Back to Journals » Clinical Ophthalmology » Volume 15
Repeatability of Vascular Density Measurement of the Three Retinal Plexus Layers Using OCT Angiography in Pathologic Eyes (OCTA Vascular Density Repeatability of Three Plexus Layers)
Authors Mukkamala L, Nguyen M , Chang M, Park SS
Received 1 October 2020
Accepted for publication 25 November 2020
Published 8 January 2021 Volume 2021:15 Pages 93—103
DOI https://doi.org/10.2147/OPTH.S284872
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Lekha Mukkamala,1 Michael Nguyen,1 Melinda Chang,1– 3 Susanna S Park1
1Department of Ophthalmology & Vision Science, University of California Davis Eye Center, Sacramento, CA, USA; 2Vision Center, Children’s Hospital of Los Angeles, Los Angeles, CA, USA; 3Roski Eye Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA
Correspondence: Susanna S Park
Department of Ophthalmology & Vision Science, University of California Davis Eye Center, 4860 Y St., Suite 2400, Sacramento, CA 95817, USA
Tel + 1-916-734-6074
Fax + 1-916-734-6197
Email [email protected]
Purpose: Although commercial optical coherence tomography angiography (OCTA) machines quantitate retinal vascular density (VD) by dividing the vasculature into superficial and deep capillary plexus (SCP, DCP), histology reveals three distinct plexus layers. This study tested the hypothesis that the VD measurement of three distinct retinal plexus layers obtained using custom segmentation has high repeatability comparable to that of automatically segmented SCP and DCP layers.
Materials and Methods: Forty-four participants (86 eyes) were enrolled – 54 eyes with retinal vasculopathy and 25 eyes with macular edema. Macular OCTA images (3x3 mm and 6x6 mm) were obtained twice within 30 minutes by the same personnel using the same instrument (AngioVue, Optovue, version 2018.0.0.18). The intraclass correlation coefficient (ICC) was calculated to access repeatability.
Results: The repeatability of VD for SCP and DCP was good-to-moderate (ICC=0.65– 0.85) and minimally affected by image quality, retinal vasculopathy, or macular edema. The repeatability of the VD of the custom-segmented intermediate and deep plexus layers (cICP and cDCP) was poor/moderate (ICC=0.40– 0.74) but better in the subset without macular edema using 3x3 mm scans with good images quality (ICC=0.58– 0.93). Repeatability of cICP and cDCP VD measurement for 6x6 mm scans was poor (ICC≤ 0.5) in eyes with retinal vasculopathy and/or macular edema.
Conclusion: Although repeatability of the VD measurement is high for the automatically segmented SCP and DCP, repeatability of VD is poor for the cICP and cDCP using larger scans in eyes with retinal vasculopathy and/or macular edema.
Keywords: deep retinal plexus, intermediate retinal plexus, macular edema, middle retinal plexus, retinal vasculopathy
Plain Language Summary
Optical coherence tomography angiography (OCTA) is a new imaging modality for visualizing the retinal vasculature in three-dimensions. It is rapid and non-invasive. Currently available commercial OCTA machines can measures the density of the blood vessels in the retina by dividing the retinal vasculature into two layers of varying depth. In reality, the retinal vasculature has three distinct vascular layers of differing retinal depth which can be identified by manually customizing the setting in the OCTA machine. Accurate measurement of the vascular density in these different retinal vascular layers is important for reliable assessment of severity of retinal vasculopathy, but it is unknown whether the vascular density measurement of these three layers obtained by manual customized setting is reliable and repeatable. This study used a commercially available OCTA instrument and found that the vascular density measurements was highly repeatable when divided into two layers of varying depth. However, when the OCTA machine is customized to measure the vascular density of the retinal vascular layers by dividing the vasculature into three layers of varying depth, the repeatability of the vascular density measurement decreased for the deeper layers. In fact, the repeatability was poor for the intermediate and deep retinal vascular layers in eyes with retinal vascular disease when using OCTA scans that cover a larger retinal area. OCTA scans with higher image resolution which cover a smaller area of the retina yielded improved repeatability of vascular density measurement of these deeper layers and recommended in eyes with retinal vasculopathy.
