Back to Journals » Research and Reports in Urology » Volume 12
Regulation of Cellular Stress Signaling in Bladder Ischemia
Authors Yang J, Li Y, Azad R, Azadzoi K
Received 17 July 2020
Accepted for publication 1 September 2020
Published 17 September 2020 Volume 2020:12 Pages 391—402
DOI https://doi.org/10.2147/RRU.S271618
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jan Colli
Jing-Hua Yang,1 Yedan Li,2 Roya Azad,2 Kazem Azadzoi3
1Department of Surgery, Boston University School of Medicine, Boston, MA, USA; 2Department of Urology, VA Boston Healthcare System, Boston, MA, USA; 3Department of Urology and Department of Pathology, VA Boston Healthcare System and Boston University School of Medicine, Boston, MA, USA
Correspondence: Kazem Azadzoi
VA Boston Healthcare System, Building 1A, Room 317 (151), 150 South Huntington Avenue, Boston, MA 02130, USA
Tel +857 364-5602
Email [email protected]
Introduction: The etiology of lower urinary tract symptoms in patients with non-obstructed non-neurogenic bladder remains largely unknown. Clinical studies divulged a significant correlation between reduced bladder blood flow and low bladder compliance. Animal models of bladder ischemia displayed structural modifications, characterized by loss of smooth muscle cells and accumulation of connective tissue in the bladder wall. The underlying mechanisms contributing to structural damage in bladder ischemia remain largely elusive. We previously reported that structural modifications in bladder ischemia correlate with upregulated stress proteins and cell survival signaling, suggesting the potential role of cellular stress in ischemic damage. However, stress response molecules and downstream pathways eliciting bladder damage in ischemia remain largely undetermined.
Methods: Using a rat model of bladder ischemia along with a cell culture hypoxia model, we investigated stress signaling molecules in the ischemic bladder tissues and hypoxic bladder smooth muscle cells.
Results: Our data suggest simultaneous upregulation of two major cellular stress-sensing molecules, namely apoptosis signal-regulating kinase 1 (ASK1) and caspase-3, implying degenerative insult via stress signaling pathway in bladder ischemia. Consistent with bladder ischemia, incubation of cultured human bladder smooth muscle cells at low oxygen tension increased both ASK1 and caspase-3 expression, insinuating hypoxia as an essential factor in ASK1 and caspase-3 upregulation. Gene deletion of ASK1 by ASK1 siRNA in cultured smooth muscle cells prevented caspase-3 upregulation by hypoxia, suggesting caspase-3 regulation by ASK1 under the ischemic/hypoxic conditions. Upregulation of ASK1 and caspase-3 in rat bladder ischemia and human bladder smooth muscle cell hypoxia was associated with subcellular structural modifications consistent with the initial stages of apoptotic insult.
Conclusion: Our data suggest that stress sensing by ASK1 and caspase-3 may contribute to subcellular structural damage and low bladder compliance. The ASK1/caspase-3 pathway may provide therapeutic targets against cellular stress and degenerative responses in bladder ischemia.
Keywords: bladder, ischemia, hypoxia, cellular stress
Introduction
The role of bladder ischemia in aging-related lower urinary tract symptoms (LUTS) has been documented in human and animal models. In clinical studies, Lin et al studied 36,042 patients and reported a significant correlation between LUTS and the prevalence of atherosclerotic arterial occlusive disease.1 Gibbons et al reported that American Urological Association Symptom Score (AUA-SS) is significantly higher in patients with vascular disease risk factors in comparison with patients without vascular risk factors.2 Other studies revealed low bladder blood flow in the elderly population and found a close correlation between decreased bladder perfusion and LUTS in both men and women.3–5 These clinical observations suggested the role of ischemia as a potential contributing factor in aging-related bladder dysfunction and micturition disorders in both genders. Basic research with animal models showed that moderate ischemia provokes overactive bladder contractions and leads to structural damage.6–8 Prolonged severe ischemia was shown to impair bladder contractile activity and trigger degenerative responses in smooth muscle cells, microvasculature and nerve fibers.9 These changes in the ischemic bladder were associated with impairment of cellular antioxidant defense system, mitochondrial structural damage, depression of mitochondrial respiration and activation of cell survival signaling.10 Hypoxia, nutrients deficiency, and accumulation of metabolic waste in bladder ischemia were shown to compromise cellular homeostasis and initiate defensive responses via rebalancing mechanisms to protect cells against the ischemic insult.6–10 However, when ischemia persists, noxious free radicals prevail and cellular protective mechanisms decline leading to activation of cell danger responses and cellular stress signaling.6–10
Cellular stress is the cell’s reaction to environmental changes that compromise cellular homeostasis and provoke complex disturbances within the cells, leading to macromolecular damage to subcellular elements.11,12 Cellular stress response is tightly associated with ischemia-mediated interruption of nutrients and oxygen delivery to the cells and accumulation of deleterious free radicals and metabolic waste. Cell survival and death after exposure to stress depends on the severity and duration of stressful stimuli and the cell’s ability to handle the stress eliciting condition to which it is exposed.11,12 Cellular stress responses are regulated by cellular stress sensors. The cell’s initial rejoinder to stress begins with survival signaling followed by defensive responses to recover cells from the stressful insult.11,12 However, if the stressful stimulus remains unresolved, cellular stress sensors initiate cell danger signals and provoke degenerative responses via cell death signaling pathways.11,12
Apoptosis signal-regulating kinase 1 (ASK1) is a prominent cellular stress sensor that integrates downstream stress response molecules into signaling pathways that regulate cell fate.13–18 ASK1 is preferentially activated by stressful conditions involving disturbed oxygen tension such as ischemia, hypoxia, and oxidative stress.13,14 Activated ASK1 plays a pivotal role in inflammatory responses and apoptotic reactions in the ischemic/hypoxic tissues.13,14,16 ASK1 was shown to promote apoptotic cell death under the chronic ischemic conditions.13,14 While the underlying mechanisms remain largely unknown, activation of caspases is consistently implicated in apoptotic reactions triggered by ischemia.19,20 Among the caspase family, caspase-3 is a cellular stress intensity sensor activated by various apoptosis inducers and known to play an essential role in the execution and completion of apoptosis.19,20 The role of ASK1 and caspase-3 in ischemia-mediated bladder dysfunction has not been previously investigated.
