Back to Journals » Therapeutics and Clinical Risk Management » Volume 12
Reduction of group A beta-hemolytic streptococcus pharyngo-tonsillar infections associated with use of the oral probiotic Streptococcus salivarius K12: a retrospective observational study
Authors Gregori G, Righi O, Risso P, Boiardi G, Demuru G, Ferzetti A, Galli A, Ghisoni M, Lenzini S, Marenghi C, Mura C, Sacchetti R, Suzzani L
Received 10 September 2015
Accepted for publication 17 November 2015
Published 19 January 2016 Volume 2016:12 Pages 87—92
DOI https://doi.org/10.2147/TCRM.S96134
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Giuseppe Gregori,1 Ornella Righi,1 Paolo Risso,2 Goffreda Boiardi,1 Giovanni Demuru,1 Anna Ferzetti,1 Antonio Galli,1 Marco Ghisoni,1 Sonia Lenzini,1 Claudio Marenghi,1 Caterina Mura,1 Roberto Sacchetti,1 Lucia Suzzani1
1Primary Care Department, Local Health Unit (ASL), Piacenza, 2Department of Health Science (DISSAL), University of Genoa, Genoa, Italy
Abstract: Recurrent pharyngo-tonsillar infections caused by group A beta-hemolytic streptococci (GABHS) occur frequently in young children, and the treatment of these infections contributes substantially to the total current requirement for antibiotic prescribing. Our study goal was to assess through a retrospective observational analysis whether the administration of the oral probiotic, Streptococcus salivarius K12 (SsK12), could reduce the occurrence of GABHS pharyngo-tonsillar infections in children who had a recent history of recurrent episodes of these infections. Twelve primary care pediatricians identified, through their databases, a total of 130 children who had experienced recurrent GABHS pharyngo-tonsillar infections over a period of at least 6–12 months prior to their inclusion in the study. Of these children, 76 then undertook a 90-day program requiring once-a-day dosing with a commercially available (Bactoblis) lozenge containing SsK12. No probiotic supplement was given to the remaining 54 (control) children. Each subject was monitored for the occurrence of GABHS pharyngo-tonsillitis and also for acute otitis media, bronchitis, sinusitis, and bronchopneumonia for at least 12 months following their entry to the study. Even 9 months after the use of SsK12 had been stopped, the probability of new GABHS infections was significantly lower (P>0.001) when compared to the period before dosing commenced. When compared to the untreated children, those taking SsK12 appear to have had significantly fewer GABHS infections both during the 90-day period of prophylaxis and during the following 9 months (P<0.001). These observations are supportive of the use of probiotic SsK12 for the control of recurrent GABHS pharyngo-tonsillar infections in children, and as an associated benefit, the use of this probiotic could lead to reduced antibiotic consumption. Follow-up controlled prospective studies should now be initiated in order to further establish the efficacy of this newly emerging prophylactic strategy.
Keywords: recurrent pharyngo-tonsillar infections, group A beta-hemolytic streptococcus, Streptococcus salivarius K12
Background
Group A beta-hemolytic streptococci (GABHS) are a frequent cause of recurrent pharyngo-tonsillar infections (RPTIs) in young children, and this is associated with the further requirements for recurrent clinical examinations, pharmacological treatments, specialist consultations, and sometimes surgical intervention.
In Italy, oral penicillin is not available, and penicillin G is only provided through the National Health Service for patients with rheumatic disease. Therefore, amoxicillin is the drug of choice for treatment of single acute episodes of GABHS.1,2
For those experiencing recurrent GABHS infections, cycles of antibiotic therapy and tonsillectomy are given consideration. Recently, an orally administered probiotic product (Bactoblis), based on Streptococcus salivarius K12 (SsK12), became available in Italy. SsK12, a normal inhabitant of the human oral cavity, produces two bacteriocins, salivaricin A2 and salivaricin B, both of which interfere with the growth of GABHS.3,4 Bacteriocins are antimicrobials having relatively specific killing activity. Their action leads to suppression of the growth of bacteria that are phylogenetically closely related to the bacteriocin-producing strain. Unlike the classical antibiotics used to treat infections, the action of bacteriocins does not extend to microbial species that are distanced phylogenetically from the producer strain.
As is often the case for nutraceutical products, reports of its efficacy are still quite limited.5
The product is provided as tablets to be sucked slowly in the evening before bedtime with a recommended dosing program of one tablet daily for 90 days.
