Back to Journals » Clinical and Experimental Gastroenterology » Volume 12
Reductive effect of ursodeoxycholic acid on bilirubin levels in neonates on phototherapy
Authors Ughasoro MD , Adimorah GN, Chukwudi NK , Nnakenyi ID , Iloh KK , Udemba CE
Received 4 March 2019
Accepted for publication 11 June 2019
Published 29 July 2019 Volume 2019:12 Pages 349—354
DOI https://doi.org/10.2147/CEG.S207523
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Koulaouzidis
Maduka Donatus Ughasoro,1 Gilbert Nwadiaka Adimorah,1 Ndubuisi Kennedy Chukwudi,2 Ifeyinwa Dorothy Nnakenyi,3 Kenechukwu Kaosisochukwu Iloh,1 Charles Ejike Udemba4
1Department of Paediatrics, University of Nigeria Enugu Campus, Enugu, Nigeria; 2Department of Paediatrics, Federal Medical Centre, Umuahia, Abia State, Nigeria; 3Department of Chemical Pathology, University of Nigeria Enugu Campus, Enugu, Nigeria; 4Department of Paediatrics, University of Nigeria Teaching Hospital, Enugu, Nigeria
Background: Phototherapy is paramount in the management of high total serum bilirubin (TSB). Whether its effectiveness can be improved with ursodeoxycholic acid (UDCA) has not been evaluated among newborns of African descent.
Methods: A double-blind-controlled study was used to evaluate the effect of UDCA on the management of high TSB in neonates. Recruited neonates were categorized into the experimental group (given UDCA plus phototherapy) and the control group (phototherapy and plain syrup), and their TSB and conjugated bilirubin levels were measured. The data were analyzed using SPSS version 20. Statistical significance was set at a p-value of <0.05.
Results: The mean (SD) percentage reductions in TSB after 24 hrs were 40.73% (18.1) and 10.21% (7.1) in the experimental and control groups, respectively, and the difference was statistically significant (p=0.001). The mean (SD) durations on therapy were 3.0 days (0.58) in the experimental group and 5.5 days (1.35) in the control group (p=0.001).
Conclusions: Phototherapy is still effective in the management of neonatal hyperbilirubinemia, but inclusion of UDCA accentuates the reductive effect of phototherapy on the TSB in neonates, reducing the duration of treatment and in-patient care.
Keywords: neonatal hyperbilirubinemia, phototherapy, ursodeoxycholic acid
Introduction
Neonatal jaundice is common among newborn babies.1 Jaundice is caused by yellowing of the baby’s skin, eyes, and other tissues due to deposition of bilirubin, which is a product of metabolism of red blood cells. Newborns cannot easily get rid of the bilirubin and it can accumulate in the blood and other tissues and fluids of the baby’s body and give rise to hyperbilirubinemia. The bilirubin can be conjugated in the liver which some proportion not being conjugated. If the proportion of unconjugated bilirubin is high and untreated, it can cross the blood–brain barrier and cause bilirubin-induced neurologic dysfunction.2 Although the risk of neurodevelopmental damage correlates with the increase in the level of unconjugated bilirubin, cases of neurological damage have been reported even at lower levels.3,4 Thus, every case requires prompt review and institution of adequate management.
The mainstay of treatment in Nigeria is hospital-based phototherapy and in severe cases exchange blood transfusion, since the option of home-based phototherapy is not available.5,6 This has both huge economic and psychological burdens on the parents/caregivers. Phototherapy is very efficient but emerging pieces of evidence have identified some pharmaceutical products that can augment its efficacy.7,8 Among some drugs that can augment phototherapy is ursodeoxycholic acid (UDCA), a bile extract that has been in use for the management of neonatal cholestasis.9,10 UDCA is more hydrophilic than the bile acids, and when administered, it gradually displaces the more hydrophobic ones in the bile that accumulate during cholestasis. This helps improve the flow of bile out of the liver/gall bladder. It is metabolized by intestinal bacteria to an insoluble form that is then excreted in feces. It has been found to have the potential to protect the newborn brain and liver cells from the damaging effects of unconjugated bilirubin.11,12 The UDCA induces biliary flow and reduces intestinal reabsorption of biliary acids.13 It also inhibits the apoptotic effect of unconjugated bilirubin on both hepatocytes and neuronal cells.14 In view of the abovementioned attributes of UDCA, its inclusion as adjuvant therapy in the management of neonatal jaundice may not only protect the brain from the damaging effect of high bilirubin levels but may also reduce the duration of treatment with phototherapy.