Stress response proteins and cell survival signaling provoked by cellular stress in bladder ischemia were documented in our previous studies.10 However, stress response molecules regulating cell fate in bladder ischemia remain largely unknown. The goal of the present study was to investigate cellular stress-sensing molecules and define the structural consequences of cellular stress in chronic bladder ischemia.
Methods
Bladder Ischemia Model
Animal care and experimental protocols were in accordance with the guidelines and approval of our Institutional Animal Care and Use Committee at VA Boston Healthcare System. The animal model of bladder ischemia was developed in hypercholesterolemic rats using balloon endothelial denudation of the iliac arteries under general anesthesia, as previously described.4,6–8 The arterial ballooning procedure results in atherosclerosis-induced pelvic ischemia and subsequent changes in bladder contractile activity and compliance.4,6–8 The sham control group underwent similar surgical procedures without arterial endothelial denudation. At 8 weeks after arterial ballooning, cystometrograms were obtained and bladder blood flow was measured in the treated and control animals, as described below. Bladder tissues were then processed for analysis.
Measurement of Bladder Contractile Activity and Compliance
Animals were anesthetized with inhalation of 1–2% isoflurane mixed with oxygen. An abdominal incision was then made. Bladder pressure was recorded through a 23 gauge angiocatheter inserted into the bladder and connected to a pressure transducer. Spontaneous bladder contractions were recorded at a constant intravesical volume of 0.8 mL without infusion. After this, the bladder was emptied then infused with saline at a rate of 6 mL per hour using an infusion pump (Harvard Apparatus Inc, MA). Changes in intravesical pressure were recorded and premicturition pressure was noted. To determine bladder compliance, the ratio of infused volume over pre-micturition pressure minus pressure at the initiation of bladder filling was calculated.
Measurement of Bladder Blood Flow
After the recording of bladder contractile activity and compliance in the anesthetized rats, a laser Doppler needle probe was inserted into the muscular layer of the bladder wall. The Doppler probe was connected to a blood flowmeter (Transonic Systems, Inc., Ithaca, NY). Bladder blood flow was recorded at five different sites of the bladder at a constant intravesical volume of 0.8 mL. Average bladder blood flow in the hypercholesterolemic rats undergoing arterial ballooning was statistically analyzed versus bladder blood flow in sham controls.
Cell Culture Hypoxia Model
We previously reported that reduction of blood flow to the bladder diminishes bladder wall oxygen tension and leads to hypoxia.6,9 In the present study, we examined the direct role of hypoxia in stress signaling using an oxycycler cell culture model. Primary human bladder smooth muscle cells (Lonza, Allendale, NJ) were grown in cell culture media at 21% O2, 5% CO2, balance N2 at 37°C. Cell passaging and subculturing were carried out according to the standard protocols and viable cells were used for experiments. Cell viability and morphology were examined by trypan blue staining and transmission electron microscopy, respectively. Viable cells were incubated under hypoxia and normoxia conditions in a computerized servo-control cell oxycycler system (BioSpherix, Parish NY). The oxycycler monitors the levels of oxygen tension for the desired duration by infusing either oxygen or nitrogen into the cell culture chambers. In this study, we exposed cultured cells to either normoxia (21% oxygen) or continuous hypoxia (2% oxygen) conditions. After 48 hours, cells were collected on ice, lysed and processed for centrifugation. The protein-containing supernatant was collected and the pellet was discarded. Protein extracts were prepared and constant protein concentration per mL was achieved in each sample. The samples were processed for analysis as described below.
Assessment of Cellular Stress Markers in Bladder Ischemia
Two cellular stress-sensing molecules were examined in bladder tissues. 1) ASK1, a well-known stress-sensing molecule, that is known to be activated by a variety of cellular stress conditions including oxidative stress, endoplasmic reticulum stress and inflammatory conditions.13–16 2) Caspase-3, a cellular stress intensity sensor, also known as the executioner of apoptosis. Caspase-3 is activated by cell death proteases and mediates programed cell death.17–20 The expression levels of ASK1 and caspase-3 in the ischemic and control bladder tissues were analyzed using Western blotting, as described below.