Introduction
Optical coherence tomography angiography (OCTA) uses motion-contrast imaging to provide three-dimensional retinal blood flow information non-invasively.1–3 Prior studies have shown that the areas of retinal nonperfusion noted on traditional fluorescein angiography correlate well with flow voids noted using OCTA.2,3
Commercial OCTA instruments include software that provides automated segmentation of the retinal vasculature into superficial and deep retinal capillary plexus layers (SCP and DCP, respectively). Automated quantitation of the vascular density (VD) in these layers is possible.4–12 Alterations in retinal vascular flow in SCP and DCP have been described in eyes with retinal vascular diseases.5–7
Histology and ultrahigh resolution OCT imaging, however, identified three distinct retinal vascular plexus layers.6,13 These three distinct layers can be imaged using commercial OCTA instruments by custom-segmentation.5,14,15 Although microvascular perfusion abnormalities are found in all three layers using OCTA in eyes with diabetic retinopathy, visual acuity appears to correlate most closely with changes in VD of the deepest plexus layer imaged by custom-segmentation (cDCP).5 The retinal vascular changes in the custom-segmented intermediate plexus layer (cICP), also called middle capillary plexus layer, was associated with disease progression.15
Repeatability of retinal VD measurement using OCTA has been studied for the SCP and DCP using various different OCTA instruments, but repeatability of VD measurement for the cDCP and cICP has not been studied to date. Furthermore, OCTA software varies among vendors and continues to be refined and upgraded. Thus, the repeatability of OCTA VD measurements obtained using an older software or a different vendor may yield different findings. Two studies reported high repeatability of SCP VD measurement in normal eyes using a OCTA instrument that uses optical microangiography.11,16 Others showed repeatability of the VD of the SCP can be affected by signal strength, visual acuity, average VD, and macular thickness in normal eyes and eyes with retinal pathology.17–21 Repeatability of the VD of the deeper retinal capillary layers was not evaluated in these studies due to concerns about projection artifacts. A few studies that did evaluate repeatability of the VD of the DCP were limited to normal eyes and showed variable findings depending on the machine used.9,10 In fact, OCTA machines made by different vendors had comparable vascular density measurement for the SCP but not for the DCP.22
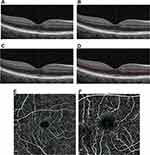
In the current study, the repeatability of the VD measurement of the three plexus layers, SCP, cICP, and cDCP, was assessed using Optovue OCTA instrument with the latest software (AngioVue, Optovue, version 2018.0.0.18, Fremont, CA). Of note, the thicker DCP obtained using Optovue OCTA is a combination of cICP and cDCP (Figure 1). Optovue OCTA uses split-spectrum amplitude decorrelation angiography for OCTA and corrects for projections artifacts in the deeper retinal layers.12 We selected this OCTA instrument since the software for VD measurement is now commercially available and was used previously to measure VD of the cDCP and cICP in eyes with diabetic retinopathy.5 Since fundus pathology can affect VD repeatability of the SCP,17–20 we enrolled eyes with retinal vasculopathy, macular edema, and optic nerve pathology as well as contralateral normal eyes. We tested the hypothesis that repeatability of VD measurement of all three plexus layers is high and unaffected by retinal vasculopathy or macular edema.
Materials and Methods
This prospective cross-sectional study enrolled individuals with retinal vascular disease (±macular edema), optic neuropathy, and normal contralateral eyes who were examined at the University of California Davis Eye Center between September 1, 2017, and April 30, 2018. The study was conducted according to a protocol approved by the University of California, Davis Office of Human Research and in adherence to the tenets of the Declaration of Helsinki and Health Insurance Portability and Accountability Act.