The reductive effect of UDCA on unconjugated hyperbilirubinemia in neonates receiving phototherapy has been documented by Honar et al,15 Hassan et al16 and Jafari et al.17 Unfortunately, none of these studies was conducted among newborn of African newborn who are more likely to be genetically different and varied in skin complexion. It has been shown that different skin colors manifest different traits to light exposure.18 Skin color is primarily determined by melanin that is synthesized in the melanosome. Melanosomes in dark skin (African) are larger and more heavily pigmented than those in light skins (Asian and Caucasian). Since melanin can present a significant competitive absorber of visible light,19,20 thus reducing the penetration of light down through the skin, neonates with black skin may need more intensive phototherapy. Therefore, the need for an intervention which has the capacity to reduce the duration of phototherapy in the newborns of African parents. In this study, we tested the hypothesis that inclusion of UDCA in phototherapy management of neonatal jaundice would not increase the rate of reduction in the serum bilirubin levels with a resulting reduction in the duration of treatment. The outcome of this study will be necessary for the review of treatment protocols for neonatal hyperbilirubinemia among our African babies.
Methods
Site selection
The study took place in the newborn special care units of University of Nigeria Teaching Hospital (UNTH), Enugu, and Enugu State and Federal Medical Centre (FMC), Umuahia, Abia State. Both hospitals have functional newborn special care units with an average admission of about 10–15 neonates weekly. The units have functional phototherapy machines that are routinely serviced.
Study design
The study was a prospective, randomized, double-blinded, controlled study conducted from May 2017 to November 2017. All full-term neonates with serum bilirubin levels that could be managed with phototherapy alone were included in the study. The systematic random sampling was used to assign subjects into either of the two groups: experimental or control group. Each of the groups received phototherapy and an oral medication. The content of the medication was blinded to the researchers. After analysis, 18 jaundiced neonates were in the experimental group that received UDCA with phototherapy while 22 others were in the control group that received phototherapy and placebo (simple syrup). Neonates who were not jaundiced or who were jaundiced but on nil-per-oral were excluded from the study.
All the jaundiced neonates received at least 10 hrs of intensive phototherapy daily. A four-letter code in a sealed envelope was drawn by the researcher each time, to ascribe a neonate to either of the study groups. Both the caregivers and the researchers were unaware of the medication administered as the UDCA and placebo were distributed in identical plastic bottles. The nurses in the newborn special care unit were trained on how to administer the medication daily and document having done so.
The experimental group received UDCA, 10 mg/kg/day in two divided doses given every 12 hrs as a compounded mixture from capsule UDCA (Laboratorio Estedi S.L, Monsey, Barcelona-Espana) to a concentration of 50 mg/5ml, while the control group received a placebo of simple syrup. The simple syrup was made out of purified water and sucrose according to specification in the British Pharmacopoeia.21 The effectiveness of each medication was measured by the rate of decline of total serum bilirubin (TSB) levels on days 2 and 3. The total duration the patient spent on admission before discharge was documented.
At baseline, all the neonates underwent a clinical evaluation, and their mothers’ blood groups and the likely cause of the jaundice were investigated and documented.
The indirect agglutination test (indirect Coomb’s test) was used to make the diagnosis of ABO incompatibility. The diagnosis of sepsis was based on the presence of fever or hypothermia and elevated total white blood cell count on complete blood count.
The baseline serum bilirubin level was documented, classified and managed according to the recommendations of the American Academy of Pediatrics.22 Those neonates with significant hyperbilirubinemia were treated with intensive phototherapy (minimum irradiance of 30 µW/cm2/nm).23 The bilirubin levels of the neonates were monitored daily according to the existing protocol in the units. Neonates with TSB level of >25 mg/dL or bilirubin ≥10 mg/dL per kg body weight or those who failed to respond to phototherapy had an exchange blood transfusion and were excluded from this study. There was a provision of other ancillary treatment like intravenous fluid therapy and appropriate antibiotics.
Sample size, data collection and analysis
The sample size was calculated using the formula for bioequivalents and bilirubin decrease rate of 2 mg/dL/week according to Brown and Kim24
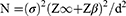
where N = is the size of sample for each group of treatment, σ = standard deviation of the effectiveness of treatment (0.45), Z∞ =1.64, Zβ =1.28, d = is the difference between the effects of normal (phototherapy only) and new treatment (UDCA and phototherapy) that is being considered clinically significant =15% (2×0.15=0.3). Therefore,
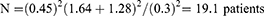
An addition of 10% attrition of 2 was added to give a minimum sample size of 21.
