Back to Journals » Research and Reports in Urology » Volume 9
Results of a randomized, prospective, double-dummy, double-blind trial to compare efficacy and safety of a herbal combination containing Tropaeoli majoris herba and Armoraciae rusticanae radix with co-trimoxazole in patients with acute and uncomplicated cystitis
Authors Stange R, Schneider B, Albrecht U, Mueller V, Schnitker J, Michalsen A
Received 31 August 2016
Accepted for publication 2 November 2016
Published 14 March 2017 Volume 2017:9 Pages 43—50
DOI https://doi.org/10.2147/RRU.S121203
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jan Colli
Rainer Stange,1 Berthold Schneider,2 Uwe Albrecht,3 Valentina Mueller,3 Joerg Schnitker,4 Andreas Michalsen1
1Internal and Complementary Medicine, Immanuel Krankenhaus Berlin-Wannsee, Berlin, 2Institute for Biostatistics, Medical University, 3Mediconomics GmbH, Hannover, 4Institute for Applied Statistics, Bielefeld, Germany
Objectives: To demonstrate non-inferiority of an herbal combination (horseradish root and nasturtium herb) to an antibiotic (co-trimoxazole) in acute uncomplicated cystitis.
Design: Randomized, prospective, double-blind, double-dummy, multicenter, phase III clinical study, using block randomization of 4 blocks (size 2).
Setting: Twenty-six centers in Germany, from May 2011 to June 2013.
Participants: Adult patients (median age, 38.5 years; 90% female) with acute uncomplicated cystitis confirmed via urinalysis and bacterial counts.
Interventions: Patients received the herbal combination (five tablets, four times per day) or the antibiotic (two tablets daily) for a period of 7 or 3 days, respectively, followed by a 21-days without drug treatment. Placebos ensured blinding.
Primary and secondary outcome measures: The primary endpoint was the percentage of responders, expressed as reduction of germ count from >105 to <103 CFU/mL of pathogens between visit 1 (day 0) and 3 (day 15). Secondary endpoints included change of symptom scores, duration of symptoms, efficacy assessments, relapse frequency, and safety. A sample size of 178 patients per group was estimated.
Results: Of the 96 randomized patients (intent-to-treat; 45 in the phytotherapy group, 51 in the antibiotic group), 51 were considered per-protocol patients (22 in the phytotherapy group, 29 in the antibiotic group). Responder rates were 10/22 (45.5%) for the phytotherapy group and 15/29 (51.1%) for the antibiotic group (group difference: –6.27% [95% CI: –33.90%–21.3%]). The study was terminated prematurely due to slow recruitment rates. Non-inferiority could not be assumed by predefined criteria. During the follow-up period, one relapse occurred in each group. Both treatments were well tolerated.
Conclusion: This clinical trial indicates comparable efficacy of the herbal combination and antibiotic, although non-inferiority was not proved. However, the results and lessons learned are important for the planning of future trials. Issues that led to the premature trial discontinuation were considered.
Keywords: urinary tract infection, herbal medicinal product, cystitis, horseradish, nasturtium herb, co-trimoxazole, randomized clinical trial
Introduction
Cystitis is defined as a symptomatic infection of the lower urinary tract due to bacterial colonization. The most common uropathogens are Escherichia coli and Staphylococcus saprophyticus.1 Common symptoms involve dysuria, alguria, pollakiuria, or desire to urinate and abnormal urinary parameters.2 Women are more often affected due to the anatomical structure of the female urinary tract. Approximately 5% of young women experience new infections each year, and the rate increases with age. Fifty to seventy percent of women experience symptoms of cystitis at least once in their life; approximately 30% of these women experience recurrent infections.3
Currently, the standard treatment for cystitis comprises oral antibiotics; the choice depends on the spectrum of uropathogens and their antibiotic resistance. A combination of trimethoprim and sulfamethoxazole is often recommended, unless resistance rates exceed 20%.4 These substances act synergistically, interfering with the metabolism of folic acid.5 Antimicrobial resistance is, however, spreading, and there is a great need for new treatment strategies.
