Back to Journals » Clinical Ophthalmology » Volume 11
Refractive predictability in eyes with intraocular gas tamponade – results of a prospective controlled clinical trial
Authors Wagenfeld L, Hermsdorf K , Stemplewitz B, Druchkiv V, Frings A
Received 18 January 2017
Accepted for publication 22 March 2017
Published 23 May 2017 Volume 2017:11 Pages 993—998
DOI https://doi.org/10.2147/OPTH.S132644
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Lars Wagenfeld,1 Kristin Hermsdorf,1 Birthe Stemplewitz,1 Vasyl Druchkiv,1 Andreas Frings2
1Department of Ophthalmology, University Medical Centre Hamburg-Eppendorf (UKE), Hamburg, 2Department of Ophthalmology, University Hospital Düsseldorf, Heinrich-Heine University, Düsseldorf, Germany
Purpose: To determine the postoperative refractive error in eyes with intraocular gas tamponade in combined phacovitrectomy using a Z-haptic intraocular lens (IOL).
Methods: This prospective non-randomized case-control study compared patients with combined phacovitrectomy with or without intraocular gas tamponade to cataract surgery-only. The main outcome measure was the IOL power prediction error (PE). Secondary outcome measures were spherical equivalent, anterior chamber depth (ACD), and axial length.
Results: Thirty-four patients with epiretinal membranes and 18 patients with cataract only were enrolled. There were no statistically significant (P>0.05) differences of IOL power PE or postoperative ACDs (P=0.952–1.00). Nevertheless, IOL power PE indicated a myopic shift in cases with phacovitrectomy independent of gas tamponade (P=1.00). No statistically significant between-group differences between secondary outcome measures were observed.
Conclusion: A myopic shift after phacovitrectomy seems to be independent of the use of intraocular gas tamponade. When using a Z-haptic IOL, aiming for slight residual hyperopia (+0.50 D) is suggested in patients having phacovitrectomy.
Keywords: IOL power prediction error, myopic shift, intraocular gas tamponade, biometry, pars plana vitrectomy, axial length
Introduction
Pars plana vitrectomy (PPV) combined with phacoemulsification and intraocular lens (IOL) implantation is routinely performed in eyes with vitreoretinal pathology and coexisting cataract. Several studies have reported that a combined vitreoretinal procedure is a safe and effective way to manage cases with vitreoretinal disease and cataract, with the functional outcomes comparable to those of sequential surgery.1–3
Biometry and IOL power calculation have been major aspects of research and have resulted in improved refractive outcomes after cataract surgery.2–4 However, how to best achieve the planned spherical equivalent (SE) in a combined surgery is still a question of vigorous scientific debate. Certain pitfalls have to be considered when planning a combined surgery as major factors limiting an IOL power calculation in combined surgery are inaccuracies in axial length (AL) measurement and the estimated lens position.
A previous study reported the inaccuracy in IOL power calculation in cases with combined surgery that was caused by an increased retinal thickness.4 Here, it is most likely that an underestimation of the cornea–photoreceptor layer distance contributed to a myopic shift. In a meta-analysis of cataract surgery results by Norrby published in 2008, preoperative estimation of postoperative IOL position, postoperative refraction determination, and preoperative AL measurement were the largest contributors of error (35%, 27%, and 17%, respectively), with a mean absolute error of 0.6 D for an eye of average dimensions.5 Thus, another major source of refractive error after cataract surgery lies in the imprecise prediction of the postoperative anterior chamber depth (ACD).5,6
The axial postoperative IOL movement in eyes treated with additional intraocular gas tamponade remains unclear. Some studies reported that eyes with a gas tamponade showed a myopic shift compared to eyes without a gas tamponade, while other studies explained that the gas tamponade resulted in more zonular elasticity, leading to a more posterior IOL position.7–9 The IOL type itself also seems to play a major role in potential IOL movement.
Drawing into this, the current prospective controlled clinical trial was designed to contribute to the notion of a possible correlation between the postoperative refractive error and intraocular gas tamponade in eyes with combined phacovitrectomy using a Z-haptic IOL.
Methods
Patients
This prospective non-randomized case-control study comprised patients presenting with vitreoretinal pathology and coexisting significant cataract (study group) and patients with significant cataract only (control group). Patients were recruited from the University Medical Center Hamburg-Eppendorf inpatient department from October 2015. The study and consent procedure were approved by the local ethics committee of the University of Hamburg, Germany (no PV4995), and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all patients after explanation of the study purpose.
