Back to Journals » Clinical Ophthalmology » Volume 10
Risk factors for visual impairment associated with corneal diseases in southern China
Authors Xu C, Chow J, Liu J, Li L, Maslin J, Chadha N, Chen B, Teng C
Received 29 December 2015
Accepted for publication 10 February 2016
Published 2 May 2016 Volume 2016:10 Pages 777—782
DOI https://doi.org/10.2147/OPTH.S103302
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Sarah C Xu,1 Jessica Chow,1 Ji Liu,1 Liang Li,2 Jessica S Maslin,1 Nisha Chadha,1 Baihua Chen,2 Christopher C Teng1
1Department of Ophthalmology and Visual Science, Yale University School of Medicine, New Haven, CT, USA; 2Department of Ophthalmology, The Second Xiangya Hospital of Central South University, Changsha, People’s Republic of China
Purpose: To identify the most common etiologies of corneal disease and the risk factors associated with worse visual outcomes in Changsha, Hunan, located in southern China.
Methods: This observational, cross-sectional study evaluated 100 consecutive patients seen at the cornea clinic of The Second Xiangya Hospital of Central South University. Ocular history, demographic information, and ocular use of traditional Chinese medicine were recorded and analyzed. Causes of infectious keratitis were diagnosed clinically. Fungal and acanthamoeba keratitis were confirmed by confocal microscopy. Visual impairment was categorized based on visual acuity according to World Health Organization recommendations. A binary logistic regression model was used to calculate odds ratio (OR).
Results: One hundred consecutive patients were evaluated. Sixty patients (60%) had noninfectious corneal diseases, most commonly dry eye syndrome (26.7%, n=16), followed by corneal abrasion (18.3%, n=11). Forty-five patients had infectious keratitis, five of whom had both infectious and noninfectious etiologies. Of the patients with infectious keratitis, viral keratitis was the most frequent cause (57.8%, n=26), followed by fungal (20%, n=9) and bacterial (20%, n=9). Older age (OR =5.08, P=0.048), male sex (OR =3.37, P=0.035), and rural residence (OR =3.11, P=0.017) had increased odds of having worse visual impairment. Rural residence was also associated with infectious keratitis (P=0.005), particularly bacterial and fungal keratitis (P=0.046), and a history of ocular trauma (P=0.003). Occupation was not a significant risk factor in this population. Fourteen patients reported use of traditional Chinese medicine, with no association with visual outcomes found.
Conclusion: Older age, male sex, and rural residence were associated with worse visual impairment. Prevalence and outcome of corneal diseases may be improved with an increased awareness in these populations.
Keywords: rural area, international ophthalmology, corneal diseases, traditional Chinese medicine
Introduction
Corneal disease is one of the major causes of blindness worldwide. Infectious keratitis, ocular trauma, and corneal opacities cause an estimated 1.5–2.0 million new cases of unilateral blindness every year.1,2 The prevalence of corneal blindness remains highest in developing countries.3 Corneal scar from trachoma is reported to cause 20.6% of all blindness in Ethiopia.4 Corneal scars from vitamin A deficiency, use of traditional medicine, and trachoma accounted for 44% of bilateral blindness and 39% of monocular blindness in Tanzania.5
While rapidly transitioning into a developed nation, the People’s Republic of China faces challenges in eliminating corneal blindness. In population studies in the People’s Republic of China, the rate of corneal blindness in at least one eye ranged from 0.3% to 0.9%,6–8 which is higher than that reported in India, a comparable nation in Asia.9 The leading causes of corneal blindness in the People’s Republic of China have been reported to be keratitis and trauma.6–8,10
There is an absence of representative clinic-based data on the magnitude and etiologies of corneal diseases managed by ophthalmologists in the People’s Republic of China. The identification of risk factors could lead to targeted improvements in public health measures, such as the promotion of eye protection to prevent trauma-related corneal injuries and hand hygiene to prevent spread of infectious keratitis, in any vulnerable group. We sought to identify the most common etiologies of corneal disease and the risk factors associated with worse visual outcomes in an outpatient setting. Additionally, we evaluated the prevalence of traditional Chinese medicine (TCM) in patients with corneal diseases in such an environment.
Methods
One hundred consecutive patients seen by one corneal specialist (BC) at The Second Xiangya Hospital of Central South University in Changsha, People’s Republic of China, were evaluated. Changsha, with a population of 7 million people, is the capital and most populous city in Hunan Province, located in the central region of southern China. The Second Xiangya Hospital is a tertiary hospital serving a mix of rural and urban patients. This cross-sectional study was conducted from November 2014 to December 2014. The study protocol was reviewed and exempted by the institutional review board at Yale University, given no patient identifiers were recorded. Additional institutional review board approval was obtained from The Second Xiangya Hospital of Central South University. Verbal consent was obtained from all patients.