The herbal combination (Angocin® Anti-Infekt N; Repha GmbH, Langenhagen, Germany) used in this trial contains as active ingredients horseradish root (Armoraciae rusticanae radix) and nasturtium (Tropaeoli majoris herba). It has been on the German market for more than 50 years and is authorized for treating acute uncomplicated urinary tract infections (UTIs). Its antibacterial efficacy against a broad range of pathogens has been demonstrated in vitro and is primarily attributed to isothiocyanates.6–8 Previous clinical observations demonstrated similar efficacy to standard antibiotics in treating acute UTI, and a significantly reduced relapse rate of recurrent lower UTI compared to placebo.9–11 Moreover, in a recent clinical trial, the herbal combination was superior to placebo when treating respiratory tract infections.12
The purpose of this non-inferiority trial was to investigate efficacy and safety of the herbal combination in comparison to co-trimoxazole in the treatment of acute, uncomplicated cystitis.
Materials and methods
Study design
This randomized, prospective, double-blind, double-dummy, active-controlled, multicenter, phase III clinical trial was conducted from May 2011 to June 2013 in 26 centers (involving urologists, gynecologists, and general practitioners) in Germany according to Guidelines for Good Clinical Practice E6 published by the International Council on Harmonisation and the German Drug Law. The study was approved by the leading Ethics Committee, “Landesamt für Gesundheit und Soziales” (LAGeSo; Berlin, Germany) on March 28, 2011 (EudraCT-number 2010-022096-54). The aim was to demonstrate non-inferiority of the herbal combination compared to co-trimoxazole in the treatment of acute, uncomplicated cystitis. Subjects were randomly allocated, using block randomization of 4 blocks of size 2, by an independent statistician who was not involved in patient recruitment and data collection. Study medication was packaged according to a randomization list by a manufacturing facility not involved in this trial. Trial sites were supplied with packages of blinded units containing randomization numbers, which were assigned to each eligible patient, always using the lowest number available at the trial site. The same batches of study medication were used throughout the trial. All individuals involved in this trial remained blinded until the database had been locked. A Blind Data Review Meeting was conducted before unblinding. Study duration was 28 days, consisting of 7 days of active treatment and 21 days without drug treatment. An additional observational period of 6 months was optional.
Participants
Outpatients of both sexes, aged 18–70 years, with symptoms of uncomplicated acute cystitis, presence of leukocytes measured via urinalysis, and bacterial counts of more than 105 CFU/mL in a midstream sample of urine (MSU) were included. Written informed consent was obtained before any study procedure. Relevant exclusion criteria comprised the following: presence of bacteria resistant to co-trimoxazole; progressive infection of the urinary tract; overactive bladder; abnormal anatomical structures or preceding surgery of the urinary tract; renal failure; nephritis; proteinuria; acute infections other than UTI or factors indicating a complicated UTI; abnormal laboratory values; intake of antibiotics 2 weeks before study inclusion and/or parallel to study treatment; presence of inhibition zones in Bacillus subtilis lawn after application of native urine as a marker for recent intake of antibiotics (positive inhibitor test); presence of at least three UTI during the past 12 months and/or at least two UTI during the past 6 months; pregnancy; lactation; and contraindications of the study medication.
Interventions
One tablet of the herbal combination contained 80 mg horseradish root powder and 200 mg nasturtium herb powder, produced by grinding of unmodified dried plants containing isothiocyanates as the active ingredients. The comparator (co-trimoxazole) consisted of a combination of 160 mg trimethoprim and 800 mg sulfamethoxazole per tablet. Placebo tablets were identical in appearance to the respective study drug. Since both drugs have different galenic properties and treatment durations, a double-dummy technique was used to maintain blinding.
