Back to Journals » International Journal of Nanomedicine » Volume 12
Role of poly(ε-caprolactone) lipid-core nanocapsules on melanoma–neutrophil crosstalk
Authors Drewes CC, Alves ACS , Hebeda CB, Copetti I, Sandri S, Uchiyama MK, Araki K , Guterres SS, Pohlmann, AR , Farsky SH
Received 27 April 2017
Accepted for publication 15 July 2017
Published 27 September 2017 Volume 2017:12 Pages 7153—7163
DOI https://doi.org/10.2147/IJN.S140557
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Carine C Drewes,1 Aline de CS Alves,2,3 Cristina B Hebeda,1 Isabela Copetti,2,3 Silvana Sandri,1 Mayara K Uchiyama,4 Koiti Araki,4 Silvia S Guterres,2 Adriana R Pohlmann,2,3 Sandra H Farsky1
1Department of Clinical and Toxicological Analyses, School of Pharmaceutical Sciences, University of São Paulo, São Paulo, 2Postgraduate Program in Pharmaceutical Sciences, 3Department of Organic Chemistry, Institute of Chemistry, Federal University of Rio Grande do Sul, Porto Alegre, 4Department of Fundamental Chemistry, Institute of Chemistry, University of São Paulo, São Paulo, Brazil
Abstract: Metastatic melanoma is an aggressive cancer with increasing incidence and limited therapies in advanced stages. Systemic neutrophilia or abundant neutrophils in the tumor contribute toward its worst prognosis, and the interplay of cancer and the immune system has been shown in tumor development and metastasis. We recently showed the in vivo efficacy of poly(ε-caprolactone) lipid-core nanocapsule (LNC) or LNC loaded with acetyleugenol (AcE-LNC) to treat B16F10-induced melanoma in mice. In this study, we investigated whether LNC or AcE-LNC toxicity could involve modifications on crosstalk of melanoma cells and neutrophils. Therefore, melanoma cells (B16F10) were pretreated with vehicle, LNC, AcE or AcE-LNC for 24 h, washed and, further, cocultured for 18 h with peritoneal neutrophils obtained from C57Bl/6 mice. Melanoma cells were able to internalize the LNC or AcE-LNC after 2 h of incubation. LNC or AcE-LNC pretreatments did not cause melanoma cells death, but led melanoma cells to be more susceptible to death in serum deprivation or hypoxia or in the presence of neutrophils. Interestingly, the production of reactive oxygen species (ROS), which causes cell death, was increased by neutrophils in the presence of LNC- and AcE-LNC-pretreated melanoma cells. LNC or AcE-LNC treatments reduced the concentration of transforming growth factor-β (TGF-β) in the supernatant of melanoma cells, a known factor secreted by cancer cells to induce pro-tumoral actions of neutrophils in the tumor microenvironment. In addition, we found reduced levels of pro-tumoral chemical mediators VEGF, arginase-1, interleukin-10 (IL-10) and matrix metalloproteinase-9 (MMP-9) in the supernatant of LNC or AcE-LNC-pretreated melanoma cells and cocultured with neutrophils. Overall, our data show that the uptake of LNC or AcE-LNC by melanoma cells affects intracellular mechanisms leading to more susceptibility to death and also signals higher neutrophil antitumoral activity.
Keywords: hypoxia, serum deprivation, apoptosis, coculture, tumor microenvironment, LNC, acetyleugenol, intravital microscopy
Introduction
Melanoma is a highly aggressive tumor and presents elevated metastatic index. It is the most severe form of skin cancer, being responsible for the majority of skin cancer deaths. The survival rate of patients with metastatic melanoma is 6–10 months, and only 20% of patients survive for about 5 years from the time of diagnosis.1–3 Melanoma is extremely resistant to the current therapies such as chemotherapy, radiotherapy, immunotherapy and/or target therapy.4–6 Therefore, the comprehension of the mechanisms underlying the disease is crucial for the establishment of novel and complementary therapeutic approaches.