Individuals qualified for this study if they were at least 18 years of age, signed informed consent, and had best corrected visual acuity (BCVA) of at least 20/200 in one eye. Individuals were excluded if pregnant or head tremor, nystagmus, or other factors resulted in poor fixation or head immobilization. Eyes with other concurrent retinopathy, advanced glaucoma, refractive error >6 diopters or intraocular surgery within 6 months of enrollment were excluded. A complete eye examination was performed on the day of enrollment and imaging.
Image Acquisition and Analysis
All participants underwent two sessions of macular OCTA imaging of both eyes performed by the same personnel using the same machine. The participants were freshly positioned at the OCTA machine for each session which included a 3x3 mm and a 6x6 mm scan centered on the fovea. All imaging was done after pupillary dilation and completed within 30 minutes. Central macular thickness (CMT) is defined as the thickness of the central zone in the automated ETDRS macular thickness map.
The OCTA instrument had a scan rate of 70,000 A-scans/seconds and axial and transverse resolution of 5 and 15 μm, respectively. The segmentation of the SCP and DCP was performed automatically using pre-set settings (Figure 1): SCP was between 3 μm below the internal limiting membrane (ILM) and 15 μm below the inner plexiform layer (IPL); DCP was underneath the SCP to 70 μm below the IPL. Segmentation for the cDCP and cICP was manually set on the OCTA machine as previously described.5 The inner boundary of cDCP was 19 μm below the inner nuclear layer-outer plexiform layer (OPL) junction, and the outer boundary was 9 μm below the OPL-outer nuclear layer (ONL) junction. The segmentation of the cICP was manually obtained from the DCP by moving the outer boundary 9 μm above the OPL-ONL junction. All images were reviewed and corrected manually for any segmentation error before recording VD measurement. OCTA scans with signal strength <6 were considered poor quality.5
The VD measurement is the proportion of the scanned macular area covered by detectable blood flow for regions of the macula corresponding to the ETDRS macular map (Figure 1). The “overall ETDRS” refers to the entire 3 mm circle of the ETDRS map for the 3x3 mm scan and entire 6 mm circle for the 6x6 mm scan. The “fovea” refers to VD of the central 1 mm zone. The foveal avascular zone (FAZ) was measured automatically by the machine after manually correcting any boundary error.
Statistical Analysis
All statistical analysis was performed using SPSS software version 21.0 (Armonk, NY: IBM Corp.). Intraclass correlation coefficient (ICC) and coefficient of variation (CV) were calculated for VD measurement. Repeatability based on ICC was poor if <0.50, moderate if 0.50–0.75, good if 0.75–0.90, and excellent if >0.90.23 A CV of <10% was considered low variability. Student’s t-test was performed for comparison of scale variables. A P-value<0.05 was considered statistically significant.
Results
Baseline Characteristics
Forty-four participants (86 eyes) were enrolled and imaged. After excluding OCTA scans with signal strength <6, eyes with good OCTA image quality (“Good Image Cohort”) included 54 eyes (34 participants) for 3x3 mm OCTA scans and 47 eyes (32 participants) for 6x6 mm OCTA scans.
Table 1 summarizes the demographics and clinical characteristics of the Total Cohort compared to the Good Image Cohort. Most of the eyes with retinal vasculopathy had diabetic retinopathy or retinal vein occlusion, the remaining having radiation retinopathy or retinal artery occlusion. Eyes with optic neuropathy included eyes with disc edema/papilledema or optic nerve atrophy. No significant difference was noted in mean BCVA and mean CMT between the Total Cohort and the Good Image Cohort.
 |
Table 1 Demographics and Clinical Characteristics of the Good Image Cohorts for Each Scan Size and Total Cohort. P-value Shown Compares the Total Cohort to the Good Image Cohort |
Vascular Density Repeatability
Effect of Image Quality and Scan Size
Table 2 summarizes the ICC and CV of VD measurement for the Total Cohort and the Good Image Cohort. For the 3x3 mm scan, repeatability of VD measurement for the overall ETDRS for SCP was good (ICC=0.853) for the Total Cohort and excellent for the Good Image Cohort (ICC=0.918), with no significant difference based on the 95% confidence interval (CI). For the DCP, repeatability of the VD measurement was moderate for the Total Cohort (ICC=0.665) and the Good Image Cohort (ICC=0.637). For cDCP, the repeatability of VD measurement was poor for the Total Cohort (ICC=0.419) and significantly improved in the Good Image Cohort (ICC=0.759) based on 95% CI. For the cICP, repeatability for the Total Cohort and the Good Image Cohort were both moderate (ICC=0.662 and 0.772, respectively).