Assessment of Cellular Stress Markers in Cultured Cells
After analysis of bladder tissues, cell culture experiments were carried out to determine the direct role of hypoxia in ASK1 and caspase-3 expression. Primary human bladder smooth muscle cells (Lonza, Allendale, NJ) were grown in cell culture media, then randomly divided into hypoxia and control groups. The two groups of cell culture dishes were processed to oxycyclers for incubation under distinct oxygen tensions, as described earlier. The first group was incubated under hypoxic conditions at 2% oxygen while the second group was incubated under normoxia condition at 21% oxygen. After 48 hours, cells were collected and processed for Western blotting of ASK1 and caspase-3, as described below.
Analysis of ASK1 and Caspase-3 Intercommunication
ASK1 senses cellular stress and activates apoptotic activity.13–16 Caspase-3 senses cellular stress and executes the apoptosis process via proteolytic mechanisms.17–20 Our goal was to examine whether ASK1 and caspase-3 crosstalk under the hypoxic conditions. Cultured human bladder smooth muscle cell dishes were prepared and randomly divided into treatment and control groups. In the treatment group, ASK1 gene deletion was achieved by transfecting cells with ASK1 small interfering RNA (siRNA, Santa Cruz Biotechnology, Dallas TX). The control group of cells was transfected with control siRNA provided by the manufacturer (Santa Cruz Biotechnology, Dallas TX). ASK1 siRNA and control siRNA transfection were carried out using lipofectamine RNAiMax (Thermo Fisher, Waltham MA), according to the manufacturer’s instructions. After transfection, treated and control groups of cells were incubated under hypoxic conditions at 2% oxygen, using the cell oxycycler system, as described earlier. At 48 hours after hypoxia, cell samples were processed for Western blotting to examine caspase-3 expression under the hypoxic condition in cells transfected with ASK1 siRNA versus cells transfected with control siRNA.
Western Blotting
Frozen bladder tissues were pulverized then homogenized and processed for centrifugation. Primary human bladder smooth muscle cells were collected from the oxycycler chambers on ice and then treated with lysis reagent (Sigma, St. Louis, MO) containing appropriate protease inhibitors. After centrifugation, pellets of the samples were discarded and the protein-containing supernatants were collected. Protein concentration was determined by NanoDrop ND-1000 Spectrophotometer (Thermo Fisher, Waltham MA). Protein extracts were diluted with Phosphate Buffered Saline (PBS) to ensure an equal concentration. Proteins were separated using sodium dodecyl sulfate-PAGE and then transferred to polyvinylidene difluoride filter membranes (Millipore, Bedford, MA). The membranes were incubated overnight with either ASK1 (Abcam, Cambridge, MA), caspase-3 (Abcam, Cambridge, MA) or β-actin (Cell Signaling, Danvers, MA) antibody at 4°C. After this, the membranes were incubated with the fluorescent-labelled secondary antibody for 2 hours and fluorescent signals were scanned with Typhoon 8600 imager (GE Healthcare, Pittsburg, PA). Protein expression was quantitated by densitometry using ImageJ software.
Morphologic Assessment
The goal was to elucidate the morphological consequences of cellular stress in the ischemic bladder tissues and hypoxic smooth muscle cells displaying ASK1 and caspase-3 upregulation. Using transmission electron microscopy (TEM), ultrastructural changes in the ischemic bladder tissues and hypoxic cells were compared versus control tissues and cells, respectively. Samples were fixed and processed for embedding and polymerization, according to the standard TEM protocols. Ultrathin sections were prepared, placed onto copper grids, and processed for staining with lead citrate. Ultrastructure of subcellular elements in the ischemic and hypoxic samples were compared versus controls, using a JEOL 1200EX microscope (JEOL USA, Inc.)
Statistical Analysis
Data are expressed as mean ± standard error of the mean. Differences in the bladder ischemia group versus control group and differences between the hypoxic cells versus control cells were determined by t-test using SigmaPlot software. Significance was determined at p ≤ 0.05 level.
Results
Effects of Ischemia on Bladder Contractile Activity and Compliance
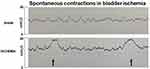
Bladder blood flow in animals undergoing aorto-iliac arterial ballooning was significantly lower in comparison with sham controls (Table 1), suggesting atherosclerosis-induced ischemia. In cystometry, the frequency of spontaneous bladder contractions, characterized by recurring episodes of intravesical pressure rises, was greater in the ischemic bladders in comparison with sham controls (Figure 1). Bladder compliance was significantly lower in the bladder ischemia group versus sham controls (Table 1). Premicturition pressure, body weight and bladder weight in the bladder ischemia group were similar to the control group (Table 1).
 |
Table 1 Decreased Blood Perfusion and Low Compliance in Bladder Ischemia |
Cellular Stress Signaling in Bladder Ischemia
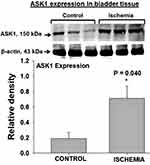
Two cellular stress-sensing molecules with proapoptotic properties were upregulated in bladder ischemia. The expression levels of ASK1, a cellular stress sensor and apoptosis initiator, was significantly greater in the ischemic bladder tissues versus controls (Figure 2). Increased levels of ASK1 in the ischemic bladder were associated with significant upregulation of caspase-3 (Figure 3), a cellular stress intensity sensor also known as the executioner of cell apoptosis. Simultaneous upregulation of ASK1 and caspase-3 in bladder ischemia suggests cellular stress and activation apoptotic degenerative activity via stress response mechanisms. Beta-actin protein expression in the ischemic bladder tissues was similar to beta-actin expression in the sham control bladder tissues.