Inclusion criteria for the study group were diagnosis of epiretinal membrane and cataract, intended combined unilateral surgery, and the use of IOLMaster 500 (Carl Zeiss Meditec AG, Jena, Germany) for preoperative IOL calculation. Exclusion criteria were diabetic vitreous hemorrhage, macular hole, posterior subcapsular and mature cataract, myopia or hyperopia >8.0 D, astigmatism >2.0 D, previous laser refractive surgery, or scleral buckle surgery.
Refractive measurements and surgery
Spherical and cylindrical refractions, and visual acuity with and without correction, were assessed pre- and postoperatively, at 1 day after surgery, and during two consecutive follow-up (FU) examinations (1 week and 1 month after cataract surgery; 4 weeks and 2–3 months after combined surgery), and were recorded electronically. Pre- and postoperative corneal topography and ACD data were obtained using Scheimpflug tomography (Pentacam HR; Oculus, Wetzlar, Germany). In addition, pre- and postoperative AL measurements and K values were determined using the IOLMaster. All refractions were acquired by subjective refraction by expert optometrists using similar refractometers, visual acuity tables, and documentation protocol. Each patient was examined pre- and postoperatively by the same team of optometrists. Examinations were carried out according to a standardized protocol. The main outcome measure was the IOL power prediction error (PE), obtained by subtracting the SE of the actual refraction from the predicted refraction, which was calculated preoperatively. Secondary outcome measures were SE, ACD, and AL. Biometric data were obtained with a laser interferometer (IOLMaster 500; Carl Zeiss GmbH) using the triple optimized Haigis formula with a0, a1, a2 constants from the ULIB website for this particular IOL model (a0=0.325, a1=0.255, a2=0.141; http://www.ocusoft.de/ulib/c1.htm) aiming at emmetropia.
In all cases, standard phacoemulsification (clear corneal incision) with in-the-bag IOL implantation was performed. All eyes received a one-piece acrylic, foldable IOL with a Z-haptic (1stQ Basis Z; 1stQ GmbH, Mannheim, Germany). This type of IOL was a standard lens in the department at the time of study and was not exclusively used for study purposes. None of the IOLs were sulcus fixated.
In the study groups, cataract surgery was performed before vitrectomy during the same session. Vitreoretinal procedures included a standard 23-gauge PPV and membrane peeling assisted by the use of dyes. Three 23-gauge trocars were inserted at 3.5-mm distance to the limbus at positions of 8, 10, and 2 o’clock (right eye) or 4, 10, and 3 o’clock (left eyes). Cataract surgery was performed using the phacoemulsification technique through a 2.4-mm clear-corneal incision followed by intracapsular implantation of a foldable acrylic posterior chamber IOL. In all cases, the diameter of the continuous curvilinear capsulorhexis was smaller than the optics of the IOL. Following cataract surgery, in combined procedures, a three-port PPV was performed. In cases with attached vitreous, detachment of the posterior hyaloid was induced with suction of the cutter. Epiretinal membranes were peeled without staining and brilliant blue-assisted internal limiting membrane (ILM) peeling was performed in all cases. At the end of the operation, the trocars were removed and sclerotomies were checked for leakage. In case of leakage, a transconjunctival 8-0 resorbable suture was made. All patients of the study and control groups were operated on by two experienced surgeons using the same technique. In all eyes with gas tamponade, sulfur hexafluoride (SF6) was injected. All eyes were filled with the same concentration (20%) of SF6 at the end of the operation. In cases without gas endotamponade, balanced salt solution (BSS) was applied. Prior to surgery, patients with phacovitrectomy were randomly subjected to either gas or BSS endotamponade.
Statistical analysis
A computer database was created as a fill-in form to record all pseudonymized patient data, and a masked statistical analysis of all outcome measures was performed. Depending on the distribution of parameters within a group, a non-parametric test (Mann–Whitney test, non-normal distributed data) or a parametric test (unpaired t-test, normal distributed data) was chosen to establish the P-value. The respective median values (including quartiles) were compared if parameters were not normally distributed; otherwise, arithmetic means (including standard deviation) were compared. In all cases, a P-value <0.05 was considered statistically significant.
Ethical standards
All data were anonymized prior to analysis and this study adhered to the tenets of the Declaration of Helsinki.