We asked the primary care pediatricians of the Local Health Unit (LHU) of Piacenza to analyze their databases retrospectively for children experiencing GABHS RPTIs, and then to compare the subsequent clinical sequelae in the children who had been treated with the recommended program of SsK12 with those who had not taken this product.
The primary objective of the study was to assess retrospectively if SsK12 use in pediatric patients with GABHS RPTIs could:
(a) | significantly reduce the occurrence of GABHS relapses during the treatment period itself and over the following 9 months, when compared to the 6- to 12-month period immediately prior to the start of their probiotic treatment; and | |
(b) | significantly reduce the occurrence of GABHS relapses during the treatment period and over the following 9 months, when compared with a control group of children experiencing GABHS RPTIs but nontreated with SsK12. |
A secondary study objective was to assess whether the subjects treated with SsK12 had experienced any significant differences in the occurrence of bronchitis, otitis, sinusitis, or bronchopneumonia.
Materials and methods
This study was performed according to the criteria contained in the Declaration of Helsinki and was approved by the Ethics Committee of the Local Health Authority of Piacenza. A written consent was obtained from parents of children enrolled in the study.
Methods and selection of patients
Twelve of the 33 primary care pediatricians of the LHU of Piacenza participated in the study. Each pediatrician collected the retrospective data of patients ranging from 3 to 7 years of age who had received a diagnosis of GABHS RPTIs during the period January 1, 2011 to December 31, 2013.
Since 2010, the primary care pediatricians of LHU have used standardized clinical and microbiological criteria for the diagnosis of GABHS infections based on the McIsaac clinical score6 and the rapid throat swab (RAD), as a requirement of the ProBA (Project Children-Antibiotics) regional project.7 According to the McIsaac clinical score and flow chart, the diagnosis of GABHS infection could, in probability terms, be excluded, confirmed, or remain questionable: in the case of a questionable clinical score, the availability of RAD enables a diagnosis to be made with reduced error rates.8 Therefore, the following definitions were adopted for the present study.
Definition of pharyngo-tonsillar infection
McIsaac score with clinical score ≥2 (adenopathy, fever >38°C, absence of cough, pharyngo-tonsillar exudate, age, season) + confirmation of GABHS presence with RAD method or McIsaac score =5.
Definition of RPTI
RPTI is defined as three or more episodes of pharyngo-tonsillitis over a period of 6 months, or four or more episodes over a period of 12 months.
For each patient, a form was completed listing infectious events observed over the 12 months following his/her RPTI diagnosis, and/or any antibiotic therapy used. Patients diagnosed as having RPTIs who then received a standard 3-month treatment with SsK12 were then observed for a further 9 months so that all patients in the study were monitored for at least 12 months after RPTI diagnosis.
Administration of SsK12
SsK12 was administered as tablets sucked slowly in the evening just before bedtime, one tablet each day for 90 days. Every tablet (Bactoblis commercial lozenges) contains one billion units forming colonies of SsK12/dose (based on the product expiry date).
Statistical analysis
For a suitable statistical assessment, it was necessary to have a sample size of at least 100 cases of RPTIs. Nonparametric tests were used. To compare the results of the 12-month observations of the two groups of patients with RPTIs (ie, those first treated for 3 months with SsK12 vs those not treated with SsK12), Fisher’s exact test was used for contingency analysis, while the Mann–Whitney test was applied for ordinal variables analysis.
Results
Included in the present study were 130 children who were established to be affected by RPTIs and whose clinical records could be followed up for a subsequent period of 12 months: 76 children were first treated for 90 days with SsK12, while 54 did not receive SsK12 and were considered to be the control group. The group of treated children consisted of 38 males, mean age 5.0±1.3 years, and 38 females, mean age 4.9±1.6 years. The group of nontreated children consisted of 25 males, mean age 5.3±1.7 years, and 29 females, mean age 5.3±1.5 years. The two groups had no statistically significant differences in age and sex.
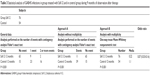
The children treated with SsK12 had a significantly lower number of pharyngo-tonsillar infections than in the period before treatment (P<0.001). Interestingly, the control group also experienced a significantly lower occurrence of GABHS infections during the observation period of 1 year following RPTI diagnosis. However, the reduction of infections obtained in the treated group was statistically higher in the SsK12-treated children (P<0.001) (Table 1).
By comparison with the control group, the group of children treated with SsK12 experienced significantly fewer GABHS infections both during the initial 90 days of inclusion in the study, during which the treatment group received SsK12 (nine relapses vs 42; P<0.001, odds ratio 0.03), and in the following 9 months (eleven relapses vs 39; P<0.001, odds ratio 0.07) (Tables 2 and 3).