The phytotherapy group received five herbal combination tablets four times per day and one placebo comparator tablet two times per day for 7 days. The antibiotic group administered five placebo herbal combination tablets four times per day for 7 days and one active comparator tablet two times per day for 3 days. For the remaining 4 days, a placebo comparator tablet was used to maintain blinding. Treatment with study medication commenced at visit 1 (day 0) and ended before visit 2 (day 8+1). Two follow-up visits were conducted: visit 3 (day 15+1) and visit 4 (day 28±1). If a relapse occurred between scheduled follow-up visits, an extra visit was carried out. During the optional 6-month observational period, monthly telephone visits were scheduled.
Endpoints
The primary endpoint was the rate of responders defined as the reduction of urinary pathogens to <103 CFU/mL in an MSU on visit 3. Secondary endpoints were change of symptom scores, duration of symptoms, physicians’ assessment of efficacy, frequency of relapse or new infection at visits 3 and 4, and physicians’ assessment of the course of the disease. Other endpoints were explorative and included rate of resistance to the comparator, the rate of patients with recurrent cystitis during the optional 6-month follow-up period, and patients’ satisfaction with the treatment. Safety endpoints were the incidence of adverse events or serious adverse events, tolerability, laboratory (hematology, blood chemistry and urinalysis), and vital signs. Compliance was also documented.
Procedure
At visit 1, subjects were screened, informed consent was obtained, demographic, anamnestic data and concomitant diseases were documented, a pregnancy test was performed in female patients of childbearing potential, resistance to co-trimoxazole and inhibitor tests were performed, and eligible patients were randomized and received the study medication and a diary for the next 7 days. Concomitant medication and therapies, vital signs, urinalyses (Combur9-Test® strips; Hoffmann-La Roche, Basel, Switzerland) and symptoms of uncomplicated cystitis (pollakiuria, dysuria, nocturia, and urinary incontinence) were assessed at each visit. Symptoms were also assessed daily in each patient’s diary (none, mild, moderate, or severe). Measurement of urine specific gravity and bacterial counts were mandatory on visits 1 and 3 and necessary during other visits if leukocytes were present. Blood samples were obtained on visits 1 and 3. Identification of pathogens in urine was performed during visit 1. Patients’ satisfaction (10-point scale) and investigators’ assessments were documented on visits 2 and 3. Adverse events or serious adverse events were checked starting from visit 2. Tolerability (good, or not good) was assessed daily in each patient’s diary and by physicians at visit 3. During the optional 6-month observational period, patients were questioned about recurrence of cystitis.
Statistical analyses
Sample size calculation and statistical analyses were carried out in accordance with the ICH-GCP guidelines. Sample size calculation was based on the assumption that the probability of therapeutic success for the comparator is 0.9 and the threshold for non-inferiority –0.1, which had to be confirmed on α=0.025 level (one-sided). Taking into account a dropout rate of 20%, a sample size of 178 patients per group was calculated.
The intent-to-treat set (ITT) consisted of all patients who received study medication at least once (Figure 1). The per-protocol set (PP) comprised patients who completed the study after visit 4 without relevant protocol violations, which resulted in the exclusion of three patients. One patient remained in the PP set, despite treatment with antibiotics (nonresponder). Furthermore, the Blind Data Review Meeting Committee decided to include four patients with complete data sets for the primary endpoint, who discontinued the study prematurely, of which two patients discontinued due to necessary treatment with antibiotics (nonresponders). To exclude potential bias, additional sensitivity analysis of the PP set without three nonresponders was performed for the primary endpoint. The safety-evaluable set was identical to the ITT set. Analysis of the primary endpoint was performed in the PP set only, because patients excluded from the PP set had no data on the primary outcome or protocol violations affecting the outcome. Analyses of secondary endpoints were performed in both sets, unless specified otherwise.
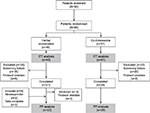  | Figure 1 Flowchart showing study design from patient screening (visit 1) to the end of follow-up (visit 4). Abbreviations: ITT, intent-to-treat; PP, per-protocol. |
Statistical analyses were conducted as was specified in the trial protocol. Analysis of non-inferiority was conducted for the primary endpoint using δ=0.1 (10%) as a threshold. Non-inferiority was assumed if the lower boundary of the 95% CI of the difference was >–δ (α=0.025, one-sided). For the analysis of other endpoints, exploratory statistics were used which comprised Mann–Whitney U-test, log-rank test, χ2-test, Wilcoxon signed-rank test, and Student’s t-test using a level of significance of α=0.05 (two-sided).