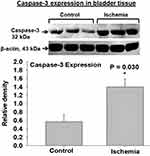 |
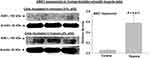
Figure 3 Caspase-3 expression was significantly upregulated in the ischemic bladder tissues in comparison with sham controls. Caspase-3 senses cellular stress intensity and regulates cell fate by either initiating cellular resistance mechanisms against stressful stimuli or activating apoptotic programmed cell death responses. Upregulated caspase-3 may play a mediating role in degenerative response to cellular stress engendered by ischemia. Simultaneous increases in caspase-3 and ASK1 expression, shown in Figure 2, may suggest crosstalk mechanisms between the two molecules under the ischemic conditions in the bladder. * represents significant change in the ischemic tissues versus controls. |
Cellular Stress Signaling in Cell Culture Hypoxia
Exposure of cultured human bladder smooth muscle cells to hypoxia caused significant increases in ASK1 (Figure 4) and caspase-3 (Figure 5) expression, suggesting low oxygen tension as an indispensable factor in ASK1 and caspase-3 upregulation. Beta-actin protein expression in cells undergoing hypoxia was similar to the control cells. Increased expression of ASK1 and caspase-3 in both ischemic bladder tissues, described earlier, and hypoxic cells may suggest a potential link between cellular stress and activation of apoptotic activity in bladder ischemia. Simultaneous upregulation of ASK1 and caspase-3 in both ischemic tissues and hypoxic cells may also imply potential crosstalk mechanisms between the two molecules. These observations suggest that hypoxia may be a major contributing factor in the regulation of cellular stress sensors and activation of stress signaling and apoptotic activity in bladder ischemia.
 |
Figure 5 Incubation of human bladder smooth muscle cells under the hypoxic condition at 2% pO2 caused a significant increase in caspase-3 expression in comparison with cells incubated in normoxia at 21% pO2. The data suggest that hypoxia may be an essential mediating factor in caspase-3 upregulation. Increased expression levels of both caspase-3 and ASK1, shown in Figure 4, suggest potential crosstalk mechanisms between the two molecules under the hypoxic conditions. * represents significant change in the hypoxic smooth muscle cells versus cells incubated in normoxia. |
ASK1 and Caspase-3 Intercommunication in Cell Culture Hypoxia
Gene deletion of ASK1 by ASK1 siRNA prevented caspase-3 upregulation by hypoxia in cultured smooth muscle cells, suggesting crosstalk between the two molecules and the essential role of ASK1 as a cellular stress sensor and apoptosis promotor under the hypoxic conditions. The expression pattern of caspase-3 in cells transfected with ASK1 siRNA versus cells transfected with control siRNA implies regulation of caspase-3 upregulation by ASK1 in the hypoxic cells. Western blotting showed caspase-3 upregulation at 48 hours hypoxia in cells transfected with control siRNA (Figure 6). However, caspase-3 expression in the hypoxic cells was significantly diminished by transfection with ASK1 siRNA, suggesting that gene deletion of ASK1 deterred caspase-3 upregulation under the hypoxic conditions (Figure 6). We also noted that the transfection of cells with ASK1 siRNA did not completely abolish caspase-3 expression, suggesting the involvement of other regulators, in addition to ASK1, in the upregulation of caspase-3 by hypoxia. Beta-actin protein was evenly expressed in cells transfected with ASK1 siRNA versus cells transfected with control siRNA (Figure 6). The data suggest activation of the ASK1/caspase-3 pathway by cellular stress and a mediating role of caspase-3 in ASK1-directed degenerative responses in bladder smooth muscle cell hypoxia.
Morphologic Hallmarks of Cellular Stress in Bladder Ischemia
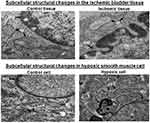
Transmission electron microscopy showed distinct subcellular ultrastructural changes in the ischemic bladder tissues in comparison with control tissues. Structural modifications in the nucleus, nuclear membrane and cytoplasmic organelles of the ischemic bladder tissues were consistent with typical morphological features of cellular stress and apoptotic degenerative responses. Prominent apoptotic hallmarks in bladder ischemia included cell shrinkage, condensation and fragmentation of the nucleus, folding and partial disruption of nuclear membrane, chromatin condensation, degradation of mitochondrial granules and increased cytoplasmic and nuclear ribosomes (Figure 7).