Results
Thirty-four patients with epiretinal membranes and 18 patients with cataract only met the inclusion criteria and were enrolled in this study. No intraoperative or postoperative complications occurred in the study groups which were designated as study group 1 (vitrectomy combined with cataract surgery with gas endotamponade), study group 2 (vitrectomy combined with cataract surgery without gas endotamponade), and study group 3 (cataract surgery-only, control group). In all cases with gas tamponade, the vitreous cavity was nearly completely filled with SF6.
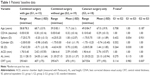
All patients of study groups 1 and 2 completed the 3-month FU, and patients of the control group were seen 4–6 weeks after surgery. Table 1 shows the patients’ baseline data. The baseline refraction differed between the study groups. Group 1 had more myopic patients, as reflected by the longer mean baseline AL and higher standard deviation of the mean baseline SE. There was a statistically significant difference (P=0.007) of central retinal thickness (CRT) between groups 2 and 3; however, differences in average preoperative ALs were not statistically significant (P>0.1).
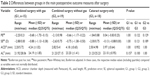
There were no statistically significant (P>0.05) differences of the resulting IOL power PE between the study groups and the control group (Table 2). Median PEs in the eyes after combined surgery were negative, indicating a myopic tendency for both the study groups. Compared to patients of the control group, eyes in group 1 had a PE of ~0.7 D thereby resulting in a median SE of −0.88 D (Table 2). There were no statistically significant differences between mean postoperative AL measurements. As shown in Tables 1 and 2, there were also no statistically significant between-group differences in pre- or postoperative ACD and SE except for preoperative SE between groups 1 and 3 (P=0.030).
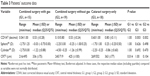
Table 3 summarizes the postoperative data. Patients with cataract only presented with a statistically significant better best corrected distance visual acuity (CDVA) compared to patients after combined surgery (P<0.05). However, there were cases of impaired postoperative CDVA which was caused by age-related dry macular degeneration. After epiretinal membrane removal, eyes of group 1 had a statistically significant (P=0.014) thinner CRT compared to eyes of group 2.
Discussion
Several studies on phacovitrectomy have described a mismatch between the expected postoperative refraction calculated preoperatively and the actually achieved refraction.9,10 Within this context, the exact postoperative IOL movement in eyes treated with additional intraocular gas tamponade remains still conflicting.8 Especially, the role of different IOL models on axial IOL movement has been discussed controversially. The purpose of this study was thus to investigate the effect of gas tamponade on postoperative refractive PE in eyes with an IOL and Z-haptic, and to identify differences in IOL positioning by measuring ACD.
In this study, a median postoperative SE of −0.88 D was obtained after phacovitrectomy with gas tamponade (group 1) compared to −0.75 D after combined surgery without gas (group 2) and −0.13 D after cataract surgery-only (control group). Compared with refractive data of the control group and group 2, surgery with gas tamponade (group 1) resulted in a higher median refractive PE and thus less predictable refractive results. In addition, the range of the IOL power PE in group 1 was higher compared to the other groups. These results are consistent with those of previous reports, which indicated that patients undergoing combined procedures with gas tamponade show more myopic shift in PE compared with patients not receiving a gas tamponade.8,11 However, the mean postoperative ACD was almost identical between groups 1 and 2 and its relative increase after surgery was deeper than after cataract surgery. Although clinically relevant, none of the before mentioned postoperative outcome measures (PE, SE, ACD, AL) were statistically significant between the study groups.