Up to 90% of the tumor mass is composed of immune cells, and an interaction of tumor/stromal/immune cells, as well the interplay of chemical mediators secreted in the tumoral microenvironment, has been fully shown to regulate the tumor growth, survival and dissemination.7–12 The role of neutrophils in the cancer development was neglected for some time; nevertheless, accumulated clinical and experimental data have shown the relevance of local infiltration of neutrophils to tumorigenesis.13 Moreover, progressive malignant cancer is associated with blood neutrophilia and the elevated neutrophil–lymphocyte ratio (NLR), which is considered a powerful prognostic parameter in several human cancers.2 Neutrophils are recruited from the blood mainly responding to chemotactic agents secreted by surrounding or tumor cells, such as chemokine CXCL12, interleukin (IL)-17 and vascular endothelial growth factor (VEGF).14–17 In the tumor microenvironment, tumor-associated neutrophils (TANs) switch to phenotype differentiation, ie, anti-tumorigenic (N1) or pro-tumorigenic (N2). The differentiation is driven by type 1 interferon γ (IFNγ) and transforming growth factor-β (TGF-β), which polarize to N1 and N2 phenotype, respectively.18–20 Actions of N1 neutrophils cause cytotoxicity, tumor rejection and immune memory, as they secrete high levels of tumor necrosis factor-α (TNF-α), produce reactive oxygen species (ROS) and release lower levels of chemokine (C–C motif) ligand 2 (CCL2), VEGF and matrix metalloproteinase-9 (MMP-9).18,21 On the other hand, pro-tumoral N2 phenotype is characterized by high expression of CXCR4, arginase-1, CCL2 and MMP-9. This phenotype promotes tumor invasion and metastasis through proteolysis of extracellular matrix components, angiogenesis and immunosuppression, which allows the cancer to escape the immune response.18,21
Recently, the role of pro-tumoral neutrophils on melanoma development and metastasis has been shown.19,22–26 The presence of neutrophils in the melanoma microenvironment is related to induction of angiogenesis, angiotropism and metastasis risk, showing that neutrophil secretion or its interaction with melanoma cells modifies the phenotype of melanoma cells.26 Moreover, neutrophilia observed in metastatic melanoma27 contributes to restrict tumor cells in the lung microcirculation, facilitating the melanoma transendothelial migration into tissue.22 Therefore, manipulating neutrophil functions in melanoma cells may be a promising target for therapy. Indeed, enhancing N1 neutrophil activation in the tumor bed may result in significant anticancer effects.28
To establish novelty in melanoma therapy, we recently administered poly(ε-caprolactone) lipid-core nanocapsules (LNCs) loaded or not with acetyleugenol (AcE-LNC) to C57Bl/6 mice carrying B16F10-induced melanoma. The LNCs are composed of an organogel as oily core surrounded by a biodegradable polymeric wall, in which dispersion in water is guaranteed by surface coating with polysorbate 80 micelles.29 Oral administration of LNC or AcE-LNC, but not AcE, did not cause any toxicity and inhibited the melanoma development. In vitro studies showed that LNC or AcE-LNC caused late apoptosis and necrosis, as well as cell cycle arrest in SK-Mel-28 cells.30
Considering the relevance of neutrophils in melanoma development and metastasis, we, in this study, investigated the effects of LNC, AcE or AcE-LNC treatments on the crosstalk between melanoma cells and neutrophils. Our data show that melanoma cells pretreated with LNC or AcE-LNC are more susceptible to death in response to serum deprivation or hypoxia and secrete lower levels of TGF-β, a known chemical mediator secreted by cancer cells to halt antitumoral properties of neutrophils. Moreover, in vivo treatment of mice with LNC or AcE-LNC impairs the interaction of neutrophils with microcirculatory vessels, which may represent an additional mechanism of LNCs to inhibit the dissemination of melanoma cells.
Materials and methods
Reagents
Roswell Park Memorial Institute 1640 (RPMI 1640) and fetal bovine serum (FBS) were purchased from Vitrocell (Sao Paulo, Brazil). Ketamine and xylazine were obtained from Ceva Santé Animale (Paulínea, Brazil). Oyster glycogen, propidium iodide (PI), 2′,7′-dichlorofluorescin diacetate (DCFH-DA), trypan blue, CoCl2 and Ringer–Locke solution were obtained from Sigma-Aldrich Co. (St Louis, MO, USA). Anti-Ly6G antibody conjugated with phycoerythrin (PE) and annexin V (AnxV)–fluorescein isothiocyanate (FITC) were purchased from BD Biosciences (San Jose, CA, USA). Percoll was obtained from GE Healthcare Bio-Sciences AB (Uppsala, Sweden). Enzyme-linked immunosorbent assay (ELISA) kits were obtained as follows: TGF-β and IL-10 were obtained from R&D Systems, Inc. (Minneapolis, MN, USA); VEGF-A was obtained from IBL (Mikasa-Shi, Japan) and ELISA, arginase-1 and MMP-9 were obtained from MyBioSource (San Diego, CA, USA). Transwell insert plate was purchased from Costar (Cambridge, MA, USA).
LNC preparation
Synthesized AcE and LNCs containing or not containing AcE were prepared using a previously reported method,30 and the physicochemical characterization of the new batches of formulations was performed as previously described.30 LNC and AcE-LNC had mean particle diameters of 208±15 nm and 194±20 nm, with polydispersity indexes of 0.11±0.01 and 0.12±0.02, respectively. Particle number densities were 4.5±0.5×1012 and 3.6±0.4×1012 nanocapsules/mL, respectively.
Melanoma cells
B16F10 malignant melanoma cell line was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were grown in culture flasks for 5–10 passages and maintained in RPMI 1640 supplemented with 10% FBS (R10). Cells were kept at 37°C, with a minimum relative humidity of 95% and an atmosphere of 5% CO2 in air. Reverse transcription polymerase chain reaction (RT-PCR) was performed in the laboratory to check cell mycoplasma contamination, and B16F10 employed in this study was in perfect assay conditions (data not shown).