One milliliter of venous blood was collected in a lithium heparin microtainer tube protected from light and processed immediately. Estimation of total and conjugated serum bilirubin levels was performed using Diazo method and reported in mg/dL. he period spent under phototherapy. This was achieved by documenting the time any child was put under phototherapy and the time the child was taken out or the phototherapy was switched off. Also, a questionnaire was used to collect information on gestational age at birth, the day of life jaundice manifested, diagnosis, the baseline bilirubin before phototherapy was commenced, the parents’ occupation, and parents’ blood group.
Data analysis
The analysis was performed using SPSS version 20 (IBM, USA). The percentage decline in the serum bilirubin level was calculated based on the difference between the preceding day level (PDL) and the succeeding day level (SDL) divided by the preceding day level, and multiplied by 100 (PDL–SDL)/PDL×100). The socioeconomic status (SES) was determined using the method by Oyedeji based on the product of both parents’ education and occupation.25 The SES was classified into five groups, where 1 is the highest SES and 5 is the lowest. Neonates below 37 weeks were categorized as preterm and excluded from the study.26 Sepsis was diagnosed based on the presence of two or more of any of these symptoms: a) fever or hypothermia, b) age-related tachycardia, c) age-related tachypnea, d) abnormal white blood cell or increase in immature forms.26 A subject was said to have cholestasis if the percentage of conjugated serum bilirubin was 20% or more. The Student’s t-test was used to compare the mean of continuous variables like the percentage reduction in the bilirubin level, while Pearson’s chi-squared test was used to compare discrete variables like the proportion that experienced reduction or rise in the bilirubin level. The confidence interval was set at 95%. Statistical significance was at p-value of <0.05.
Ethical considerations
The study protocol was reviewed and approved by the Health Research Ethics Committee of UNTH, Enugu, with an Institutional Review Board registration number of NHREC/05/01/2008B-FWA00002458-IRB00002323 and the study was conducted in accordance with the Declaration of Helsinki. The study was explained to the parents of the neonates and written informed consent was obtained before enrolling in the study. The recorded information was handled with confidentiality and no research team member was denied access to the data.
Results
Out of the total 46 neonates that enrolled in the study after consent was given by their parents, 2 left the study due to early discharge, 4 had incomplete data, thus 40 were finally analyzed. Among the 40 subjects analyzed, 18 were in the experimental group while 22 were in the control group (Table 1). The mean age was 5.22 days. The commonest causes of neonatal jaundice identified were ABO incompatibility and neonatal sepsis at 46.2% and 46.2%, respectively. Most mothers (95.5%) were blood group O rhesus D positive, while most fathers (76.9%) were blood group B rhesus D positive.
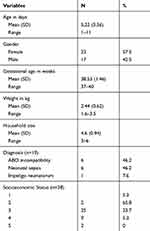 |
Table 1 Socio-demographic characteristics of the subjects |
The mean (SD) total bilirubin level for each group on day 1 was 10.2 mg/dL (3.0) for the experimental group and 10.5 mg/dL (2.1) for the control group, while on day 2, the experimental group was 6.4 mg/dL (5.8) and 10.8 mg/dL (1.6). The mean percentage fall was 40.73% and 10.21% among the experimental and control groups, respectively, and the difference was statistically significant (p=0.0001) as presented in Table 2. On the 2nd day, 14 (77.8%) of the experimental group and 15 (68.2%) of the control group experienced a drop in their bilirubin level and the difference was not statistically significant (p=0.825). On the 3rd day, all the neonates in the experimental group and 18 (81.8%) in the control group experienced a drop in their total bilirubin level. The mean duration of treatment in both experimental group and control group were 3.0 days and 5.5days, respectively (p=0.0001). The experimental group that received UDCA with phototherapy exhibited a better overall reduction in TSBlevel as represented in Table 2.
 |
Table 2 Comparative analysis of the change in bilirubin level between the experimental and control groups |
Discussion
The study revealed that the experimental group (phototherapy and UDCA) reported a higher percentage decline in the bilirubin level compared to the control group (phototherapy and placebo). The observed difference was statistically significant. This is similar to what has been reported in previous studies.15,16 The use of placebo in the control group of this study has further proven that the previously reported reductive effect of UDCA on unconjugated hyperbilirubinemia level by Honar et al15 and Hassan et al16 is a verifiable attribute of UDCA, not a placebo effect. Furthermore, it also is proven that whatever may be the effect of differences in skin color and melanin level, that UDCA has an additive effect on the effectiveness of phototherapy on newborn of African descent. This reductive effect of UDCA on serum bilirubin levels could be attributed to its ability to induce bile flow, reduce intestinal reabsorption of biliary acids13 and help reduce bilirubin concentration. In the studies by Maldonado et al,28 it was reported that combination of phototherapy, UDCA at the dose of 10 mg/kg/day in two divided doses given every 12 hrs and polyethylene glycol (PEG) decrease plasma bilirubin concentration in laboratory animals (rats) by the acceleration of the gastrointestinal transit. PEG has been found to be a safe and effective therapy for constipation in infants at a maintenance dose of 0.78 g/kg/day. None of the neonates in the experimental group experienced any bilirubin rise on day 3 compared to the control group where few still experienced some increase in bilirubin level despite being on phototherapy.