It is worth mentioning that PEs should be characterized in terms of the mean PE (or median) and spread of PEs (standard deviation or interquartile range depending on the type of data available). The median error depends on systematic errors and the spread on random errors. IOL formula constants can be modified to reduce systematic errors but not random errors. Therefore, if the PE in the study group(s) is on average significantly different to a control group, then this is significant information as the median error can be reduced by modifying the IOL formula constant. This study has demonstrated that the median PE in groups 1, 2, and 3 were −0.68, −0.66, and +0.04, thereby obtaining myopic PEs in the vitrectomy groups, which failed to reach statistical significance (Table 2). There are different explanatory models on hypothesized axial IOL movement in eyes with gas tamponade. It was suggested that the gas bubble pushes the IOL implant forward, thus accounting for myopic shift in PE. Falkner-Radler et al reported a statistically significant myopic shift between eyes with intraocular tamponade and eyes without intraocular tamponade.9 In contrast, Jeoung et al found no statistically significant difference in postoperative myopic shift.12 This finding is in agreement with the results of the present study. The IOL type seems to play a major role in potential IOL movement. As with eyes undergoing cataract surgery alone, lenses in eyes treated with combined surgery have been shown to be stable in terms of decentration and tilt measurements postoperatively. On the other hand, it has been reported that one-piece IOLs are shifted in an axial direction by fluid–gas exchange to a larger extent than three-piece IOLs and that IOLs with 4-haptic design provide the best stability in cataract surgery.13 However, Watanabe et al discussed that even different types of three-piece IOLs show different degrees of shift caused by fluid–gas exchange.14 In the current study, a one-piece IOL type with Z-haptic was used in all eyes and implanted into the capsular bag. IOLs with Z-haptic have a tendency for greater rotational stability than C-loop haptic.15 By applying the same IOL model to all eyes, potential bias which could be caused by different IOL parameters like hydrophilicity, IOL optic diameter, haptic properties, and angulation of the IOL was ruled out. This is the first study to demonstrate that IOLs with Z-haptic design applied in a combined surgery result in similar magnitudes of postoperative PE independent of intraocular gas tamponade.
The measurements indicate deeper ACDs after combined surgery compared to cataract-only eyes. Postoperative ACD was always deeper than the preoperative ACD. There was no eye in which the postoperative ACD was equal to the preoperative ACD which could be expected assuming that the gas bubble pushes the IOL implant forward. Applying the ratio of mean post- to preoperative ACD, an increase in depth was obtained by a factor 1.6 for groups 1 and 2, respectively; the mean ACD of group 3 increased by a factor of 1.4. This means that, in relation to preoperative baseline ACD, eyes of both groups 1 and 2 had deeper postoperative ACDs than one could expect, because there was no statistically significant difference in preoperative ACD between all study groups. This observation could be explained by removal of the aqueous body during vitrectomy during which backward traction and shear forces are applied to the anterior segment of the eye thereby altering biomechanical stability. However, this is an assumption and should be analyzed by other studies focussing on biomechanical changes after vitrectomy. Moreover, CRT after membrane peeling is usually thinner than before surgery. This implication on biometry has been studied before.4 What is essential for the current study is that both the combined surgery groups showed a very similar increase in depth of AC by a factor of 1.6; this means that the IOL implant forward movement by gas tamponade is less likely but is rather caused by combined surgery itself. The increased deepening of the ACD in combined vitrectomized eyes would be expected to result in a hyperopic PE as the IOL is located more posteriorly and its effective power is reduced. However, this assumption is not observed in the actual postsurgical data (Table 2).
Table 2 shows the PE of the IOL calculation for the target refraction for emmetropia using the Haigis formula with constants recommended by the ULIB website. In a previous study on biometry in combined surgery with air endotamponade, the median postoperative PE achieved with the Haigis formula was compared with the PE that would have been obtained had other formulas been used. It was found that, on average, the emmetropia target refraction was calculated most precisely with the Haigis formula, because the PE was lowest here.
There are different ways to determine AL for IOL power calculation. In the department, IOLMaster 500 (Carl Zeiss Meditec) was applied. This device is based on partial coherence interferometry (PCI) and AL is measured using a signal that is reflected off the retinal pigment epithelium (RPE) of the patient’s eye. In this way, the IOLMaster measures the distance from the corneal epithelium to the RPE.16 The focal plane of the eye corresponds to the external limiting membrane (ELM), but the distance between the ELM and RPE is negligible and stable.16 The distance between the ILM and ELM is variable due to the macular pathology.17 PCI should not be influenced by changes in the overlying retinal layers in cases with macular edema. However, epiretinal tractions have, by definition, an impact on the RPE/ELM and alter its anatomical structure. The impact of epiretinal membranes with and without accompanying macular edema has been reported previously.4 The current study did not assess the role of macular anatomy on refractive outcome; both the study groups included patients with mild to intermediate traction without macular edema and thus this parameter did not bias group results. However, the authors acknowledge limitations to this study. It is worth noting that this study included a small number of eyes and was not adequately powered to detect the difference in PE, as seen by P-values which approached clinical significance. Also, patients’ compliance was not assessed during the first days after surgery as body position may influence vectorial forces induced by gas movement.