For the 6x6 mm OCTA scans, the repeatability of overall ETDRS SCP VD measurement was moderate for the Total Cohort (ICC=0.651) and significantly improved to excellent for the Good Image Cohort (ICC=0.929) based on the 95% CI. For the DCP and cDCP, the repeatability was moderate for the Total Cohort (ICC=0.697 and 0.744, respectively) and not improved in the Good Image Cohort (ICC=0.697 and 0.609, respectively). For cICP, repeatability was good (ICC=0.820) for the Total Cohort and moderate for the Good Image Cohort (ICC=0.689), with no significant difference.
For the Good Image Cohort, repeatability of VD measurement was evaluated for the regions within the ETDRS map including the fovea, 3 mm inner ring, and 6 mm outer ring for 6x6 mm scans (Table 2). All analyzed regions of both size scans had good-to-excellent repeatability of VD measurement for the SCP (ICC range=0.863–0.929) except for the 6 mm outer ring which had moderate repeatability (ICC=0.666). Repeatability of DCP was also good-to-excellent for both size scans (ICC range=0.703–0.927) and similar to that for SCP. Repeatability of cDCP was moderate for all regions of the 3x3 mm scan (ICC=0.699–0.720) and moderate-to-poor for the 6x6 mm scan (ICC=0.0405–0.529), with the 6 mm outer ring showing poor repeatability (ICC=0.405). For cICP, repeatability was good using the 3x3 mm scan and trended lower to moderate for the 6x6 mm scans (ICC=0.508–0.737), the difference only being significant for the fovea. Variability was low overall with CV ≤10% in all categories except for the fovea for the cDCP on both size scans (CV 12.7% and 14.3%, respectively).
Effect of CME and Retinal Vasculopathy
In order to determine whether the presence of CME affected the repeatability of VD measurements on OCTA, ICC was calculated for the subset of eyes with CME and compared to eyes without CME among eyes in the Good Image Cohort (Table 3). The eyes without CME included normal eyes and eyes with optic neuropathy or retinal vasculopathy. These eyes had good-to-excellent repeatability for SCP and DCP for both the 3x3 mm and 6x6 mm scans (ICC=0.783–0.953). For the cDCP and cICP, repeatability was lower, in the moderate/good range (0.613–0.877) except for the 6x6 mm scan overall ETDRS (ICC=0.492 for cDCP; ICC=0.338 for cICP) and 6 mm outer ring for cICP (ICC=0.416), which showed poor repeatability.
 |
Table 3 Repeatability of Macular Vascular Density Measurement Obtained Using OCTA for Eyes with and without CME or Retinal Vasculopathy. (A) 3x3 mm OCTA Scans. (B) 6x6 mm OCTA Scans |
For eyes with CME, repeatability of VD measurement for SCP was good-to-excellent overall for 3x3 mm and 6x6 mm scans except for the 6 mm outer ring which had poor repeatability (ICC=0.257). Repeatability for DCP was good for all regions of both size scans (ICC range=0.75–0.93) except for poor repeatability noted for the 3x3 mm scan overall ETDRS (ICC=0.188) and 6x6 mm scan of fovea (ICC=0.399). For cDCP and cICP, repeatability was good-to-moderate for the 3x3 mm scan (ICC=0.944– 0.746) except for the overall ETDRS which had poor repeatability (ICC=0.342). For the 6x6 mm scan, repeatability was poor (ICC=0.451–0.058) for all regions but moderate for the overall ETDRS (ICC=0.574 for cDCP; ICC=0.774 for cICP). When compared to eyes without CME, the repeatability of VD of cDCP of the fovea was significantly reduced in eyes with CME (based on the 95% CI). Variability was also high for the fovea for cDCP and cICP and the 6 mm outer ring of cDCP (CV=18.1–25.6).