The average duration of treatment for those in the experimental group was shorter than those in the control group and this has been reported by previous studies15,16 This may be due to the adjunctive effect of UCDA. This can be explained by the efficacy of phototherapy being proportional to the concentration of bilirubin in the skin. Therefore, as the level of bilirubin is reduced, the relative effectiveness of phototherapy is reduced. This decline in efficacy after 48 hrs can be attributed to the fact that excreted unstable bilirubin isomers (photobilirubin) that are formed revert to natural bilirubin in the intestine and are reabsorbed via the enterohepatic circulation and contribute to the bilirubin load the hepatocytes have to handle.9 The UDCA has been shown to inhibit the enterohepatic recirculation.13 Although phototherapy has been assumed to be an innocuous form of treatment and thus should be instituted freely and as long as the jaundice persists, the economic cost of each day spent in the hospital by both mothers and their neonates has not been evaluated. Apart from direct medical costs, other intangible costs like inconveniences, psychological exhaustion, deprived care for the elder siblings of the jaundiced neonate on treatment, etc. can be reduced if the duration of hospitalization is reduced.
A limitation of this study was lack of follow-up on these neonates to monitor for rebound bilirubin levels in both groups as well as to check for any adverse events due to the use of UDCA. However, studies have shown that the average rebound bilirubin level after phototherapy is often below 1 mg per day. Furthermore, previous studies where UDCA was used for cholestasis reported no adverse events.
Conclusion
Phototherapy is still an appropriate and recommended management of uncomplicated hyperbilirubinemia in the newborns. The inclusion of UDCA as an adjuvant to phototherapy has the capacity to quicken the rate of reduction of the high bilirubin level and shorten the duration required to be under phototherapy. This intervention can improve the parental acceptance of phototherapy considering the possibility of a reduced hospital stay.
Acknowledgments
We are grateful to the parents who gave their consent for their babies to be part of this study. We are also thankful to pharmacists Ijeoma Akwukwaegwu and Iheoma Ejiofor, of the Pharmacy Department, Federal Medical Centre, Umuahia, Abia State, for their assistance during the study.
Authors contributions
All authors contributed towards data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Tikmani SS, Warraich HJ, Abbasi F, Rizv A, Darmstadt GL, Zaidi AKM. Incidence of neonatal hyperbilirubinemia: a population-based prospective study in Pakistan. Trop Med Int Health. 2010;15:502–507. doi:10.1111/j.1365-3156.2010.02584.x
2. Shapiro SM. Definition of the clinical spectrum of kernicterus and bilirubin-induced neurologic dysfunction (BIND). J Perinatol. 2005;25:54–59. doi:10.1038/sj.jp.7211157
3. Bratlid D, Nakstad B, Hansen TWR. National guidelines for treatment of jaundice in the newborn. Acta Paediatr. 2011;100:499. doi:10.1111/j.1651-2227.2010.02104.x
4. Rennie JM, Sehgal A, De A, Kendall GS, Cole TJ. Range of UK practice regarding thresholds for phototherapy and exchange transfusion in neonatal hyperbilirubinaemia. Arch Dis Child Fetal Neonatal Ed. 2009;94:F323–7. doi:10.1136/adc.2008.147686
5. Snook J. Is home phototherapy in the term neonate with physiological jaundice a feasible practice? A systematic literature review. J Neonatal Nurs. 2016; Accessed December 2nd 2016. doi:10.1016/j.jnn.2016.08.001
6. Eggert LD, Pollary RA, Folland DS, Jung AL. Home phototherapy treatment of neonatal jaundice. Pediatrics. 1985;76:579–584.