To conclude, a myopic shift after phacovitrectomy seems to be independent of the use of intraocular gas tamponade and should, with limitations, be taken into account in calculating IOL power. When using a Z-haptic IOL, aiming for slight residual hyperopia (+0.50 D) is suggested in patients having phacovitrectomy with or without gas tamponade. Further studies should recalculate PEs based on postoperative AL measurements to quantify the influence of errors of preoperative AL measurement.
Acknowledgment
The authors thank Dr Christos Skevas, a surgeon in this study, who advised on the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Treumer F, Bunse A, Rudolf M, Roider J. Pars plana vitrectomy, phacoemulsification and intraocular lens implantation. Comparison of clinical complications in a combined versus two-step surgical approach. Graefes Arch Clin Exp Ophthalmol. 2006;244:808–815. | ||
Findl O. Biometry and intraocular lens power calculation. Curr Opin Ophthalmol. 2005;16:61–64. | ||
Pereira FAS, Cronemberger S. Ultrasound biomicroscopic study of anterior segment changes after phacoemulsification and foldable intraocular lens implantation. Ophthalmology. 2003;110:1799–1806. | ||
Frings A, Dulz S, Skevas C, et al. Postoperative refractive error after phacovitrectomy for epiretinal membrane with and without macular oedema. Graefes Arch Clin Exp Ophthalmol. 2015;253:1097–1104. | ||
Norrby S. Sources of error in intraocular lens power calculation. J Cataract Refract Surg. 2008;34:368–376. | ||
Olsen T. Calculation of intraocular lens power: a review. Acta Ophthalmol Scand. 2007;85:472–485. | ||
Shioya M, Ogino N, Shinjo U. Change in postoperative refractive error when vitrectomy is added to intraocular lens implantation. J Cataract Refract Surg. 1997;23:1217–1220. | ||
Patel D, Rahman R, Kumarasamy M. Accuracy of intraocular lens power estimation in eyes having phacovitrectomy for macular holes. J Cataract Refract Surg. 2007;33:1760–1762. | ||
Falkner-Radler CI, Benesch T, Binder S. Accuracy of preoperative biometry in vitrectomy combined with cataract surgery for patients with epiretinal membranes and macular holes: results of a prospective controlled clinical trial. J Cataract Refract Surg. 2008;34:1754–1760. | ||
Kovács I, Ferencz M, Nemes J, Somfai G, Salacz G, Récsán Z. Intraocular lens power calculation for combined cataract surgery, vitrectomy and peeling of epiretinal membranes for macular oedema. Acta Ophthalmol Scand. 2007;85:88–91. | ||
Schweitzer KD, García R. Myopic shift after combined phacoemulsification and vitrectomy with gas tamponade. Can J Ophthalmol. 2008;43:581–583. | ||
Jeoung JW, Chung H, Yu HG. Factors influencing refractive outcomes after combined phacoemulsification and pars plana vitrectomy: results of a prospective study. J Cataract Refract Surg. 2007;33:108–114. | ||
Kim SW, Oh J, Song JS, Kim YY, Oh IK, Huh K. Risk factors of iris posterior synechia formation after phacovitrectomy with three-piece acrylic IOL or single-piece acrylic IOL. Ophthalmologica. 2009;223:222–227. | ||
Watanabe A, Shibata T, Ozaki M, Okano K, Kozaki K, Tsuneoka H. Change in anterior chamber depth following combined pars plana vitrectomy, phacoemulsification, and intraocular lens implantation using different types of intraocular lenses. Jpn J Ophthalmol. 2010;54:383–386. | ||
Warlo I, Krummenauer F, Dick HB. Rotationsstabilität monofokaler Intraokularlinsen mit C-Haptik versus Z-Haptik nach Kataraktchirurgie. [Rotational stability in intraocular lenses with C-loop haptics versus Z haptics in cataract surgery. A prospective randomised comparison]. Ophthalmologe. 2005;102:987–992. German. | ||
Williams DR, Brainard DH, McMahon MJ, Navarro R. Double-pass and interferometric measures of the optical quality of the eye. J Opt Soc Am A Opt Image Sci Vis. 1994;11:3123–3135. | ||
Chaber S, Helbig H, Gamulescu M. Messunterschiede der Makuladicke bei Normalprobanden. [Time domain OCT versus frequency domain OCT: measuring differences of macular thickness in healthy subjects]. Ophthalmologe. 2010;107:36–40. German. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.