To assess if the presence of retinal vasculopathy affected VD repeatability, the ICC was calculated for the subset of eyes with vasculopathy without CME (Table 3). The repeatability of the SCP and DCP was good-to-excellent in all regions of the 3x3 mm and 6x6 mm scans (ICC range=0.909–0.947 for SCP; 0.793–0.940 for DCP). For the cDCP and cICP, repeatability using the 3x3 mm scan was also good-to-excellent (ICC=0.581–0.933). However, for the 6x6 mm scan, repeatability was poor for all regions (ICC=0.147–0.499) except for the 3 mm inner ring which had moderate repeatability (ICC=0.701). When compared to repeatability of VD of 3x3 mm scans of cDCP, the difference was statistically significant for the overall ETDRS based on 95% CI.
Foveal Avascular Zone
The repeatability of FAZ measurement obtained using OCTA software was calculated (Tables 2 and 3). It was excellent for both 3x3 mm and 6x6 mm scans (ICC=0.935–0.921, respectively). The presence of CME did not affect the repeatability of the FAZ size measurement (ICC=0.958 and 0.895 for 3x3 mm scan and 6x6 mm scan, respectively), but the variability in FAZ measurement was on the high side among these eyes with CME (CV=24.2% for the 3x3 mm scan; CV=19.1% for the 6x6 mm scan). In eyes with retinal vasculopathy but no CME, the FAZ size measurements had good repeatability (0.793–0.800 for both size scans).
Discussion
This study used a commercial OCTA instrument (Optovue) with the latest software to compare the repeatability of VD measurement for the three retinal plexus layers (SCP, cICP, and cDCP) and assess the impact of OCTA image quality and presence of macular edema and retinal vasculopathy on repeatability of these VD measurements. The study findings are important since changes in VD of cDCP and cICP using OCTA have been correlated with vision loss and progression of retinopathy in eyes with diabetic retinopathy.5,15 Furthermore, various studies have demonstrated that early vascular changes in retinal vascular disease likely occur in deeper plexus layers.24–28 Thus, it would be important to know whether the VD of these deep, thin plexus layers within the retina can be measured accurately and reliably using a commercial OCTA instrument.
The repeatability of VD measurement for cICP and cDCP had not been studied previously. Most prior studies on repeatability of OCTA VD measurement were limited to the analysis of the SCP due to concerns about project artifacts in the deeper layers.11,–16–20 These prior studies consistently showed high repeatability of VD measurement of the SCP. Among diabetic patients, SCP VD measurement repeatability was high within a session and between visits 1 month apart.29 For the DCP somewhat variable results were reported depending on the type of OCTA device used. A study showed good repeatability of VD measurement in DCP in normal eyes using a Nidek RS-3000 OCTA instrument.9 A study using Optovue OCTA showed that repeatability of the VD measurement of DCP in healthy eyes could be improved using the eye tracking option available in the machine for the second scan.10 Yet another study used three different OCTA instruments to show that repeatability of VD measurement was good for the SCP but not DCP among machines made by different vendors.22
In our current study, we used the latest OCTA software that corrects for projection artifacts to evaluate the repeatability of VD measurement for the three retinal capillary plexus layers (SCP, cICP, and cDCP). We found that VD repeatability varies somewhat with the size and quality of the OCTA scan, the capillary plexus layer imaged, and the presence of macular edema and/or retinal vasculopathy. We found that a smaller 3x3 mm OCTA scan tended to have higher repeatability of VD measurement when compared to a larger 6x6 mm scan. This is likely due to higher image resolution of the smaller OCTA scan. A similar observation was noted previously for SCP in normal eyes.11 We also noted that the subgroup of eyes with good OCTA images showed a somewhat higher repeatability of VD measurement. This difference was statistically significant for the cDCP of the 3x3 mm scan and the SCP for the 6x6 mm scan (Table 2). A similar finding was reported previously for the VD measurement of SCP in normal eyes.21 The most noteworthy finding of our study is that repeatability of VD measurement varied depending on the depth and thickness of the retinal vascular plexus layers with the SCP tending to have highest repeatability of VD measurement when compared to that for the DCP, cDCP, and cICP. This observation may be due to the smaller size and density of vessels in the deeper plexus layers and the narrower band of retina represented by the cDCP and cICP compared to the SCP (Figure 1). Consistent with this hypothesis, a prior study of SCP in normal eyes showed that the average VD of the layer affected repeatability of the VD measurement.21 However, they did not evaluate factors affecting repeatability of the deeper plexus layers.