A comprehensive medical interview was conducted, focusing on patient’s history of present illness, ocular history, social history, and use of TCM for their ocular disease. A comprehensive ocular examination was performed including best-corrected visual acuity tested with tumbling E chart and slit lamp examination with fluorescein staining. Visual acuity in decimal notations was recorded. Causes of infectious keratitis were diagnosed clinically as according to the standard of practice in the People’s Republic of China. All fungal and acanthamoeba keratitis were confirmed by confocal microscopy. The ophthalmic equipment was calibrated and standardized at regular intervals to minimize any potential observer or measurement bias.
Visual acuity of the affected eye or the worse eye (if both eyes were involved) was analyzed. Visual impairment was graded based on World Health Organization recommendations.11 Mild or no visual impairment was defined as vision equal to or better than 20/70 or 0.3. Moderate visual impairment was defined as vision worse than 20/70 (0.3) and equal to or better than 20/200 (0.1). Severe visual impairment was defined as vision worse than 20/200 (0.1) and equal to or better than 20/400 (0.05). Vision worse than 20/400 (0.05) was considered as blindness.
Statistical analysis
Data were analyzed using SPSS (Version 21.0; IBM Corporation, Armonk, NY, USA). P<0.05 was considered clinically significant. For risk factor analysis, a binary logistic regression model was used to investigate associations between sociodemographic/clinical variables and the presence of moderate, severe, or blind vision impairment (visual acuity worse than 20/70). A univariate analysis was conducted first for individual potential risk factors. A multivariate analysis was subsequently conducted adjusting for clinically significant variables and for variables significant in the univariate analysis. Specifically, the multivariate regression model accounted for sex, age, location, occupation, TCM use, history of ocular trauma, and corneal disease etiology. There was a concordance between the adjusted and unadjusted analyses in identifying the variables that have significant odds ratios (ORs). Prevalence rate and adjusted OR with corresponding 95% confidence interval (CI) are presented. Student’s t-test was used to compare the mean visual acuity between groups and the mean patient age by sex. One-way analysis of variance was used to compare the mean visual acuity between age groups. One-sample chi-square (χ2) test was used to compare the distribution of demographic characteristics. Pearson’s χ2 test was used to compare the distribution of corneal diseases by rural and urban locations. Five patients with both infectious and noninfectious corneal diseases were excluded from analyses comparing infectious and noninfectious etiologies.
Results
Demographic profile
Of the 100 consecutive patients evaluated, there were 50 female and 50 male patients. The average age of patients was 46.0±20.1 years. There was no difference in sex, average age of the patients by sex, and laterality of eye affected (Table 1). More patients had mild or no visual impairment (n=48) compared to those with moderate (n=12) or severe impairment (n=10) or blindness (n=30).
Risk factors for significant visual impairment
Binary logistic regression analysis was conducted for patients who had moderate, severe, or blind visual impairment (n=52). The analysis accounted for variables including sex, age, location, occupation, TCM use, history of ocular trauma, and corneal disease etiology. Adjusting for these variables, the odds of having moderate, severe, or blind visual impairment were higher in males (P=0.035, adjusted OR: 3.37, 95% CI [1.09–10.43]), patients ≥60 years of age (P=0.048, adjusted OR: 5.08, 95% CI [1.01–25.55]), and those living in a rural location (P=0.017, adjusted OR: 3.108, 95% CI [1.22–7.89]; Table 2). Occupation was not a significant risk factor in this population. There was no difference in visual impairment when comparing agricultural workers, indoor workers, or unemployed patients.
In addition, a similar binary logistic analysis was conducted for patients with unilateral or bilateral blindness (n=30). However, adjusted OR for sex (P=0.145), age 60 years and older (P=0.067), and rural location (P=0.258) were not significant.
Males had lower visual acuity compared to females (0.22±0.27 vs 0.46±0.37, P=0.002), and rural residents had lower visual acuity than urban residents (0.24±0.28 vs 0.48±0.37, P=0.003), by Student’s t-test. Additionally, patients 60 years of age and older had the worst visual acuity (0.21±0.26) compared to other age groups (one-way analysis of variance, P=0.005).
