloroquine retinopathy (the “flying saucer” sign). Clin Ophthalmol. 2010;4:1151–1158. | ||
Kellner U, Renner AB, Tillack H. Fundus autofluorescence and mfERG for early detection of retinal alterations in patients using chloroquine/ hydroxychloroquine. Invest Ophthalmol Vis Sci. 2006;47:3531–3538. | ||
Marmor MF, Kellner U, Lai TY, Lyons JS, Mieler WF; American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118:415–422. | ||
Browning DJ. Impact of the revised american academy of ophthalmology guidelines regarding hydroxychloroquine screening on actual practice. Am J Ophthalmol. 2013;155:418–428.e1. | ||
Lyons JS, Severns ML. Using multifocal ERG ring ratios to detect and follow Plaquenil retinal toxicity: a review : Review of mfERG ring ratios in Plaquenil toxicity. Doc Ophthalmol. 2009;118:29–36. | ||
Browning DJ, Lee C. Scotoma analysis of 10–2 visual field testing with a white target in screening for hydroxychloroquine retinopathy. Clin Ophthalmol. 2015;9:943–952. | ||
Browning DJ. Hydroxychloroquine and chloroquine retinopathy: screening for drug toxicity. Am J Ophthalmol. 2002;133:649–656. | ||
Browning DJ, Lee C, Rotberg D. The impact of different algorithms for ideal body weight on screening for hydroxychloroquine retinopathy in women. Clin Ophthalmol. 2014;8:1401–1407. | ||
Browning DJ. Reply to defining ideal body weight. Am J Ophthalmol. 2002;134:935–936. | ||
Rao HL, Yadav RK, Begum VU, et al. Role of visual field reliability indices in ruling out glaucoma. JAMA Ophthalmol. 2015;133:40–44. | ||
Heijl A, Patell VM. Statpac. In: Essential Perimetry Third Edition. Dublin, California: Carl Zeiss Meditec, 2002:57–58. | ||
Murdoch IE, Morris SS, Cousens SN. People and eyes: statistical approaches in ophthalmology. Br J Ophthalmol. 1998;82:971–973. | ||
Yam JC, Kwok AK. Ocular toxicity of hydroxychloroquine. Hong Kong Med J. 2006;12:294–304. | ||
Marmor MF, Chien FY, Johnson MW. Value of red targets and pattern deviation pots in visual field screening for hydroxychloroquine retinopathy. JAMA Ophthalmol. 2013;131:476–480. | ||
Wolfe F, Marmor MF. Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2010;62:775–784. | ||
Saunders LJ, Russell RA, Crabb DP. Measurement precision in a series of visual fields acquired by the standard and fast versions of the Swedish interactive thresholding algorithm: analysis of large-scale data from clinics. JAMA Ophthalmol. 2015;133:74–80. | ||
Browning DJ, Lee C. The relative sensitivity and specificity of 10–2 visual fields, multifocal electroretinography, and spectral domain optical coherence tomography in detecting hydroxychloroquine and chloroquine retinopathy. Clin Ophthalmol. 2014;8:1389–1399. | ||
Easterbrook M, Trope G. Value of Humprey perimetry in the detection of early chloroquine retinopathy. Lens Eye Toxic Res. 1989;6:255–268. | ||
Anderson C, Blaha GR, Marx JL. Humphrey visual field findings in hydroxychloroquine toxicity. Eye (Lond). 2011;25:1535–1545. | ||
Browning DJ, Lee C. Test-retest variability of multifocal electroretinography in normal volunteers and short-term variability in hydroxychloroquine users. Clin Ophthalmol. 2014;8:1467–1473. | ||
Lai TY, Ngai JW, Chan WM, Lam DS. Visual field and multifocal electroretinography and their correlations in patients on hydroxychloroquine therapy. Doc Ophthalmol. 2006;112:177–187. | ||
Tanga L, Centofanti M, Oddone F, et al. Retinal functional changes measured by frequency-doubling technology in patients treated with hydroxychloroquine. Graefes Arch Clin Exp Ophthalmol. 2011;249:715–721. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.