Animals
Male C57Bl/6 mice (25–30 g; 7–10 per group) were provided by the Central Animal House of School of Pharmaceutical Sciences and the Chemistry Institute of the University of São Paulo. Mice were housed in polycarbonate cages (four animals per cage; Tecniplast, Buguggiate, Italy) at room temperature (22°C±0.1°C) and humidity (50%±10%) with a 12 h light/dark cycle, receiving standard food and water ad libitum. Animals were anesthetized with a combination of ketamine:xylazine solution (20:2 mg/kg, intraperitoneal [i.p.]) before each experimental procedure. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the School of Pharmaceutical Sciences, University of São Paulo (protocol number: 309) and were performed according to the Brazilian Society of Science of Laboratory Animals guidelines for the proper care and use of experimental animals.
Collection and characterization of neutrophils
Neutrophils were obtained from adult mice 4 h after i.p. injection of 3 mL sterile 1% oyster glycogen solution, previously prepared in phosphate-buffered saline (PBS). The animals were anesthetized, and the cells were collected by rinsing the abdominal cavity with 10 mL of sterile culture medium. Cells were separated using a gradient of Percoll followed by centrifugation at 600× g for 20 min at 4°C. Neutrophils were washed once with 10 mL of culture medium, stained with trypan blue and observed under conventional light microscopy to evaluate their viability. Using anti-Ly6G antibody conjugated with PE, the neutrophil purity was >95% as observed by flow cytometry.
Melanoma cell culture and treatments
For cell viability assays, melanoma cells (1×104 cells/well) were seeded into 24-well plates and maintained in culture for 12 h for attachment. Then, the cells were treated with R10 (vehicle), LNC (9×109 particles/mL), AcE (30 μM) or AcE-LNC (30 μM of AcE; 9×109 particles/mL) for 24 h.
In another set of experiments, melanoma cells were incubated with CoCl2 (100 μM in culture medium) to induce hypoxia31 or with culture medium with serum deprivation (RPMI 1640 plus 1% of FBS) for 24 h. After this period, cells were washed three times with fresh culture medium and incubated with R10 (vehicle), LNC (9×109 particles/mL), AcE (30 μM) or AcE-LNC (30 μM of AcE; 9×109 particles/mL) for 24 h.
In vitro cellular uptake
The internalization of LNC or AcE-LNC by B16F10 cells was confirmed by enhanced dark-field hyperspectral microscopy. Melanoma cells (5×105 cells) were seeded in extra clean dust-free Nexterion® Glass D coverslips (#D263T; Schott, New York, NY, USA) present in 12-well plates. After adherence, cells were incubated with medium containing LNC (9×109 particles/mL) or AcE-LNC (30 μM of AcE; 9×109 particles/mL) for 2 h at 37°C under 5% CO2 atmosphere. A control group was maintained in the presence of culture medium (vehicle). Immediately after the incubation and washing four times with PBS, the coverslip was placed on extra clean dust-free Nexterion® Glass B slides containing 10 μL of PBS, and melanoma cells were imaged using a CytoViva Ultra Resolution Imaging System (CytoViva, Inc., Auburn, AL, USA). It was mounted on an Olympus BX51 microscope (×1,500 magnification; Olympus Corporation, Tokyo, Japan) equipped with fluorite 100× oil iris 0.6–1.30 numerical aperture (NA) objective and a 75 W Xe light source. Optical images were taken using a Q-imaging Retiga EXi CCD camera (Olympus Corporation, Center Valley, PA, USA) and Dage XL CCD digital camera with Image Processing Software (Dage®; DAGE-MTI of MC, Inc., Michigan City, IN, USA).
Coculture of melanoma cells and neutrophils
First, melanoma cells were previously treated as described in the “Melanoma cell culture and treatments” section. Freshly isolated neutrophils and pretreated tumor cells were cocultured in R10 at 37°C in a humidified CO2 incubator (5% CO2) for 18 h in 24-well tissue culture plates. To avoid cell-to-cell contact, neutrophils were separated from melanoma cells by a 0.4 μM membrane in a Transwell plate (Corning Incorporated, Corning, NY, USA). Neutrophils (5×105 cells/insert) were placed in the upper chambers of the Transwell culture inserts.32 After 18 h of coculture, neutrophils were removed and melanoma cells were harvested, washed three times, resuspended in PBS and immediately employed in all experiments. Supernatants were also collected and stored at −20°C until the experiments were performed.
Cell viability
The melanoma cells viability was determined after pretreatment with vehicle (R10), LNC, AcE or AcE-LNC and cells were further incubated with serum deprivation or hypoxia or cocultured with neutrophils. Briefly, melanoma cells were harvested and apoptosis and necrosis were measured by adding annexin V–FITC (1:100) and PI (50 μg/mL), respectively. Results are presented as the percentage of marked cells. All experiments were conducted using a BD FACSCanto Flow Cytometer (BD, Franklin Lakes, NJ, USA) and analyzed using the Flow Jo software (version 9.1; TreeStar Inc., Ashland, OR, USA). Data from 10,000 cells were obtained.