Results
Ninety-six patients (nine male; median age, 38.5 years) were randomized (ITT), and all of them completed the treatment period of 7 days (Figure 1). Patients were randomized if the available results (from test strips and description of symptoms) permitted study inclusion. Results of urine specific gravity, bacterial counts, inhibitor test, resistance tests, and identification of pathogens were available a couple of days after the screening. If eligibility status was changed due to updated results, the patient was considered a retrospective screening failure and was excluded from the study (phytotherapy group: 18 patients; antibiotic group: 20 patients).
The PP set included 51 patients with negative inhibitor tests, confirmed presence of pathogenic microorganisms, cystitis confirmed by physician, pathogens count ≥105 CFU/Ml, and no resistance to co-trimoxazole.
Fifty (52.1%) patients completed the trial at visit 4; 44 of them agreed to the optional 6-month observational period and 41 (42.7%) patients completed it. Before reaching the planned sample size, the study was terminated due to slow recruitment rate. Baseline data and clinical characteristics are presented in Table 1. Compliance was high in both groups (>97%). The test for homogeneity revealed significant differences for only eight (11.3%) variables, which was below the expected value of 15% and can be considered random. Thus, a homogeneous population was evaluated.
Efficacy
The responder rates in the PP set were 10/22 (45.5%) for the phytotherapy group and 15/29 (51.7%) for the antibiotic group. The difference in responder rates was –6.27% [95% CI: –33.90%–21.37%], suggesting that non-inferiority of the primary endpoint cannot be assumed.
Sensitivity analysis in the PP set showed responder rates of 10/19 (52.6%) for the phytotherapy group and 15/29 (51.7%) for the antibiotic group (0.91% [95% CI: –27.99%–29.80%]), still indicating that non-inferiority cannot be assumed.
Non-inferiority of the herbal combination versus the antibiotic was demonstrated in the ITT set for improvement of the cardinal symptoms (dysuria and pollakiuria; Table 2). Non-inferiority analyses were not performed in the PP set, because of a CI range of >20% and subsequently low statistical power. In the phytotherapy group, 18/20 (90.0%) were free of symptoms at visit 3 (PP set). In the antibiotic group, the numbers were 29/29 (100.0%) patients (PP set).
Median duration to symptom resolution was significantly shorter in the antibiotic group (antibiotic group: 4 days; phytotherapy group: 6.5 days; PP set: P=0.0122). Similar results were observed in the ITT set (P=0.0046).
The course of the disease during the 1st week as assessed by the physician showed a tendency for better improvement in the antibiotic group. Seventeen out of twenty (85.0%) patients were free of symptoms compared with 16/22 (72.7%) patients in the phytotherapy group (PP set: P=0.0313), which was also observed in the ITT set (P=0.0792).
Physicians’ assessment of efficacy on visit 3 was significantly better for the antibiotic group (very good: 55.2%; good: 41.4%) compared with the phytotherapy group (very good: 25.0%; good: 50.0%). Patients’ satisfaction was similar in both groups (P=0.1824).
At visit 4, one relapse of cystitis was documented in each group. No relapses or new infections occurred during the optional 6-month observational period.
Further analyses were exploratory and performed in the ITT set. Resistance to the antibiotic was observed in 21 (25.0%) patients who were retrospectively considered as screening failures (twelve in the phytotherapy group and nine in the antibiotic group). The most common resistant pathogen in both groups was Escherichia coli (six in the phytotherapy group and three in the antibiotic group).
Safety
Side effects occurred in three patients (dyspepsia, decreased appetite, and/or headache) in the phytotherapy group, and in five patients (diarrhea, gastrointestinal pain, disturbance in attention, headache, restlessness, exfoliative dermatitis, facial skin alteration, and/or nausea) of the antibiotic group. No serious side effects were documented.