Morphologic Hallmarks of Cellular Stress in Cell Culture Hypoxia
Ultrastructural changes in the hypoxic bladder smooth muscle cells were identical to ultrastructural modifications seen in the ischemic bladder tissues, suggesting cellular stress and apoptotic degeneration of subcellular elements. Transmission electron microscopy of cultured hypoxic cells showed morphological changes consistent with apoptosis, characterized by nuclear condensation, partial loss of nuclear membrane, chromatin condensation, swollen and splintered endoplasmic reticulum and accumulation of lysosomes (Figure 7).
Discussion
A close link between reduced blood flow and low bladder compliance has been documented in both human and animal models,6,21 suggesting structural modifications by ischemia and subsequent loss of bladder elasticity. On the cellular level, ischemia was shown to upregulate cytokines and profibrotic growth factors involved in collagen production and deterioration of smooth muscle cells.6 The evidence of such structural modifications in bladder ischemia was associated with markers of cellular stress, characterized by increased expression of stress response protein Hsp70, upregulation of mitochondrial stress protein mtHsp70 also known as glucose-regulated protein 75 (GRP75), depression of mitochondrial respiration rate and activation of cell survival signaling.10 Our present study suggests the role of cellular stress as an important intervening variable in the instigation of structural modifications in bladder ischemia. Structural damage provoked by cellular stress may contribute to low bladder compliance recorded under the ischemic conditions. Downstream mechanisms appear to involve stress sensing by ASK1 and caspase-3 and degenerative cellular stress responses that seem to compromise the structural integrity of subcellular elements via the ASK1/caspase-3 apoptosis signaling pathway.
The induction of cellular stress involves a variety of adverse conditions, including ischemia, that disrupts cellular homeostasis and elicits damage to proteins, DNA, RNA and lipids.11,12 Cellular stress responses comprise well-coordinated reactions by elements of the cellular defense and survival system incited by stressful stimuli that compromise the structure and function of macromolecules in the native cellular environment.11,12 These reactions involve a cascade of downstream mechanisms regulated by stress sensors, stress response molecules, stress-sensitive signaling cascades, cell danger response and survival signaling.11 Stress response molecules coordinate defensive mechanisms against stress-activated endogenous cell danger signals to promote cell survival.11 However, cellular defensive mechanisms and cell survival pathways fail when stressful stimuli persist and deteriorating insult to subcellular elements continue.11,12 Persisting cellular stress and lack of protective mechanisms initiate cell danger signaling via deteriorating stress responses that activate catalytic activities to eliminate damaged cellular components.11,12 If the stressful stimuli remain unresolved, then the cells activate apoptotic death signaling pathways to terminate damaged cells and remove them.11 Oxidative insult via free radicals is one of the leading redox-based inducers of cellular stress under the ischemic conditions.12 Cellular defensive mechanisms in ischemia involve antioxidants, stress-activated kinases and stress response proteins such as heat shock proteins that, cumulatively, promote cell survival.12 Efficacy of the defensive mechanisms depend on the duration of cellular stress and the ability of cells to coordinate an appropriate response against environmental and intracellular stress inducers.11,12
Among the various stress-activated signaling molecules, apoptosis signal-regulating kinase 1 (ASK1) has been characterized as a leading sensor of free radical-mediated cellular stress and a major player in the regulation of endoplasmic reticulum (ER) stress response signaling.13–16 ASK1 was shown to be preferentially activated by free radicals and tightly regulated by redox proteins.13 Inhibition of ASK1 appeared to diminish free radical injury, protect the ischemic heart from ischemia-reperfusion injury and reduce myocardial infarct size.14 Mice lacking the ASK1 gene were shown to have normal homeostatic functions and were less vulnerable to kidney injury and renal interstitial fibrosis caused by ischemia-reperfusion.15,16 These observations have suggested the rational for targeting ASK1 as a potential prophylactic and therapeutic strategy to prevent cellular stress and protect against ischemic damage.
In our study, the upregulation of ASK1 in bladder ischemia insinuates cellular stress-sensing that seems to activate degenerative responses via apoptotic mechanisms with consequential damage to key cellular components and depreciation of the integrity of subcellular elements. Upregulation of ASK1 by reduced oxygen tension in cultured bladder smooth muscle cells suggests the role of hypoxia as an essential factor in ASK1 upregulation under the ischemic conditions. Overexpression of ASK1 has been shown to provoke DNA fragmentation and increase ischemia reperfusion-induced injury to the heart by greater than two-fold in a mouse model.14 Downstream signaling mechanisms were shown to involve activation of c-June N-terminal kinase pathway, inhibition of calcineurine-nuclear factor of activated T-cells and the induction of Bax-mediated apoptotic cell death.14 Studies in mice showed upregulation of ASK1 in the inflammatory phase of the ischemic stroke, suggesting a regulating role of ASK1 in neuronal structural damage and degenerative responses in the advanced stage of brain ischemia.22 Suppression of ASK1 was shown to preserve neuronal size and promote cell survival after ischemic stroke.22 Our observations in bladder ischemia along with aforementioned studies in the ischemic heart and brain suggest a regulating role of ASK1 in cellular stress and degeneration in conditions involving interrupted blood flow and disturbed oxygen tension. These studies, collectively, support the concept that ASK1 may be a key factor in the instigation of cell death when stressful cellular stimuli prevail and protective cellular mechanisms disintegrate. Intermediating molecules, downstream of ASK1, arbitrating its cellular stress-sensing properties and degenerative activities remain largely unknown.