Oxidative burst evaluation
Cocultured neutrophils were harvested and washed with PBS, and incubated with 10 μM DCFH-DA for 30 min at 37°C. After the incubation period, the fluorescence was analyzed using flow cytometry. Results are presented as arbitrary units of fluorescence. All experiments were conducted with a FACS Canto Flow Cytometer and analyzed using the Flow Jo software (version 9.1). Data from 10,000 cells were obtained, and only the morphologically viable cells were considered in the analysis.
Quantification of chemical mediators
Levels of TGF-β, IL-10, VEGF, arginase-1 and MMP-9 were quantified by immunoassay in the supernatants of coculture, according to the instructions of the supplier. Samples obtained from each experimental group were analyzed in duplicate. The absorbance was monitored using a multiwell plate reader (PowerWaveX340; Biotek Instruments, Inc., Winooski, VT, USA).
Intravital microscopy assay
Intravital microscopy assay was carried out to evaluate the effects of treatments on neutrophil interaction with post-capillary venules in vivo. Animals were anesthetized, cremaster muscle was surgically exteriorized and animals were maintained on a special board that was thermostatically controlled at 37°C, which included a transparent platform on which tissue was placed. The preparation was kept moist and warmed by irrigating the tissue with a warmed (37°C) Ringer–Locke solution (pH 7.2–7.4). The rate of solution outflow onto the exposed tissue was controlled to keep the preparation in continuous contact with a film of the solution. Trans-illuminated images were obtained by optical microscopy (Axioplan II; Carl Zeiss Meditec AG, Jena, Germany; equipped with 5.0/0.30 plan-neofluar or ×10.0/0.25 Achroplan longitudinal distance objectives/numeric aperture and ×1.0, 1.25 or 1.60 optovar). The images were captured with a video camera (ZVS, 3C75DE; Carl Zeiss Meditec AG) and simultaneously transmitted to a computer. Interaction between leukocytes and vessel walls were examined using image-analyzing software (KS 300; Kontron, Hamburg, Germany). The number of rolling leukocytes on the post-capillary venule wall (20–30 μm diameter, 100 μm length) of the cremaster muscle just before the administration of treatments (time 0) and 10, 30, 60 and 120 min after the intravenous (i.v.) administration of vehicle (saline), LNC (0.9±0.5×1012), AcE (20 mg/kg) or AcE-LNC (20 mg/kg; 0.72±0.4×1012) were counted. Leukocytes moving in the periphery of the axial stream, in contact with the endothelium, were considered to be rollers, and their number was determined in a 5 min period.
Statistical analyses
Statistical analyses were performed using Graph Pad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). One- or two-way analyses of variance (ANOVA) followed by Tukey’s post hoc test were used to compare the data. Data were presented as mean ± standard error mean, and P-values lower than 0.05 were considered significant.
Results
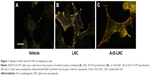
LNC and AcE-LNC were taken up by melanoma cells
To certify that nanocapsules were internalized by melanoma cells, CytoViva microscopy analysis was employed. As shown in Figure 1, LNC and AcE-LNC were taken up by cells after 2 h of incubation.
LNC or AcE-LNC pretreatments increased melanoma cell death in stress or neutrophil cocultured conditions
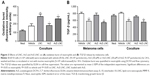
In this study, we employed nontoxic concentrations of LNC, AcE or AcE-LNC to treat melanoma cells, as percentages of cell viability (AnxV−/PI−), apoptosis (AnxV+/PI−), late apoptosis (AnxV+/PI+) and necrosis (AnxV−/PI+) were equivalent to those found in untreated cells (Figure 2A). The tumor microenvironment in general presents regions with serum deprivation and reduced oxygen supply. To address these conditions in vitro, melanoma cells were previously treated with LNC, AcE or AcE-LNC and further maintained with serum deprivation or hypoxia induced by CoCl2.31 The nanocapsule pretreatment decreased the cell viability in both conditions in comparison to vehicle or AcE pretreatments (Figure 2B and C). The loss of cell viability was related to increased apoptosis caused by LNC or AcE-LNC pretreatments (Figure 2B and C). Neutrophils are present in tumor microenvironment,11,33 and their role needs to be elucidated. Hence, B16F10 were pretreated with vehicle, nanocapsules or AcE and further cocultured with neutrophils. It is noteworthy that melanoma cells treated with vehicle and cocultured with neutrophils presented reduced viability when compared to melanoma cells in the absence of neutrophils (Figure 2A and D). Furthermore, lower cell viability was detected in LNC-, AcE- or AcE-LNC-pretreated melanoma cells cocultured with neutrophils, mainly due to late apoptosis (Figure 2D).
LNC or AcE-LNC pretreatments increased the oxidative burst in cocultured neutrophils
After coculture with pretreated melanoma cells, neutrophils were assayed to evaluate the oxidative burst. Data obtained showed that neutrophils cocultured with LNC- or AcE-LNC-pretreated melanoma cells produced higher ROS levels than those pretreated with vehicle or AcE (Figure 3A).