In eyes with CME, we found that the repeatability of VD measurement of cDCP and cICP was poor, particularly for the fovea using the 6x6 mm scan (Table 3). This is likely due to increased disruption of the retinal vascular layer from CME within the thin segment of cDCP and cICP. The presence of CME also can result in errors in segmentation, although this was minimized in our study by manually correcting any segmentation errors seen before VD data collection. The repeatability of the VD measurement for the thicker SCP and DCP also was somewhat reduced in eyes with CME for the larger 6x6 mm OCTA scans but to a lesser extent. A recent study in eyes with retinal vein occlusion also showed some reduction in repeatability of VD measurement with increasing macular thickness, but this study was limited to SCP and did not evaluate the deeper plexus layers.20
To evaluate the effect of retinal vasculopathy on repeatability of VD measurement, we evaluated the ICC of the subset of eyes with retinal vasculopathy without CME. Repeatability of VD measurement for SCP and DCP was high, but the repeatability of VD measurement for the cDCP and cICP was reduced using the 6x6 mm OCTA scan but not as severely reduced as that noted among eyes with CME. This is not surprising since all eyes with CME in our study also had retinal vasculopathy. In our study eyes with CME, the structural changes within the retina resulting from retinal vasculopathy as well as CME likely both contributed to reduced repeatability of VD measurement for the cDCP and cICP. In eyes with retinal vasculopathy, macular ischemia resulting in reduced visual acuity and reduced retinal VD, may contribute to the reduced repeatability of VD measurement.20 Based on our study observations, we recommend using the higher image quality 3x3 mm OCTA scan in eyes with retinal vasculopathy and/or CME for more accurate and reliable VD measurement of the cDCP and cICP.
Our study has some limitations. First, our sample size was limited and included eyes with various pathologies. The size was further reduced for subset analysis to study the impact of image quality, retinal vasculopathy, and macular edema on repeatability. Therefore, the study may not be powered to detect more subtle differences among study subgroups. Nonetheless, we identified several factors that were associated with significant reduction in repeatability of the VD measurement for the thinner cDCP and cICP when compared to SCP or DCP. Second, the pathology in our study eyes was limited to retinal vasculopathy or optic disc pathology. The impact of other retinal pathologies on repeatability of VD measurement for the three retinal plexus layers remains unknown. Next, our study did not explore the impact of eye-tracking on repeatability of VD measurement. A prior study showed improved repeatability of the VD measurement of the DCP in normal eyes when eye-tracking was turned on using this OCTA machine.10 Thus, it is possible that the VD repeatability for the cICP and cDCP in eyes retinal vasculopathy and/or CME may be improved using this feature for follow-up imaging. Finally, the study used the latest OCTA software, but the OCTA software development and refinement continue. Thus, a future OCTA software may provide further improvement in repeatability of VD measurements.
Conclusions
Although repeatability of VD measurement using OCTA for SCP and DCP is high in normal and pathologic eyes, repeatability for cDCP and cICP is poor using larger OCTA scans, especially in eyes with retinal vasculopathy and/or CME. The study highlights the limitations for VD measurement of the deeper, thinner plexus layers using the current commercial OCTA system. As OCTA software continues to improve, future studies may shed light on whether repeatability of VD measurement of the deeper retinal plexus layers can be improved for eyes with retinal pathology.