Our data show the simultaneous upregulation of ASK1 and caspase-3 in both rat bladder ischemia and human bladder smooth muscle cell hypoxia. Upregulation of these two molecules were associated with morphologic markers of cell damage consistent with apoptotic insult and degeneration of subcellular elements. ASK1 is a stress sensor that activates apoptotic responses to cellular stress.13–16 Caspase-3 is a cellular stress intensity sensor that is perpetually implicated in the execution of apoptosis.17–20 Our studies with cultured bladder smooth muscle cells showed that ASK1 gene silencing by ASK1 siRNA prevents caspase-3 upregulation under the hypoxic condition. This observation implies potential intercommunication between the two molecules, suggesting caspase-3 regulation by ASK1 and potential role of caspase-3 as a mediating factor in ASK1-elicited structural damage in both bladder ischemia and cell culture hypoxia. We also noted that ASK1 siRNA did not completely abolish caspase-3 expression, implying the involvement of additional regulatory factors in hypoxia-mediated upregulation of caspase-3. Other studies have delineated the role of caspase-3 as a crucial factor in the activation of proteolytic and apoptotic activities by oxidative stress.19,20 Caspase-3 was shown to mediate degenerative responses triggered by ischemia and hypoxia in the brain.19,20 Downstream degenerative actions of caspase-3 in brain ischemia and hypoxia were shown to involve DNA damage and protein degradation.17–20 Interrelationship between ASK1 and caspase-3 is further supported by studies of lung epithelial cells showing that caspase inhibition prevents ASK1-induced cell death. It was also shown that the upregulation of ASK1 stimulates the release of mitochondrial cytochrome c, which in turn leads to the activation of caspase-3.23 Our studies along with the aforementioned reports suggest that caspases may be required for the implementation of degenerative responses initiated by ASK1.23
Among the known members of the caspase family, caspase-3 is known to act as a crucial cellular stress intensity sensor with the ability to determine cell fate, by either assembling appropriate tools to instigate a defensive response against the stressful stimuli or activating the execution of apoptotic degeneration of subcellular elements that leads to cell death. Cell survival or death in response to stressful stimuli involves a series of complex and tightly regulated mechanisms. It was shown that caspase-3 action in cellular stress depends on the intensity of stressful stimuli.24,25 Caspase-3 is also known to protect cells from mild stressful stimuli by activation of defensive mechanisms via cell survival pathway.24,25 On the other hand, caspase-3 activity is required for the execution of apoptotic cell death in response to abrasive cellular stress stimuli as it has the ability to cleave a large number of the caspase substrates.24 In addition to its apoptotic properties, caspase-3 is capable of regulating critical homeostatic functions and protects cells when the stressful stimuli are transient and manageable.25 Caspase-3 was shown to negatively regulate B cell proliferation and preserve its homeostasis in mild cellular stress conditions.25 The precise mechanisms by which caspase-3 regulates homeostasis in response to mild stress in non-dying cells remain unclear. Cell culture studies have suggested that low caspase-3 activity in response to mild cellular stress may promote cell survival by activating protein kinase B, also known as Akt.26 Activation of Akt in response to low cellular stress was prevented by chemical inhibition of caspase-3 and by caspase-3 knockout in mice. It was shown that higher caspase-3 activity in severe cellular stress is incapable of stimulating Akt due to subcellular fragmentation and widespread stress responses.26 These observations suggest that the configuration of caspase-3 response is an important modulating factor in differential cellular responses to mild and severe stressful stimuli under unfavorable cellular conditions such as hypoxia and oxidative stress.
Upregulation of stress-sensing molecules, ASK1 and caspase-3, in bladder ischemia were associated with subcellular structural changes consistent with cellular stress and apoptotic responses. Marked ultrastructural modifications were present in the nucleus, nuclear membrane and cytoplasmic organelles. These changes appeared to be typical of apoptotic degeneration characterized by cell shrinkage, condensation and fragmentation of the nucleus, folding and partial disruption of nuclear membrane, chromatin condensation, degradation of mitochondrial granules and increased cytoplasmic and nuclear ribosomes. Congruently, exposure of cultured bladder smooth muscle cells to hypoxia provoked ultrastructural modifications identical to those caused by bladder ischemia, including nuclear condensation, partial loss of nuclear membrane, chromatin condensation, swollen and splintered endoplasmic reticulum and accumulation of lysosomes. The precise link between cellular stress and downstream pathways contributing to ultrastructural damage in bladder ischemia remains to be thoroughly elucidated. Our data suggest that ASK1 may be an important factor in ischemia-mediated inflammation, fibrosis and smooth muscle damage.26 Inflammatory responses and structural changes in ischemia were shown to be prevented by chemical inhibition of ASK1 and by ASK1 knockout in mice.27 Activation of ASK1 by cellular stress in ischemia and subsequent degenerative responses are tightly regulated by redox due to the nature of the dithiol oxidoreductase binding partners of ASK1.26 Downstream pathways contributing to inflammation, fibrosis and cell death were shown to involve ASK1-regulated caspase-3, p38 mitogen-activated protein kinases (p38α and β) and c-June N-terminal kinases (JNK1, 2 and 3).26,27 Degenerative responses to cellular stress in ischemia were shown to be triggered by redox and modulated by ASK1 via prolonged activation of p38 and JNK.26,27 Immunohistochemical studies have shown that positive caspase-3 staining cells exhibit morphologic features consistent with fibrosis, deterioration of subcellular structures and apoptosis.28 Mitochondrial stress was shown to activate caspase-3 by the release of proapoptotic molecules such as cytochrome c.29 Activated caspase-3 promoted the cleavage of Akt, an important regulator of antiapoptotic transcription factors.30 Impairment of Akt facilitated activation of caspase-3 via posttranslational modification of cytochrome c-regulated cytosolic factors leading to the deterioration of subcellular elements.30 Detailed investigation of molecules and pathways, downstream of ASK1 and caspase-3, contributing to cellular stress responses and apoptotic degeneration in bladder ischemia remain as the focus of our future studies.