No significant changes were observed for any laboratory value or vital sign, except for significant declines of leukocytes in urine (phytotherapy group: P=0.0025; antibiotic group: P<0.0001) and erythrocytes in urine (phytotherapy group: P=0.0167; antibiotic group: P=0.0007). Overall, both treatments were well tolerated.
Discussion
Principal findings
Efficacy of the herbal preparation in the treatment of uncomplicated UTI was previously demonstrated in two cohort studies.9,10 Although non-inferiority for the primary endpoint could not be proved in this trial, the responder rates for both therapies were around 50% and a similar improvement of UTI symptoms was seen in both groups. Cystitis relapse rates were similar for both treatments during the follow-up and the optional 6-month observational period, suggesting similarly effective long-term management of UTI. Resistance rates to co-trimoxazole (22%) were comparable to published literature.13–16 Resistance to the herbal combination or isothiocyanates is unknown.17
Antibiotics are the first-line agents for the treatment of acute uncomplicated UTI. Other treatment modalities exist (ibuprofen, cranberry, or delayed treatment), but play a minor role due to limited available evidence.18 The increasing rate of antibiotic resistance, however, has promoted the search for alternative therapies that aim to prevent UTI. Such approaches include herbal treatment, D-mannose, hyaluronic acid instillation, probiotics, or vaccines. The use of these alternatives may help to circumvent common side effects associated with repeated use of antibiotics such as gut dysbiosis and drug resistance.
Strengths and limitations
The strength of our study was the use of several objective tests combined with subjective parameters as inclusion criteria. The sole use of subjective parameters may result in bias toward patients with less severe symptoms, with more severely affected symptomatic patients misjudged to be unfit for participation. A standardized method of urine collection and handling of urine samples was introduced to ensure similar quality. We included males because, although an uncomplicated cystitis is rare, it sometimes occurs in younger men.4 Inclusion of male subjects provided a better representation of the general population.
A major limitation of the trial is that the planned sample size could not be reached due to insufficient recruitment rates; this impaired statistical significance and, therefore, the results of this study must be interpreted with caution. The data can only provide preliminary explorative evidence, which must be examined further in future clinical trials.
We identified several barriers and difficulties that may have influenced patient recruitment. For instance, a delay of therapy was not justified for symptomatic patients because there is a risk of ascension of bacteria through the urinary tract. Patients who were eligible at screening (UTI symptoms and positive urine test strips) were included before all test results (bacterial counts and urinalysis) were available. This resulted in an unexpectedly high proportion of randomized patients who were later excluded as screening failures without data on outcome.
Another recruitment barrier was the selection of inclusion criteria, which was too strict. We used the urinary germ count of >105 CFU/mL, which has been a standard criterion to detect significant bacteriuria,4,19 as an inclusion criterion. However, it could not be detected in approximately 14% of patients despite sufficient UTI symptom scores. A lower threshold may be more appropriate. In one study, ≥102 CFU/mL was found to be the best diagnostic criterion when clinical symptoms of UTI were present.20 Another study established that low-count bacteriuria was statistically more frequent among young women with UTI symptoms.21 Furthermore, the initial inclusion criterion “positive nitrite test” was abandoned with the Ethics Committee’s consent, due to increased incidence of false negative results that occurred when micturated volume was too small despite sufficient count of nitrite-producing germs. Newer clinical trials rely more on clinical symptoms as inclusion criteria, which may be preferable when the patient’s quality of life is the main study endpoint.22
Early trial closure is a common problem seen in many acute care clinical trials, which are more frequently discontinued compared to nonacute care trials.23 Slow recruitment is the main reason for most study discontinuations.22,24,25 In our trial, practitioners reported limited staff availability, complex protocol design, and a limited number of eligible cases as the main reasons for slow recruitment, with the latter due to a different pool of patients often encountered in centers other than those containing general practitioners. For instance, of the eight active recruiting centers, 92 patients were recruited by general practitioners, whereas only 5/18 nonrecruiting centers housed general practitioners. This association was confirmed in another recently published study.26 From the participants’ view, strong individual treatment preferences, lack of interest, high number of tablets and visits, and low payment were vital barriers. Similar motives were seen in other clinical trials.26–28
Conclusion
This clinical trial suggests a possible role of the herbal combination in the treatment of uncomplicated UTI. Other clinical trials with sufficient statistical power are required to assure this assumption.