Associations between visual acuity and etiology of corneal disease
Virus was the most frequent cause of infectious keratitis (n=26 [57.8%], χ2 test, P<0.001), followed by fungus (n=9 [20%]) and bacteria (n=9 [20%]). Patients with viral keratitis had significantly better mean visual acuity compared to those with fungal keratitis (0.35±0.05 vs 0.13±0.09, Student’s t-test, P=0.036) or bacterial keratitis (0.11±0.09, Student’s t-test, P=0.022; Figure 1).
  | Figure 1 Mean visual acuity of patients with various etiologies of infectious keratitis. |
In comparison, patients with noninfectious etiologies of corneal diseases had significantly better visual acuity compared to those with infectious keratitis (0.42±0.05 vs 0.27±0.04, Student’s t-test, P=0.035). The most common noninfectious corneal disease was dry eye syndrome (DES, n=16 [26.7%]), followed by corneal abrasion (n=11 [18.3%]; Figure 2). Patients with DES had significantly higher mean visual acuity compared to those with corneal trauma (0.61±0.09 vs 0.13±0.06, Student’s t-test, P<0.001), peripheral corneal ulceration or thinning (0.10±0.04, Student’s t-test, P=0.004), neurotrophic ulcer (0.11±0.10, Student’s t-test, P=0.005), or chemical burn (0.02±0.01, Student’s t-test, P=0.004). Similarly, patients with corneal abrasion (0.65±0.12) or filamentary keratopathy (0.60±0.15) also had significantly higher mean visual acuity compared to those with corneal trauma (0.13±0.06), peripheral corneal ulceration or thinning (0.10±0.04), neurotrophic ulcer (0.11±0.10), or chemical burn (0.02±0.01, Student’s t-test, P<0.05 in all pairwise comparisons).
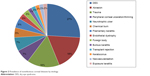  | Figure 2 Prevalence of noninfectious corneal diseases by etiology. |
Geographic differences in corneal diseases
The majority of patients with a history of ocular trauma (n=22 [81.5%], χ2 test, P=0.003) or infectious keratitis were from a rural location (n=29 [72.5%], χ2 test, P=0.005). Specifically, patients with bacterial keratitis or fungal keratitis were predominantly from a rural location (χ2 test, P=0.046; Table 3). Of the 14 patients who used topical and/or systemic TCM, 71.4% were from a rural location.
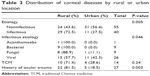  | Table 3 Distribution of corneal diseases by rural or urban location |
Traditional Chinese medicine
Four TCMs were named by patients: Jin Yin Hua, Yu Xing Cao, Qi Ju Di Huang Wan, and Wu Xing Dan. Of these, Wu Xing Dan was dissolved with distilled water and used topically (Table 4). TCM was not associated with visual impairment (binary logistic regression, P=0.225; Table 2) or visual acuity (Student’s t-test, P=0.283).
  | Table 4 Traditional Chinese medicine used by patients for treating various corneal diseases |
Discussion
We found that age 60 years and older, male sex, and living in a rural location are risk factors for worse visual impairment in an outpatient population in the central region of southern China. Previous epidemiology studies in India and the People’s Republic of China have reported age and male sex as risk factors for poor visual outcome.6–9 These studies examined corneal diseases in rural populations only; thus, they were unable to discern whether rural location was associated with disease prevalence. Another study examined the prevalence of corneal blindness in Ningxia, People’s Republic of China but did not show any significant association between rural living and corneal blindness.6
Our results demonstrate that rural living was associated with worse visual impairment, ocular trauma, and a diagnosis of infectious keratitis, particularly bacterial and fungal keratitis. This may be because rural residents are exposed to more organic matter on a daily basis than urban residents. They also may be more predisposed to ocular trauma due to rural lifestyle. Ocular trauma may predispose them to infectious keratitis, particularly fungal and bacterial keratitis, which were difficult to treat and carried a poor visual prognosis. Our study is consistent with others that identified a high prevalence of infectious keratitis and history of ocular trauma in the People’s Republic of China.7,9,12 The identification of the rural population as a particularly vulnerable group with worse visual outcome emphasizes the need for safety measures and use of eye protection to prevent injuries.
The use of traditional medicine, particularly Indian and African traditional medicine, has been reported as a risk factor for corneal blindness.13–16 An estimated 26% of childhood blindness in Malawi16 and 25% of corneal ulcers in Tanzania were associated with the ocular use of traditional medicine.17 African traditional eye medicine also increased the risk of developing peripheral corneal ulcers.13 TCM has shown to be effective in treating DES.18,19 In a randomized controlled study involving 80 patients with DES, Chi Ju Di Huang Wan was shown to improve tear breakup time after 2–4 weeks of use by Rose Bengal staining.18 A meta-analysis of seven randomized controlled trials showed that 198 patients with DES who underwent acupuncture had an improved tear breakup time, Schirmer I test, and cornea fluorescein staining compared to 185 patients with DES treated with artificial tears.20 The treatment duration in studies included ranged from 8 weeks to 8 months.20 Our study did not show any association between TCM and either better or worse visual outcome.
It is important to interpret the results of our study by understanding the following limitations. The prevalence of corneal diseases in a referring subspecialty clinic cannot be extrapolated to estimate the prevalence of corneal diseases in the general population in southern China. It is likely that the prevalence of corneal diseases and any associated decreased visual acuity is lower in the general population than our patient sample. Our findings were based on the observation and analysis of patients who were referred to one tertiary hospital in the People’s Republic of China. Difficulty in accessing ophthalmic care at a tertiary center may explain any difference in prevalence and patient demographics in community clinics.