Conclusion
Cellular stress resulting from lack of nutrients, hypoxia, redox, and accumulation of metabolic waste in bladder ischemia increased the expression levels of ASK1 and caspase-3, two cellular stress-sensing molecules with proven degenerative properties. Upregulation of ASK1 and caspase-3 in bladder ischemia was associated with ultrastructural changes consistent with apoptotic degeneration of subcellular elements. Congruently, exposure of cultured human bladder smooth muscle cells to low oxygen tension also upregulated ASK1 and caspase-3 simultaneously, suggesting the induction of cellular stress by low oxygen tension and the role of hypoxia as a critical factor in increased expression of the two molecules. Gene deletion of ASK1 by ASK1 siRNA in cultured bladder smooth muscle cells prevented caspase-3 upregulation by hypoxia, implying intercommunication between the two molecules and potential regulation of caspase-3 by ASK1 under the ischemic and hypoxic conditions. Ultrastructural modifications in tissues and cells displaying upregulation of ASK1 and caspase-3 may suggest degenerative responses to cellular stress via ASK1/caspase-3 apoptosis pathway in both bladder ischemia and smooth muscle cell hypoxia. Our data suggest that cellular stress provoked by ischemia compromises subcellular structures and may play a role in low bladder compliance. The ASK1/caspase-3 pathway may provide novel therapeutic targets against cellular stress and degenerative responses in bladder ischemia.
Acknowledgment
This work was supported by Merit Review Award Number I01 BX004372 from the United States (US) Department of Veterans Affairs Biomedical Laboratory R&D (BLRD) Service.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lin W-Y, Andersson KE, Lin C-L, Kao C-H, Wu H-C. Association of lower urinary tract syndrome with peripheral arterial occlusive disease. PLoS One. 2017;12(3):e0170288. doi:10.1371/journal.pone.0170288
2. Gibbons EP, Colen J, Nelson JB, Benoit RM. Correlation between risk factors for vascular disease and the American Urological Association Symptom Score. BJU Int. 2007;99:97–100. doi:10.1111/j.1464-410X.2007.06548.x
3. Pinggera GM, Mitterberger M, Pallwein L, Frauscher F, Aigner F, Bartsch G. Strasser H: association of lower urinary tract symptoms and chronic ischaemia of the lower urinary tract in elderly women and men: assessment using colour Doppler ultrasonography. Brit J Urol. 2008;102:470. doi:10.1111/j.1464-410X.2008.07587.x
4. Thurmond P, Yang JH, Azadzoi KM. LUTS in pelvic ischemia: a new concept in voiding dysfunction. Am J Physiol Renal Physiol. 2016;310(8):F738. doi:10.1152/ajprenal.00333.2015
5. Andersson KE, Boedtkjer DB. Forman Axel: the link between vascular dysfunction, bladder ischemia, and aging bladder dysfunction. Ther Adv Urol. 2017;9(1):11. doi:10.1177/1756287216675778
6. Azadzoi KM, Tarcan T, Kozlowski R, Krane RJ, Siroky MB. Overactivity and structural changes in the chronically ischemic bladder. J Urol. 1999;162:1768. doi:10.1016/S0022-5347(05)68236-5
7. Nomiya M, Yamaguchi O, Andersson KE, et al. The effect of atherosclerosis-induced chronic bladder ischemia on bladder function in the rat. Neurourol Urodyn. 2012;31(1):195. doi:10.1002/nau.21073
8. Azadzoi KM, Chen B, Radisavljevic Z, Mike S. Molecular reactions and ultrastructural damage in the chronically ischemic bladder. J Urol. 2011;186:2115. doi:10.1016/j.juro.2011.06.047
9. Zhao Z, Azad R, Yang JH, Siroky MB, Azadzoi KM. Progressive changes in detrusor function and micturition patterns with chronic bladder ischemia. Investig Clin Urol. 2016;57(4):249. doi:10.4111/icu.2016.57.4.249
10. Yang JH, Siroky MB, Yall SV. Azadzoi KM: mitochondrial Stress and Activation of Phosphoinositol 3 Kinase (PI3K) and Protein Kinase B (Akt) Survival Pathway in Bladder Ischemia. Res Rep Urol. 2017;9:93.