A poor recruitment rate is one of the main obstacles encountered in many clinical studies. Several issues must be taken into account when conducting trials in patients with acute uncomplicated UTI. Less strict inclusion criteria, study site selection, and simplified study designs are essential elements. Study sites should be constantly motivated to recruit more patients.
Acknowledgment
This work was supported by Repha GmbH.
Disclosure
Repha GmbH has not been involved in trial design, data collection, analysis, or decision to publish this manuscript. The authors report no conflicts of interest in this work.
References
Schito GC, Naber KG, Botto H, et al. The ARESC study: an international survey on the antimicrobial resistance of pathogens involved in uncomplicated urinary tract infections. Int J Antimicrob Agents.2009;34(5):407–413. | ||
DEGAM-Guideline No. 1. Brennen beim Wasserlassen [Sharp pain when urinating]. Düsseldorf: 2009. Available from: http://www.degam.de/files/Inhalte/Leitlinien-Inhalte/Dokumente/DEGAM-S3-Leitlinien/Leitlinien-Entwuerfe/Brennen%20beim%20Wasserlassen/LL-01_Langfassung_mit_KV_ZD.pdf. German. | ||
Renz-Polster H, Krautzig S, editors. Basislehrbuch Innere Medizin [Basic textbook of internal medicine]. 4th ed. Munich: Elsevier, Urban & Fisher Verlag; 2008:5:941–950. | ||
S3-Guideline AWMF-Register-No. 043/044. Harnwegsinfektionen [Urinary tract infections]. 2010. Available from: http://www.awmf.org/leitlinien/detail/ll/043-044.html. German. | ||
Bushby SR. Synergy of trimethoprim-sulfamethoxazole. Can Med Assoc J. 1975;112(13 Spec No):63–66. | ||
Aires A, Mota VR, Saavedra MJ, Rosa EA, Bennett RN. The antimicrobial effects of glucosinolates and their respective enzymatic hydrolysis products on bacteria isolated from the human intestinal tract. J Appl Microbiol. 2009;106(6):2086–2095. | ||
Conrad A, Biehler D, Nobis T, et al. Broad spectrum antibacterial activity of a mixture of isothiocyanates from nasturtium (Tropaeoli majoris herba) and horseradish (Armoraciae rusticanae radix). Drug Res (Stuttg). 2013;63(2):65–68. | ||
Conrad A, Kolberg T, Engels I, Frank U. [In vitro study to evaluate the antibacterial activity of a combination of the haulm of nasturtium (Tropaeoli majoris herba) and of the roots of horseradish (Armoraciae rusticanae radix)]. Arzneimittelforschung. 2006;56(12):842–849. German. | ||
Goos KH, Albrecht U, Schneider B. [Efficacy and safety profile of a herbal drug containing nasturtium herb and horseradish root in acute sinusitis, acute bronchitis and acute urinary tract infection in comparison with other treatments in the daily practice/results of a prospective cohort study]. Arzneimittelforschung. 2006;56(3):249–257. German. | ||
Goos KH, Albrecht U, Schneider B. [On-going investigations on efficacy and safety profile of a herbal drug containing nasturtium herb and horseradish root in acute sinusitis, acute bronchitis and acute urinary tract infection in children in comparison with other antibiotic treatments]. Arzneimittelforschung. 2007;57(4):238–246. German. | ||
Albrecht U, Goos KH, Schneider B. A randomised, double-blind, placebo-controlled trial of a herbal medicinal product containing Tropaeoli majoris herba (Nasturtium) and Armoraciae rusticanae radix (Horseradish) for the prophylactic treatment of patients with chronically recurrent lower urinary tract infections. Curr Med Res Opin. 2007;23(10):2415–2422. | ||