By selecting consecutive patients from one examiner, we minimized observer and measurement bias. Additionally, patients’ diagnoses were confirmed by clinical and/or objective data. This study is unique in that it reports consecutive cases managed by a corneal specialist in the People’s Republic of China and the risk factors associated with worse visual outcome in an outpatient setting. This study may be useful in planning public health efforts, such as education on corneal diseases and eye protection use. Our results may also be helpful in allocating resources and improving health care access for rural residents to maximize the impact on visual impairment reduction or prevention in the People’s Republic of China. Future studies involving a larger sample size and with a multicenter approach would broaden our understanding of the epidemiology of corneal disease in the People’s Republic of China.
Acknowledgments
The authors would like to thank Jing Luo and Junyi Ouyang for their help in data collection. A part of this study was presented at the Association for Research in Vision and Ophthalmology (ARVO) annual meeting on May 7, 2015, Denver, CO, USA. This work is supported in part by an unrestricted departmental grant from Research to Prevent Blindness (RPB), Inc.
Disclosure
The authors report no conflicts of interest in this work.
References
Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96(5):614–618. | ||
World Health Organization [webpage on the Internet]. Prevention of Blindness and Visual Impairment. Priority Eye Diseases – Corneal Opacities. http://www.who.int/blindness/causes/priority/en/index9.html. Accessed April 17, 2015. | ||
Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79(3):214–221. | ||
Zerihun N, Mabey D. Blindness and low vision in Jimma Zone, Ethiopia: results of a population-based survey. Ophthalmic Epidemiol. 1997;4(1):19–26. | ||
Rapoza PA, West SK, Katala SJ, Taylor HR. Prevalence and causes of vision loss in central Tanzania. Int Ophthalmol. 1991;15(2):123–129. | ||
Sheng XL, Li HP, Liu QX, et al. Prevalence and associated factors of corneal blindness in Ningxia in northwest China. Int J Ophthalmol. 2014;7(3):557–562. | ||
Li Z, Cui H, Zhang L, Liu P, Bai J. Prevalence of and associated factors for corneal blindness in a rural adult population (the southern Harbin eye study). Curr Eye Res. 2009;34(8):646–651. | ||
Wang H, Zhang Y, Li Z, Wang T, Liu P. Prevalence and causes of corneal blindness. Clin Experiment Ophthalmol. 2014;42(3):249–253. | ||
Gupta N, Vashist P, Tandon R, Gupta SK, Dwivedi S, Mani K. Prevalence of corneal diseases in the rural Indian population: the corneal opacity rural epidemiological (CORE) study. Br J Ophthalmol. 2015;99(2):147–152. | ||
Li X, Wang L, Wei Q. Corneal disease in China. Ophthalmology. 2012; 119(8):1712–1712.e1. | ||
World Health Organization [webpage on the Internet]. Change the Definition of Blindness. Available from: http://www.who.int/blindness/Change%20the%20Definition%20of%20Blindness.pdf. Updated 2015. Accessed April 17, 2015. | ||
Song X, Xie L, Tan X, et al. A multi-center, cross-sectional study on the burden of infectious keratitis in China. PLoS One. 2014;9(12):e113843. | ||
Lewallen S, Courtright P. Peripheral corneal ulcers associated with use of African traditional eye medicines. Br J Ophthalmol. 1995;79(4):343–346. | ||
Prajna NV, Pillai MR, Manimegalai TK, Srinivasan M. Use of traditional eye medicines by corneal ulcer patients presenting to a hospital in south India. Indian J Ophthalmol. 1999;47(1):15–18. | ||
Courtright P, Lewallen S, Kanjaloti S, Divala DJ. Traditional eye medicine use among patients with corneal disease in rural Malawi. Br J Ophthalmol. 1994;78(11):810–812. | ||
Chirambo MC, Benezra D. Causes of blindness among students in blind school institutions in a developing country. Br J Ophthalmol. 1976;60(9):665–668. | ||
Yorston D, Foster A. Traditional eye medicines and corneal ulceration in Tanzania. J Trop Med Hyg. 1994;97(4):211–214. | ||
Chang YH, Lin HJ, Li WC. Clinical evaluation of the traditional Chinese prescription Chi-Ju-Di-Huang-Wan for dry eye. Phytother Res. 2005;19(4):349–354. | ||
Zhou WY, Li YH. A survey on treatment of dry eye by traditional Chinese medicine and integrative Chinese and western medicine. Chin J Integr Med. 2006;12(2):154–159. | ||
Yang L, Yang Z, Yu H, Song H. Acupuncture therapy is more effective than artificial tears for dry eye syndrome: evidence based on a meta-analysis. Evid Based Complement Alternat Med. 2015;2015:143858. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