11. Sawa T, Naito Y, Kato H, Amaya F. Cellular stress responses and monitored cellular activities. Shock. 2016;46(2):113. doi:10.1097/SHK.0000000000000603
12. Hai Chen H, Yoshioka H, Kim GS, et al. Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal. 2011;14(8):1505–1517. doi:10.1089/ars.2010.3576
13. Soga M, Matsuzawa A, Ichijo H. Oxidative stress-induced diseases via the ASK1 signaling pathway. Int J Cell Biol. 2012;2012:439587. doi:10.1155/2012/439587
14. Liu Q, Sargent MA, York AJ, Molkentin JD. ASK1 regulates cardiomyocyte death but not hypertrophy in transgenic mice. Circ Res. 2009;105(11):1110. doi:10.1161/CIRCRESAHA.109.200741
15. Tesch GH, Ma FY, Nikolic-Paterson DJ. ASK1: a new therapeutic target for kidney disease. Am J Physiol Renal Physiol. 2016;311:F373. doi:10.1152/ajprenal.00208.2016
16. Hattori K, Naguro I, Runchel C, Ichijo H. The roles of ASK family proteins in stress responses and diseases. Cell Commun Signal. 2009;24(7):9. doi:10.1186/1478-811X-7-9
17. Su JH, Zhao M, Anderson AJ, Srinivasan A, Cotman CW. Activated caspase-3 expression in Alzheimer’s and aged control brain: correlation with Alzheimer pathology. Brain Res. 2001;898:350–357. doi:10.1016/S0006-8993(01)02018-2
18. Chien JW, et al. Urinary 8-hydroxy-2’-deoxyguanosine (8-oxodG) level can predict acute renal damage in young children with urinary tract infection. Biomarkers. 2014;19(4):326–331. doi:10.3109/1354750X.2014.910552
19. Carvour M, Song C, Kaul S, Anantharam V, Kanthasamy A, Kanthasamy A. Chronic low dose oxidative stress induces caspase-3 dependent PKCδ proteolytic activation and apoptosis in a cell culture model of dopaminergic neurodegeneration. Ann N Y Acad Sci. 2008;1139:197–205. doi:10.1196/annals.1432.020
20. Gill R, Soriano M, Blomgren K, et al. Role of caspase-3 activation in cerebral ischemia-induced neurodegeneration in adult and neonatal brain. J Cereb Blood Flow Metab. 2002;22(4):420–430. doi:10.1097/00004647-200204000-00006
21. Kershen R, Azadzoi K, Siroky M. Blood flow, pressure and compliance in the human bladder. J Urol. 2002;168:121–125. doi:10.1016/S0022-5347(05)64843-4
22. Song J, Cho KJ, Cheon SY, et al. Apoptosis signal-regulating kinase 1 (ASK1) is linked to neural stem cell differentiation after ischemic brain injury. Exp Mol Med. 2013;45(12):e69. doi:10.1038/emm.2013.134
23. Hatai T, Matsuzawa A, Inoshita S, et al. Execution of Apoptosis Signal-Regulating Kinase 1 (ASK1)-induced apoptosis by the mitochondria-dependent caspase activation. J Biol Chem. 2000;275(34):26576–26581. doi:10.1074/jbc.M003412200
24. Slee EA, Adrain C, Martin SJ. Executioner caspase-3, −6, and −7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem. 2001;276:7320–7326. doi:10.1074/jbc.M008363200
25. Kuranaga E, Miura M. Nonapoptotic functions of caspases: caspases as regulatory molecules for immunity and cell-fate determination. Trends Cell Biol. 2007;17:135–144. doi:10.1016/j.tcb.2007.01.001
26. Shiizaki S, Naguro I, Ichijo H. Activation mechanisms of ASK1 in response to various stresses and its significance in intracellular signaling. Adv Biol Regul. 2013;53(1):135–144. doi:10.1016/j.jbior.2012.09.006
27. Terada Y, Inoshita S, Kuwana H, et al. Important role of apoptosis signal-regulating kinase 1 in ischemic acute kidney injury. Biochem Biophys Res Commun. 2007;364(4):1043–1049. doi:10.1016/j.bbrc.2007.10.122
28. Yang B, Jain S, Ashra SY, Furness PN. Nicholson, Apoptosis and caspase-3 in long-term renal ischemia/reperfusion injury in rats and divergent effects of immunosuppressants. transplantation. 2006;81(10):1442–1450.
29. Li Z, Jo J, Jia JM, et al. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell. 2010;141:859–871.
30. Chu J, Lauretti E, Praticò D. Caspase-3-dependent cleavage of Akt modulates tau phosphorylation via GSK3β kinase: implications for Alzheimer’s disease. Mol Psychiatry. 2017;22:1002–1008. doi:10.1038/mp.2016.214
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.