Acknowledgment
The research was supported in part by the University of California Davis Eye Center Kohl Medical Student Scholarship (MN). The authors would like to thank the retinal imaging team at the University of California Davis Eye Center for data collection. This was an investigator-initiated study without external financial support. However, we thank Optovue for providing the OCT/OCTA instrument and software as a loan for the study duration.
Previous presentations
Presented in part as a poster at the annual meeting of the American Society of Retinal Specialists, July 22, 2018, Vancouver, Canada and as a paper presentation at the UC Davis Annual Research Symposium, June 22, 2019, Sacramento, CA and at the UC Davis Kohl Medical Scholar Research Symposium, September 29, 2020.
Disclosures
Susanna S Park reports research grant support via employer (Novartis/Roche; Allergan; Greybug) investigating anti-VEGF therapy for retinal disorders. The authors report no other potential conflicts of interest for this work.
References
1. Savastano MC, Lumbroso B, Rispoli M. In vivo characterization of retinal vascularization morphology using optical coherence tomography angiography. Retina. 2015;35:2196–2203. doi:10.1097/IAE.0000000000000635
2. Spaide RF, Klancnik JM, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133:45–50. doi:10.1001/jamaophthalmol.2014.3616
3. Schwartz DM, Fingler J, Kim DY, et al. Phase-variance optical coherence tomography: a technique for noninvasive angiography. Ophthalmology. 2014;121:180–187. doi:10.1016/j.ophtha.2013.09.002
4. Huang D, Jia Y, Gao SS, Lumbroso B, Rispoli M. Optical coherence tomography angiography using the Optovue device. Dev Ophthalmol. 2016;56:6–12.
5. Dupas B, Minvielle W, Bonnin S, et al. Association between vessel density and visual acuity in patients with diabetic retinopathy and poorly controlled type 1 diabetes. JAMA Ophthalmol. 2018;136:721–728. doi:10.1001/jamaophthalmol.2018.1319
6. Onishi AC, Nesper PL, Roberts PK, et al. Importance of considering the middle capillary plexus on OCT angiography in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2018;59:2167–2176. doi:10.1167/iovs.17-23304
7. Vujosevic S, Muraca A, Alkabes M, et al. Early microvascular and neural changes in patients with type 1 and type 2 diabetes mellitus without clinical signs of diabetic retinopathy. Retina. 2019;39:435–445. doi:10.1097/IAE.0000000000001990
8. Corvi F, Pellegrini M, Erba S, Cozzi M, Staurenghi G, Giani A. Reproducibility of vessel density, fractal dimension, and foveal avascular zone using 7 different optical coherence tomography angiography devices. Am J Ophthalmol. 2018;186:25–31. doi:10.1016/j.ajo.2017.11.011
9. Al-Sheikh M, Tepelus TC, Nazikyan T, Sadda SR. Repeatability of automated vessel density measurements using optical coherence tomography angiography. Br J Ophthalmol. 2017;101:449–452. doi:10.1136/bjophthalmol-2016-308764
10. Alnawaiseh M, Brand C, Bormann E, Sauerland C, Etes N. Quantification of macular perfusion using optical coherence tomography angiography: repeatability and impact of an eye-tracking system. BMC Ophthalmol. 2018;18:123. doi:10.1186/s12886-018-0789-z
11. Lei J, Durbin M, Shi Y, et al. Repeatability and reproducibility of superficial macular retinal vessel density measurements using optical coherence tomography angiography en face images. JAMA Ophthalmol. 2017;135:1092–1098. doi:10.1001/jamaophthalmol.2017.3431
12. Zhang A, Zhang Q, Chen CL, Wang RK. Methods and algorithms for optical coherence tomography-based angiography: a review and comparison. J Biomed Opt. 2015;20:100901. doi:10.1117/1.JBO.20.10.100901
13. Gilmour DF. Familial exudative vitreoretinopathy and related retinopathies. Eye (Lond). 2015;29(1):1–14. doi:10.1038/eye.2014.70
14. Chung CS, Nesper PL, Park JJ, Fawzi AA. Comparison of Zeiss Cirrus and Optovue RTVue OCT angiography systems: a quantitative and qualitative approach examining the three capillary networks in diabetic retinopathy. Ophthalmic Surg Lasers Imaging Retina. 2018;49:e198–e205. doi:10.3928/23258160-20181101-18
15. Park JJ, Chung CS, Fawzi AA. Visualizing structure and vascular interactions: macular nonperfusion in three capillary plexuses. Ophthalmic Surg Lasers Imaging Retina. 2018;49:e182–190. doi:10.3928/23258160-20181101-16
16. Zhao Q, Yang W, Wang XN, et al. Repeatability and reproducibility of quantitative assessment of the retinal microvasculature using optical coherence tomography angiography based on optic microangiography. Biomed Environ Sci. 2018;31:407–412.