LNC or AcE-LNC pretreatments reduced the secretion of TGF-β by melanoma cells
TGF-β is a known growth factor secreted by cancer cells to induce the N2 pro-tumoral phenotype.18–21 Data obtained, in this study, show that melanoma cells pretreated with LNC or AcE-LNC secrete lower levels of TGF-β than cells incubated with vehicle or AcE (Figure 3B).
Release of pro-tumoral chemical mediators in the supernatant was reduced in cocultures of LNC- and AcE-LNC-pretreated melanoma cells
Secreted chemical mediators modulate the tumor microenvironment inducing anti- or pro-tumoral effects and, in this study, we investigated the role of nanocapsule pretreatments on the secretion of pro-tumoral mediators.
LNC, AcE or AcE-LNC pretreatments did not modify the levels of secreted VEGF by melanoma cells, and neutrophils did not secrete the growth factor (Figure 4A). Nevertheless, levels of VEGF were markedly enhanced in the supernatant of all cocultures, but with lesser magnitude in cocultures of melanoma cells pretreated with LNC or AcE-LNC (Figure 4A). Melanoma cells secreted low levels of IL-10, which was not altered by pretreatments. Neutrophils were able to secrete high amounts of IL-10, and the same pattern of secretion was observed when neutrophils were cocultured with melanoma cells pretreated with vehicle or AcE. Conversely, the pretreatment of melanoma cells with LNC or AcE-LNC reduced the secretion of IL-10 in the supernatant of cocultured cells (Figure 4B). Melanoma cells and neutrophils were able to secrete arginase-1; however, neutrophils secreted augmented levels of the enzyme (Figure 4C). LNC, AcE or AcE-LNC did not modify the levels of secreted arginase-1 by melanoma cells, but the pretreatment of melanoma cells with LNC or AcE-LNC reduced the amount of arginase-1 in the supernatant of cocultures (Figure 4D). Finally, MMP-9 was not detected in the supernatant of melanoma cells (data not shown). On the other hand, levels of MMP-9 were detected in the supernatants of melanoma cells and neutrophils coculture, and these levels were reduced when neutrophils were cocultured with LNC- or AcE-LNC-pretreated melanoma cells (Figure 4D).
In vivo LNC or AcE-LNC treatments impair neutrophil interaction with the microcirculatory network
As neutrophil interaction with the microcirculatory vessel wall improves the dissemination of melanoma cells to metastatic tissues,22 the role of in vivo LNC or AcE-LNC treatments on the interactions of neutrophils to endothelial cells was investigated. Our data show that i.v. administration of LNC or AcE-LNC reduced the number of rolling leukocytes in the post-capillary venules of the cremaster microcirculation 10 min after injections, which was maintained until 120 min. AcE injection reduced the number of cell interactions with vessels only 120 min after i.v. administration (Figure 5A). Representative images are shown in Figure 5B. It is important to mention that i.v. injections of the nanocapsule formulations or AcE did not cause any systemic or local toxic alteration (data not shown).
Discussion
Limited distribution of drugs into sites of tumor contributes to the inefficacy of treatment to cancers, and nanotechnology has been recently developed to provide effective nanocarriers to deliver drugs into tumors.34 Increasing numbers of studies have revealed the effectiveness of different nanoparticles carrying drugs in cancer treatment, including melanoma.35 In this context, we recently showed that oral treatment with LNC or AcE-LNC reduced the melanoma growth in mice.30 In this study, we extended the investigation showing that LNC pretreatments led to death of melanoma cells under hypoxia and serum deprivation conditions and in the presence of neutrophils. Furthermore, LNC directly affects in vivo neutrophil rheology in the microcirculatory network, which may impair the transendothelial invasion of melanoma cells into tissues. To our knowledge, the effects of nanocapsules on tumor cells and their crosstalk with neutrophils proposed in this study have not been described to other nanomaterial until now.
The efficacy of LNC carrying drugs has been shown in different diseases, pointing out that LNC is an efficient drug delivery system.29,36 Moreover, in this study, we confirmed that LNC easily penetrates cell cytoplasm, indicating that intracellular pathways are the target of LNC or delivered drugs. Indeed, data presented in this study clearly show that LNC or AcE-LNC treatments improve the apoptosis of melanoma cells in different conditions associated with tumor microenvironment. It is noteworthy that eugenol acts as an inducer of apoptosis in different cancer cells, such as melanoma,37,38 acetylation of eugenol favors the nanoencapsulation process to LNC and AcE-LNC also induces melanoma apoptosis.30 Induction of cell death by apoptosis is a desired mechanism, as intracellular content is not released to the tissue, avoiding further damage of the host tissues.39,40 In this study, we used concentrations of LNC or AcE-LNC, which did not cause cell death; however, the uptake of nanocapsules sensitized melanoma cells to apoptosis in serum deprivation and hypoxia, both restrictive conditions found in the tumor microenvironment.41 It is interesting to mention that concentrations of LNC or AcE-LNC, higher than employed in this study, also caused SK-Mel-28 apoptosis as a mechanism of death.30 As effects caused by LNC or AcE-LNC were equivalents, and AcE treatment evokes lower cell death, we suggest that the supramolecular structure of LNC is responsible for the toxic effects, as previously suggested.30
It has been shown that neutrophils are plastic cells and can switch the phenotype depending on tumor microenvironment, exerting pro- or antitumoral activities.42 Blood neutrophilia and massive accumulation of neutrophils in melanoma tissue favor the development of the tumor, angiogenesis and melanoma dissemination.19,22–26 Therefore, a crosstalk between melanoma cells and neutrophils may be pivotal to tumor progression.7–12 Herein, we showed that pre-incubation of melanoma cells with the nanocapsules affects the crosstalk between neutrophils/melanoma, as we observed by higher percentage of death in LNC- or AcE-LNC-pretreated melanoma cells co-incubated with neutrophils. Some mechanisms may be proposed, as LNC pretreatments could lead melanoma cells to be more susceptible to toxic actions of neutrophils, or LNC treatments could impair the signaling of melanoma cells to halt neutrophil toxic actions.