Fintelmann V, Albrecht U, Schmitz G, Schnitker J. Efficacy and safety of a combination herbal medicinal product containing Tropaeoli majoris herba and Armoraciae rusticanae radix for the prophylactic treatment of patients with respiratory tract diseases: a randomised, prospective, double-blind, placebo-controlled phase III trial. Curr Med Res Opin. 2012;28(11):1799–1807. | ||
Naber KG, Schito G, Botto H, Palou J, Mazzei T. Surveillance study in Europe and Brazil on clinical aspects and Antimicrobial Resistance Epidemiology in Females with Cystitis (ARESC): implications for empiric therapy. Eur Urol. 2008;54(5):1164–1175. | ||
Hummers-Pradier E, Koch M, Ohse AM, Heizmann WR, Kochen MM. Antibiotic resistance of urinary pathogens in female general practice patients. Scand J Infect Dis. 2005;37(4):256–261. | ||
Moroh J-LA, Fleury Y, Tia H, et al. Diversity and antibiotic resistance of uropathogenic bacteria from Abidjan. Afr J Urol. 2014;20:18–24. | ||
Manjunath GN, Prakash R, Annam V, Shetty K. Changing trends in the spectrum of antimicrobial drug resistance pattern of uropathogens isolated from hospitals and community patients with urinary tract infections in Tumkur and Bangalore. Int J Biol Med Res. 2011;2(2):504–507. | ||
Kurepina N, Kreiswirth BN, Mustaev A. Growth-inhibitory activity of natural and synthetic isothiocyanates against representative human microbial pathogens. J Appl Microbiol. 2013;115(4):943–954. | ||
Grigoryan L, Trautner BW, Gupta K. Diagnosis and management of urinary tract infections in the outpatient setting: a review. JAMA. 2014;312(16):1677–1684. | ||
Johansen T, Botto H, Cek M, et al. Critical review of current definitions of urinary tract infections and proposal of an EAU/ESIU classification system. Int J Antimicrob Agents. 2011;38 Suppl:64–70. | ||
Stamm WE, Counts GW, Running KR, Fihn S, Turck M, Holmes KK. Diagnosis of coliform infection in acutely dysuric women. N Engl J Med. 1982;307(8):463–468. | ||
Kunin CM, White LV, Hua TH. A reassessment of the importance of “low-count” bacteriuria in young women with acute urinary symptoms. Ann Intern Med. 1993;119(6):454–460. | ||
Gágyor I, Bleidorn J, Kochen MM, Schmiemann G, Wegscheider K, Hummers-Pradier E. Ibuprofen versus fosfomycin for uncomplicated urinary tract infection in women: randomised controlled trial. BMJ. 2015;351:h6544. | ||
Schandelmaier S, von Elm E, You JJ, et al. Premature Discontinuation of Randomized Trials in Critical and Emergency Care: A Retrospective Cohort Study. Crit Care Med. 2016;44(1):130–137. | ||
Kasenda B, von Elm E, You J, et al. Prevalence, characteristics, and publication of discontinued randomized trials. JAMA. 2014;311(10):1045–1051. | ||
Williams RJ, Tse T, DiPiazza K, Zarin DA. Terminated Trials in the ClinicalTrials.gov Results Database: Evaluation of Availability of Primary Outcome Data and Reasons for Termination. PLoS One. 2015;10(5):e0127242. | ||
Gágyor I, Bleidorn J, Wegscheider K, Hummers-Pradier E, Kochen MM. Practices, patients and (im)perfect data – feasibility of a randomised controlled clinical drug trial in German general practices. Trials. 2011;12:91. | ||
Wilson S, Delaney BC, Roalfe A, et al. Randomised controlled trials in primary care: case study. BMJ. 2000;321(7252):24–27. | ||
Bleidorn J, Bucak S, Gágyor I, Hummers-Pradier E, Dierks ML. Why do - or don’t - patients with urinary tract infection participate in a clinical trial? A qualitative study in German family medicine. Ger Med Sci. 2015;13:Doc 17. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