Multivaried analysis has also demonstrated the absence of any dependency on sex and age variables. Regarding documented episodes of acute otitis media, bronchitis, sinusitis, and bronchopneumonia, no significant differences were found between the group treated with SsK12 and the nontreated group.
Discussion
GABHS pharyngo-tonsillitis is one of the most frequently occurring infectious diseases in the pediatric age. Although RPTIs are a well-recognized problem in geographical areas such as the North of Italy, there are relatively little epidemiological data on this topic. Cardiac and rheumatic complications, although still present in developing countries, have been greatly reduced in recent decades in most western countries, and so their prevention is no longer the primary goal of pharyngo-tonsillitis therapy.9 Appropriate antibiotic therapy typically effects a rapid healing of acute pharyngo-tonsillitis, and this, in practice, is the most pressing need for parents, as a consequence of current social and economic patterns differing from those of the past.
The secondary prevention of GABHS RPTIs typically focuses upon tonsillectomy as a therapeutic option, although this is less frequently applied than it was 20–30 years ago. In Italy, the use of penicillin G has been gradually neglected because of increased concern about the risk of adverse anaphylactic reactions, and it is not granted by National Health Service, with a high cost per vial. At present, there are no validated alternative pharmacological options for the secondary prevention of GABHS RPTIs.
Studies of a group of school children in New Zealand10 demonstrated that some of the children had bacteriocin-producing S. salivarius present in their saliva, which had strong inhibitory action against Streptococcus pyogenes. S. salivarius are known to be harmless, frequently occurring inhabitants of the human oral cavity, and one isolate, named SsK12, was shown to produce two bacteriocins, salivaricin A2 and salivaricin B, both having strong inhibitory action against GABHS.3,4
The ability of SsK12 to colonize the upper respiratory tract when taken as a probiotic preparation in tablet form has been established both in adults and in children.11,12 The presence of SsK12 and of its released bacteriocins is detectable, through use of bacterial culture analysis and polymerase chain reaction methodologies for at least 32 days after its last administration.12
The safety profile of SsK12 has been assessed in several studies, and according to the Ministry of Health directives, it is considered safe for human use in probiotic formulations intended to achieve oral colonization.13,14
During our study, compliance rate assessed throughout the 3-month period on SsK12 was very good: no child has stopped therapy earlier than established.
The present retrospective observational study has indicated that the use of SsK12 has significantly reduced the occurrence of GABHS pharyngo-tonsillitis in a group of children established to have GABHS RPTIs, by comparison to the occurrence of these infections both in this group of children in the period prior to their use of SsK12 and in a control group of children characterized by the same clinical history of RPTIs, but untreated with SsK12.
Control group also showed reduced rate of GABHS infections over a period of 12 months: this is more likely due to immune competence that physiologically increases in childhood; nevertheless, there remained a very high statistical difference when compared with group treated with SsK12. Ours is a retrospective observational study, and hence, the different number of subjects in the two groups. Anyway, we are not concerned about the effects of numerical imbalance between the two groups because, according to Ruvuna,15 we calculated the statistical impact on the power of our test discovering it as minimal.
According to our data, SsK12 assumption makes four times less likely the need for antibiotic therapy against GABHS infections providing the chance of reducing antibiotic pressure in the era of multi-resistant germs.
It is recognized that this retrospective, observational study has less validity than a double-blind, controlled, prospective, and randomized investigation and also that it may contain significant bias: for example, no account has been taken of poorly compliant subjects who may either have stopped taking inconsistently used SsK12 during the recommended 90-day course of treatment. Also, no account has been made of the use by the test or control subjects of any other nutritional and/or probiotic products. Moreover, entry to the study occurred in a voluntary way, and the criteria established by the pediatricians for the recommendation for SsK12 use by each patient with an RPTI diagnosis were purely subjective. On the other hand, the treatment and control groups were uniform for age and sex, and the diagnostic–therapeutic management protocols for pharyngo-tonsillitis (ie, clinical assessment with McIsaac score and the use of RAD) have been well established for all of the pediatricians participating in the study. Few studies are available about clinical evaluation of the oral probiotic SsK12 in preventing recurrent pharyngitis and/or tonsillitis in childhood caused by S. pyogenes, all of them not randomized or placebo-controlled and also not blinded and with fewer children enrolled.5 Up to now, our study is the one with the largest number of children enrolled.