Neutrophils actions as ROS and nitric oxide production and granules proteases release are associate to microbicide activity and cancer regression.43–46 In this study, we show that LNC or AcE-LNC treatments on melanoma cells favor the ROS production by neutrophils. As ROS signaling induces apoptosis, we suppose that enhanced ROS production by neutrophils could, at least in part, evoke melanoma cell death after nanocapsule treatment.
It has been shown that secretion of TGF-β by cancer cells mediates the switch of neutrophils to pro-tumoral N2 phenotype and immune escape.18–20 As elevated production of ROS is one feature of N1 neutrophil phenotype, we hypothesized that LNC or AcE-LNC treatments could reduce the ability of melanoma cells to activate N2 neutrophils. Indeed, the pretreatment with LNCs impaired the secretion of the growth factor, showing a direct effect of LNC on melanoma cells.
To corroborate the melanoma/neutrophil crosstalk, we investigated other chemical mediators related to tumor microenvironment. VEGF is an angiogenic mediator in the tumor microenvironment47,48 and relevant to melanoma survival and growth.49 It was in this study evident that neutrophils do not secrete VEGF. Nevertheless, the coculture of melanoma cells and neutrophils markedly enhanced the concentration of the growth factor in the supernatant, clearly showing that the crosstalk of both cells is fundamental to the secretion of VEGF. Interestingly, the pretreatment of melanoma cells with LNC or AcE-LNC reduced the amount of VEGF. The same profile was, in this study, observed for the IL-10 secretion, a crucial cytokine in the tumor microenvironment related to immune escape.50
Arginase-1, an enzyme that hydrolyzes L-arginine into urea and L-ornithine, is related to production of substances required to cellular cycle progression, and its enhanced activity is associated to tumor cells proliferation and immune escape.51 Pro-tumoral neutrophil phenotype secretes arginase. In this study, we showed that LNC- or AcE-LNC-pretreated melanoma cells reduced the levels of arginase in the supernatant of cocultures with neutrophils. Therefore, reduced levels of IL-10 associated with low levels of arginase induced by LNC or LNC-AcE pretreatment pointed out the alterations evoked by nanocapsules, resulting in impaired immune escape.
Matrix metalloproteinases (MMPs) are a family of endopeptidases produced by different cell types, which degrade components of the extracellular matrix being involved in physiologic and pathologic processes.52–54 MMP-9 belongs to a subgroup of MMPs called gelatinases, and it is strongly related to invasion and malignancies in tumor microenvironment.55 Pro-tumoral neutrophils produce MMP-9, and in this study we also corroborate the antitumoral role of the nanocapsules by observing that pretreated melanoma cells showed a reduced production of MMP-9 by neutrophils. Overall, our data show that LNC or AcE-LNC treatments alter the melanoma/neutrophil crosstalk and may block pathways on melanoma cells responsible for the signaling of pro-tumoral neutrophil phenotype. An inverse correlation is established between neutrophil antitumoral activities and the secretion of TGF-β by cancer cells, as also shown in this work.
Neutrophils are rapidly recruited from the blood into tissue, as they present huge ability to bind to the endothelial cells covering the microcirculatory network. Adhesion molecules are expressed on activated neutrophils and interact with ligands on the endothelial cell surface, favoring cells interactions on vessel wall of the injured area.56 It has been shown that IL-8, secreted by metastatic melanoma cells, recruits neutrophils into microcirculatory beds, inducing the expression of β2 integrin on neutrophils and ICAM-1 on melanoma cells, promoting the anchoring of cancer cells to vascular endothelium.22 Using intravital microscopy, we observed that i.v. administration of LNC or AcE-LNC reduced the number of neutrophils interacting with the post-capillary venules of the cremaster muscle, showing that LNC or AcE-LNC impairs neutrophils interaction with vessel wall. The hypothesis that nanocapsules may induce a systemic protection to cancer dissemination will be further evaluated in the model of metastatic melanoma in mice.
Overall, our data point out the relevance of LNC and LNC carrying drugs by affecting melanoma cell survival in the tumor microenvironment. For the first time, it is shown that LNCs improves the immune response against tumor by reducing the secretion of mediators associated with tumor progression/invasion and immune tolerance. The proposed in vitro and in vivo mechanisms are shown in Figure 6.