7. Murki S, Dutta S, Narang A, Sarkar U, Garewal G. A randomized, triple-blind, placebo-controlled trial of prophylactic oral phenobarbital to reduce the need for phototherapy in G6PD-deficient neonates. J Perinatol. 2005;25:325–330. doi:10.1038/sj.jp.7211258
8. Kappas A, Drummond GS, Valaes T. A single dose of Sn-mesoporphyrin prevents development of severe hyperbilirubinemia in glucose-6-phosphate dehydrogenase-deficient newborns. Pediatrics. 2001;108:25–30. doi:10.1542/peds.108.6.1367
9. Bhatia V, Bavdekar A, Matthal J, Waikar Y, Sibal A. Management of neonatal cholestasis: consensus statement of the pediatric gastroenterology chapter of Indian Academy of Pediatrics. Indian Pediatr. 2014;15:203–210. doi:10.1007/s13312-014-0375-2
10. Best C, Gourley GR. Management of neonatal cholestasis. Therapy. 2009;6:75–81. doi:10.2217/14750708.6.1.75
11. Lirussi F, Okolicsanyi L. Cytoprotective with ursodeoxycholic acid: effect in chronic non-cholestatic and chronic cholestatic liver disease. Ital J Gastroenterol. 1992;24:31–35.
12. Hillaire S, Ballet F, Franco D, Setchell KD, Poupon R. Effects of ursodeoxycholic acid and chenodeoxycholic acid on human hepatocytes in primary culture. Hepatology. 1995;22:82–87. doi:10.1002/hep.1840220113
13. Cuperus FJC, Iemhoff AA, Verkade HJ. Combined treatment strategies for unconjugated hyperbilirubinemia in gunn rats. Pediatr Res. 2011;70:560–565. doi:10.1203/PDR.0b013e31822e63b3
14. Palmela I, Correla L, Silva R, et al. Hydrohilic bile acids protect human blood brain barrier endothelial cells from disruption by unconjugated bilirubin: an in vitro study. Front Neurosci. 2015;9:80. doi:10.3389/fnins.2015.00080
15. Honar N, Ghashghaei Saadi E, Saki F, Pishva N, Shakibazad N, Hosseini Teshnizi S. Effect of ursodeoxycholic acid on indirect hyperbilirubinemia in neonates treated with phototherapy. J. Pediatr Gastroenterol Nutr. 2016;62:97–100. doi:10.1097/MPG.0000000000000874
16. Hassan A, Babadoko AA, Ahmed AJ, Suleiman AM. The pattern of distribution of ABO blood groups in North Western Nigeria. Ann Niger Med. 2005;1:17–18.
17. Jafari S, Khan KA, Bhatnagar S, Srivastava G, Nanda C, Chandra A. Role of ursodeoxycholic acid in neonates with indirect hyperbilirubinemia-an open labelled randomized control trial. Int J of Contemp Pediatr. 2018;5(2):432–435. doi:10.18203/2349-3291.ijcp20180530
18. Yurdakok M. Phototherapy in the newborn: what’s new? J Pediatr Neonatal Individualized Med. 2015;4(2):e040255. doi:10.7363/040255
19. Maisels MJ, McDonagh A. Phototherapy for neonatal jaundice. N Engl J Med. 2008;358(9):920–928. doi:10.1056/NEJMct0708376
20. Zonios G, Bykowski J, Kollias N. Skin melanin, hemoglobin and light scattering properties can be quantitatively assessed in vivo using diffuse reflectance spectroscopy. J Invest Derm. 2001;117(6):1452–1457. doi:10.1046/j.1523-1747.2001.01378.x
21. British Pharmacopoeia. Available from: https://www.pharmacopoeia.com. Accessed March 9, 2018.
22. American Academy of Pediatrics. Subcommittee on hyperbilirubinemia. Clinical practice guideline: management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297–316. doi:10.1542/peds.114.1.297
23. Maisels MJ, Bhutani VK, Bogen D, Newman TB, Stark AR, Watchko JF. Hyperbilirubinemia in the newborn infant > or =35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124:1193–1198. doi:10.1542/peds.2009-0329
24. Brown AK, Kim BM, Paul Y, Wu K, Dolores AB. Efficacy of phototherapy in prevention and management of neonatal hyperbilirubinemia. Pediatrics. 1985;75:393–400.
25. Oyedeji GA. Socioeconomic and cultural background of hospitalized children in Ilesa. Nig J Paediatr. 1995;12:111–117.
26. Montquin JM. Classification and heterogeneity of preterm births. BJOG. 2003;110(20):30–33. doi:10.1046/j.1471-0528.2003.00021.x
27. Chlesa C, Panero A, Osborn JF, Simoneti AF, Pacifico SL. Diagnosis of neonatal sepsis: a clinical and laboratory challenges. Clin Chem. 2004;50(2). doi:10.1373/clinchem2003.025171
28. Maldonado SR, Tellez NCG, Yescas-Buendia G, Rios ERR. Effectiveness of ursodeoxycholic acid vs phenobarbital for the treatment of neonatal cholestasis: a cross-randomized clinical trial. Bol Med Hosp Infant Mex. 2010;67:418–424.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