Conclusion
On the basis of the results of this observational and retrospective study, it appears that oral preparations containing SsK12 may provide a beneficial option for the prevention of pediatric GABHS RPTIs: their use may be particularly useful in patients who would otherwise be forced to undergo frequent cycles of antibiotic therapy. Hopefully, further investigations of this new approach to prophylaxis against GABHS infection will follow, also bearing in mind the ever-increasing need to reduce our antibiotic usage in patients of all ages in order to reduce the risk of antibiotic resistance development.
Author contributions
GG and OR designed the study and have made substantial contributions in drafting manuscript. PR performed statistical analysis. GB, GD, AF, AG, SL, CMa, and CMu performed data acquisition and validation and contributed to interpretation of data. MG and LS verified data analysis and revised the manuscript critically. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Van Driel ML, De Sutter AIM, Keber N, Habraken H, Christiaens T. Different antibiotic treatments for group A streptococcal pharyngitis. Cochrane Database Syst Rev. 2013;(4):CD004406. | ||
Spinks A, Glasziou PP, DelMar CB. Antibiotics for sore throat. Cochrane Database Syst Rev. 2013;(11):CD000023. | ||
Tagg JR. Streptococci as effector organism for probiotic and replacement therapy. In: Versalovic J, Wilson M, editors. Therapeutic Microbiology: Probiotics and Related Strategies. Washington, DC: ASM Press; 2008: 61–81. | ||
Tagg JR, Dierksen KP. Bacterial replacement therapy: adapting ‘germ warfare’ to infection prevention. Trends Biotechnol. 2003;21(5):217–223. | ||
Di Pierro F, Donato G, Fornia F, et al. Preliminary pediatric clinical evaluation of the oral probiotic Streptococcus salivarius K12 in preventing recurrent pharyngitis and/or tonsillitis caused by Streptococcus pyogenes and recurrent acute otitis media. Int J Gen Med. 2012;5:991–997. | ||
McIsaac WJ, Kellner JD, Aufricht P, Vanjaka A, Low DE. Empirical validation of guidelines for the management of pharyngitis in children and adults. JAMA. 2004;291(13):1587–1595. | ||
E-R Agenzia sanitaria e sociale regionale. Dossier n.153/2007 – Faringotonsillite in età pediatrica. Linea Guida regionale [update February 15, 2013]. Available from: http://assr.regione.emilia-romagna.it/it/servizi/pubblicazioni/dossier/doss153. Accessed March 24, 2015. | ||
Di Mario S, Gagliotti C, Moro ML. Nuovelinee Guidafaringotonsillite – Regione Emilia – Romagna. June 2015. Available from: http://assr.regione.emilia-romagna.it/it/servizi/pubblicazioni/rapporti-documenti/faringotonsillite-guida-rapida-2015 | ||
National Institute for Health and Clinical Excellence. Centre for Clinical Practice. Respiratory tract infections – antibiotic prescribing. Prescribing of antibiotics for selflimiting respiratory tract infections in adults and children in primary care (Clinical guideline; no. 69). London, UK: NICE; 2008. | ||
Tagg JR. Prevention of streptococcal pharyngitis by anti-Streptococcus pyogenes bacteriocin-like inhibitory substances (BLIS) produced by Streptococcus salivarius. Indian J Med Res. 2004;119(Suppl):13–16. | ||
Power DA, Burton JP, Chilcott CN, Dawes PJ, Tagg JR. Preliminary investigations of the colonization of upper respiratory tract tissues of infants using a paediatric formulation of the oral probiotic Streptococcus salivarius K12. Eur J Clin Microbiol Infect Dis. 2008;27(12): 1261–1263. | ||
Horz HP, Meinelt A, Houben B, Conrads G. Distribution and persistence of probiotic Streptococcus salivarius K12 in the human oral cavity as determined by real-time quantitative polymerase chain reaction. Oral Microbiol Immunol. 2007;22(2):126–130. | ||
Burton JP, Wescombe PA, Moore CJ, Chilcott CN, Tagg JR. Safety assessment of the oral cavity probiotic Streptococcus salivarius K12. Appl Environ Microbiol. 2006;72(4):3050–3053. | ||
Burton J, Chilcott C, Wescombe P, Tagg J. Extended safety data for the oral cavity probiotic Streptococcus salivarius K12. Probiot Antimicrob Proteins. 2010;2:135–144. | ||
Ruvuna F. Unequal center sizes, sample size, and power in multicenter clinical trials. Drug Inf J. 2004;38(4):387–394. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.