Conclusion
Data presented in this study, associated with our previous publications, provide evidence that LNC or LNC-loaded drugs may be complementary tools in melanoma treatments. Therefore, further investigations must be carried out to test the role of LNCs directly on neutrophil plasticity and to open new perspectives to investigate the effects of LNC treatments on the immune escape in other models of cancer. Moreover, novel perspectives may be addressed on the systemic role of LNCs on cancer progression.
Acknowledgments
This work was supported by grants from Sao Paulo Research Foundation (FAPESP, São Paulo, grant no 2014/07328-4); National Counsel of Technological and Scientific Development (CNPq, Brasília) and Coordination of Improvement of Higher Education Personnel (CAPES, Brasília). Carine C Drewes and Mayara Uchiyama are fellows from FAPESP (projects 2010/19802-1, 10/50072-0); Cristina B Hebeda and Silvana Sandri are fellows from PNPD-CAPES. Aline de CS Alves is fellow from CAPES. Koiti Araki, Adriana R Pohlmann, Silvia S Guterres and Sandra H Farsky are research fellows from CNPq.
Disclosure
The authors report no conflicts of interest in this work.
References
Senovilla L, Vacchelli E, Galon J, et al. Prognostic and predictive value of the immune infiltrate in cancer. Oncoimmunology. 2012;1(8):1323–1343. | ||
Donskov F. Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin Cancer Biol. 2013;23(3):200–207. | ||
Ladányi A. Prognostic and predictive significance of immune cells infiltrating cutaneous melanoma. Pigment Cell Melanoma Res. 2015;28(5):490–500. | ||
Quinn C, Ma Q, Kudlac A, Palmer S, Barber B, Zhao Z. Relative efficacy of granulocyte-macrophage colony-stimulating factor, dacarbazine, and glycoprotein 100 in metastatic melanoma: an indirect treatment comparison. Adv Ther. 2017;34(2):495–512. | ||
Polkowska M, Czepielewska E, Kozłowska-Wojciechowska M. Drug combinations as the new standard for melanoma treatment. Curr Treat Options Oncol. 2016;17(12):61. | ||
Sandri S, Faião-Flores F, Tiago M, et al. Vemurafenib resistance increases melanoma invasiveness and modulates the tumor microenvironment by MMP-2 upregulation. Pharmacol Res. 2016;111:523–533. | ||
Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 2005;65(19):8896–8904. | ||
Wels J, Kaplan RN, Rafii S, Lyden D. Migratory neighbors and distant invaders: tumor-associated niche cells. Genes Dev. 2008;22(5):559–574. | ||
Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010;316(8):1324–1331. | ||
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. | ||
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. | ||
Galdiero MR, Bonavita E, Barajon I, Garlanda C, Mantovani A, Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218(11):1402–1410. | ||
Moses K, Brandau S. Human neutrophils: their role in cancer and relation to myeloid-derived suppressor cells. Semin Immunol. 2016;28(2):187–196. | ||
Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65(8):3437–3446. | ||
Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. 2006;103(33):12493–12498. | ||
De Palma M, Mazzieri R, Politi LS, et al. Tumor-targeted interferon-alpha delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer Cell. 2008;14(4):299–311. | ||
Benevides L, da Fonseca DM, Donate PB, et al. IL17 promotes mammary tumor progression by changing the behavior of tumor cells and eliciting tumorigenic neutrophils recruitment. Cancer Res. 2015;75(18):3788–3799. | ||
Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183–194. | ||
Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest. 2010;120(4):1151–1164. | ||
Andzinski L, Kasnitz N, Stahnke S, et al. Type I IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. Int J Cancer. 2016;138(8):1982–1993. | ||
Piccard H, Muschel RJ, Opdenakker G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit Rev Oncol Hematol. 2012;82(3):296–309. | ||
Huh SJ, Liang S, Sharma A, Dong C, Robertson GP. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res. 2010;70(14):6071–6082. | ||
Garcia-Hernandez Mde L, Hamada H, Reome JB, Misra SK, Tighe MP, Dutton RW. Adoptive transfer of tumor-specific Tc17 effector T cells controls the growth of B16 melanoma in mice. J Immunol. 2010;184(8):4215–4227. | ||
D’Alterio C, Barbieri A, Portella L, et al. Inhibition of stromal CXCR4 impairs development of lung metastases. Cancer Immunol Immunother. 2012;61(10):1713–1720. | ||
Jensen TO, Schmidt H, Møller HJ, et al. Intratumoral neutrophils and plasmacytoid dendritic cells indicate poor prognosis and are associated with pSTAT3 expression in AJCC stage I/II melanoma. Cancer. 2012;118(9):2476–2485. | ||