17. Lee MW, Kim KM, Lim HB, Jo YJ, Kim JY. Repeatability of vessel density measurements using optical coherence tomography angiography in retinal diseases. Br J Ophthalmol. 2018.
18. Conti FF, Young JM, Silva FQ, Rodrigues EB, Singh RP. Repeatability of split-spectrum amplitude-decorrelation angiography to assess capillary perfusion density within optical coherence tomography. Ophthalmic Surg Lasers Imaging Retina. 2018;49:e9–e19. doi:10.3928/23258160-20180907-02
19. You Q, Freeman W, Weinreb R, et al. Reproducibility of vessels density measurements with optical coherence tomography angiography in eyes with and without retinopathy. Retina. 2017;37(8):1475–1482. doi:10.1097/IAE.0000000000001407
20. Kim KM, Lee MW, Lim HB, Koo HM, Shin YZ, Kim JY. Repeatability of measuring the vessel density in patients with retinal vein occlusion: an optical coherence tomography angiography study. PloS One. 2020;15(6):e0234933. doi:10.1371/journal.pone.0234933
21. Lee TH, Bin Lim H, Nam KY, Kim K, Kim JY. Factors affecting repeatability of assessment of the retinal microvasculature using optical coherence tomography angiography in healthy subjects. Sci Rep. 2019;9(1):16291. doi:10.1038/s41598-019-52782-6
22. Anegondi N, Kshirsagar A, Mochi TB, Sinha Roy A. Quantitative comparison of retinal vascular features in optical coherence tomography angiography images from three different devices. Ophthalmic Surg Lasers Imaging Retina. 2018;49:488–496. doi:10.3928/23258160-20180628-04
23. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–163. doi:10.1016/j.jcm.2016.02.012
24. de Carlo TE, Romano A, Waheed NK, Duker JS. A review of optical coherence tomography angiography (OCTA). Int J Retina Vitreous. 2015;1:5. doi:10.1186/s40942-015-0005-8
25. Kim AY, Chu Z, Shahidzadeh A, Wang RK, Puliofito CA, Kashani AH. Quantifying microvascular density and morphology in diabetic retinopathy using spectral-domain optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:362–370. doi:10.1167/iovs.15-18904
26. Simonett JM, Scarinci F, Picconi F, et al. Early microvascular retinal changes in optical coherence tomography angiography in patients with type 1 diabetes mellitus. Acta Ophthalmol. 2017;95:e751–e755. doi:10.1111/aos.13404
27. Freiberg FJ, Pfau M, Wons J, Wirth MA, Becker MD, Michels S. Optical coherence tomography angiography of the foveal avascular zone in diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2016;254:1051–1058. doi:10.1007/s00417-015-3148-2
28. Rosen RB, Andrade Romo JS, Krawitz BD, et al. Earliest evidence of preclinical diabetic retinopathy revealed using optical coherence tomography angiography perfused capillary density. Am J Ophthalmol. 2019;203:103–115. doi:10.1016/j.ajo.2019.01.012
29. Czako C, Sandor G, Ecsedy M, et al. Intrasession and between-visit variability of retinal vessel density values measured with OCT angiography in diabetic patients. Sci Rep. 2018;8(1):10598. doi:10.1038/s41598-018-28994-7
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.