Bald T, Landsberg J, Lopez-Ramos D, et al. Immune cell-poor melanomas benefit from PD-1 blockade after targeted type I IFN activation. Cancer Discov. 2014;4(6):674–687. | ||
Schmidt H, Sorensen BS, Fode K, Nexo E, von der Maase H. Tyrosinase messenger RNA in peripheral blood is related to poor survival in patients with metastatic melanoma following interleukin-2-based immunotherapy. Melanoma Res. 2005;15(5):409–416. | ||
Manuel ER, Blache CA, Paquette R, et al. Enhancement of cancer vaccine therapy by systemic delivery of a tumor-targeting Salmonella based STAT3 shRNA suppresses the growth of established melanoma tumors. Cancer Res. 2011;71(12):4183–4191. | ||
Pohlmann AR, Fonseca FN, Paese K, et al. Poly(∈-caprolactone) microcapsules and nanocapsules in drug delivery. Expert Opin Drug Deliv. 2013;10(5):623–638. | ||
Drewes CC, Fiel LA, Bexiga CG, et al. Novel therapeutic mechanisms determine the effectiveness of lipid-core nanocapsules on melanoma models. Int J Nanomedicine. 2016;11:1261–1279. | ||
Wu D, Yotnda P. Induction and testing of hypoxia in cell culture. J Vis Exp. 2011;(54):1–4. pii: 2899. | ||
Chen YL, Chen SH, Wang JY, Yang BC. Fas ligand on tumor cells mediates inactivation of neutrophils. J Immunol. 2003;171(3):1183–1191. | ||
Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49(5):6449–6465. | ||
Neagu M, Piperigkou Z, Karamanou K, et al. Protein bio-corona: critical issue in immune nanotoxicology. Arch Toxicol. 2017;91(3):1031–1048. | ||
Pautu V, Leonetti D, Lepeltier E, Clere N, Passirani C. Nanomedicine as a potent strategy in melanoma tumor microenvironment. Pharmacol Res. Epub 2017 Feb 20. | ||
Rodrigues SF, Fiel LA, Shimada AL, et al. Lipid-core nanocapsules act as a drug shuttle through the blood brain barrier and reduce glioblastoma after intravenous or oral administration. J Biomed Nanotechnol. 2016;12(5):986–1000. | ||
Pisano M, Pagnan G, Loi M, et al. Antiproliferative and pro-apoptotic activity of eugenol-related biphenyls on malignant melanoma cells. Mol Cancer. 2007;18:6–8. | ||
Jaganathan SK, Supriyanto E. Antiproliferative and molecular mechanism of eugenol-induced apoptosis in cancer cells. Molecules. 2012;17(6):6290–6304. | ||
Greenlee-Wacker MC. Clearance of apoptotic neutrophils and resolution of inflammation. Immunol Rev. 2016;273(1):357–370. | ||
Akhtar MJ, Alhadlaq HA, Kumar S, Alrokayan SA, Ahamed M. Selective cancer-killing ability of metal-based nanoparticles: implications for cancer therapy. Arch Toxicol. 2015;89(11):1895–1907. | ||
Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27(45):5904–5912. | ||
Powell DR, Huttenlocher A. Neutrophils in the tumor microenvironment. Trends Immunol. 2016;37(1):41–52. | ||
Kettritz R. Neutral serine proteases of neutrophils. Immunol Ver. 2016;273(1):232–248. | ||
Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121(1):1–14. | ||
Wink DA, Hines HB, Cheng RY, et al. Nitric oxide and redox mechanisms in the immune response. J Leukoc Biol. 2011;89(6):873–891. | ||
Shime H, Matsumoto M, Seya T. Double-stranded RNA promotes CTL-independent tumor cytolysis mediated by CD11b(+)Ly6G(+) intratumor myeloid cells through the TICAM-1 signaling pathway. Cell Death Differ. 2017;24(3):385–396. | ||
Mittal K, Ebos J, Rini B. Angiogenesis and the tumor microenvironment: vascular endothelial growth factor and beyond. Semin Oncol. 2014;41(2):235–251. | ||
Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;3:4–10. | ||
Ott PA, Hodi FS, Buchbinder EI. Inhibition of immune checkpoints and vascular endothelial growth factor as combination therapy for metastatic melanoma: an overview of rationale, preclinical evidence, and initial clinical data. Front Oncol. 2015;5:202. | ||
Graells J, Vinyals A, Figueras A, et al. Overproduction of VEGF concomitantly expressed with its receptors promotes growth and survival of melanoma cells through MAPK and PI3K signaling. J Invest Dermatol. 2004;123:1151–1161. | ||
Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14:e218–e228. | ||
Rodriguez PC, Quiceno DG, Zabaleta J, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64(16):5839–5849. | ||
Kahari VM, Saarialho-Kere U. Matrix metalloproteinases in skin. Exp Dermatol. 1997;6(5):199–213. | ||
Hofmann UB, Houben R, Bröcker EB, Becker JC. Role of matrix metalloproteinases in melanoma cell invasion. Biochimie. 2005;87(3–4):307–314. | ||
Pittayapruek P, Meephansan J, Prapapan O, Komine M, Ohtsuki M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int J Mol Sci. 2016;17(6):E868. | ||
Roomi MW, Monterrey JC, Kalinovsky T, Rath M, Niedzwiecki A. Patterns of MMP-2 and MMP-9 expression in human cancer cell lines. Oncol Rep. 2009;21(5):1323–1333. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.