Back to Journals » International Journal of Nanomedicine » Volume 15
Recent Progress and Future Directions: The Nano-Drug Delivery System for the Treatment of Vitiligo
Authors Sun MC, Xu XL , Lou XF, Du YZ
Received 13 January 2020
Accepted for publication 8 April 2020
Published 8 May 2020 Volume 2020:15 Pages 3267—3279
DOI https://doi.org/10.2147/IJN.S245326
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Ming-Chen Sun,1,* Xiao-Ling Xu,1,* Xue-Fang Lou,2 Yong-Zhong Du1
1Institute of Pharmaceutics, College of Pharmaceutical Sciences, Zhejiang University, Hangzhou 310058, People’s Republic of China; 2School of Medicine, Zhejiang University City College, Hangzhou 310015, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yong-Zhong Du
Institute of Pharmaceutics, College of Pharmaceutical Sciences, Zhejiang University, 866 Yu-Hang-Tang Road, Hangzhou 310058, People’s Republic of China
Tel +86-571-88208435
Fax +86-571-88208439
Email [email protected]
Xue-Fang Lou
School of Medicine, Zhejiang University City College, 51 Hu-Zhou Street, Hangzhou 310015, People’s Republic of China
Tel +86-571-88013011
Fax +86-571-88018442
Email [email protected]
Abstract: Vitiligo is a depigmentation disease that seriously affects the physical health, mental health and quality of life of a patient. Therapeutic aim at control immunoreaction by relieving oxidative stress. Unfortunately, the cuticle barrier function and lack of specific accumulation lead to unsatisfactory therapeutic outcomes and side effects. The introduction and innovation of nanotechnology offers inspiration and clues for the development of new strategies to treat vitiligo. However, not many studies have been done to interrogate how nanotechnology can be used for vitiligo treatment. In this review, we summarize and analyze recent studies involving nano-drug delivery systems for the treatment of vitiligo, with a special emphasis on liposomes, niosomes, nanohydrogel and nanoparticles. These studies made significant progress by either increasing drug loading efficiency or enhancing penetration. Based on these studies, there are three proposed principles for topical nano-drug delivery systems treatment of vitiligo including the promotion of transdermal penetration, enhancement of drug retention and facilitation of melanin regeneration. The presentation of these ideas may provide inspirations for the future development of topical drug delivery systems that will conquer vitiligo.
Keywords: vitiligo, nano-drug delivery system, transdermal penetration, liposomes, skin
Introduction
Vitiligo is an acquired idiopathic dermatological disorder characterized by the appearance and development of white macules related to the apoptosis or selective damage of melanocytes. Approximately 0.5–1% of the individuals are diagnosed with vitiligo.1 The highest reported prevalence has been recorded in India (up to 8.8%), followed by Mexico (2.6–4%) and then Japan (1.68%2). Depigmentation usually occurs on exposed areas of the body including the face, neck and arms. The extreme effects of vitiligo often bring dramatic psychological burden to afflicted patients. Although males and females are equally affected by this disease, women more often openly express and address vitiligo for cosmetic purposes and are more likely to seek treatment.3 The incidence of vitiligo often presents through distinct familial clustering with reports that 20% of the vitiligo patients also have relatives diagnosed with the disorder.4 Individuals with a positive family history usually have an earlier age of onset as well as a longer duration5 compared to individuals that do not have a family history of the disease.
Thus far, the etiology of vitiligo has been found to result from multiple factors and has not yet fully been elucidated. In the 1950s, Lerner investigated 600 vitiligo patients and found that most patients with segmental vitiligo suffered from emotional imbalances or hyperhidrosis, eventually giving rise to the neural theory.6 As research progressed, factors including stress, autoimmune diseases, melanocyterrhagy and autoinflammation have been identified as important factors contributing to vitiligo.7 Of all theories, autoimmune disease or autoinflammation8–11 and oxidative stress12 as well as interactions among and between them have been accepted as some of the most important factors contributing to the disease.13 In the disease, antigen-presenting cells activate T cells through the presentation of melanocyte antigens where T cells then directly kill the melanocytes. It has been reported that endogenous killer and inflammatory dendritic cells are in a hyperactive state in patients with vitiligo.9,10 Various cytokines including INF-γ,14–16 CXCL10,14,17,18 TNF-α, IL-6 and IL-1719–22 are also secreted by innate cells through an autoimmune response.
Separate from the autoinflammation theory, oxidative stress is also an important risk factor for vitiligo. Melanin synthesized by melanocytes is toxic which stimulates the cell stress signaling pathway in these cells. Moreover, active energy metabolism in the mitochondria leads to an excessive accumulation of reactive oxygen species (ROS). This also gives rise to the development of vitiligo.23,24
Briefly, when tiny lesions generated through a sunburn, viral infection or physical trauma occur, epidermal damage-associated molecules are released into the body and oxidative stress levels are increased. The adhesion of melanocytes is lost and inflammasomes are activated by the release and induction of molecular and oxidative stress products. After a series of immunoreactions, specific cytotoxic T cells are accumulated on the skin, regulatory T cell activity is down-regulated and inflammatory cytokines as well as autoantibodies are produced, ultimately leading to immune-based melanocyte destruction (Figure 1).25 Likewise, oxidative stress contributes to the onset of depigmentation, and subsequent undesired autoimmune responses lead to the progression of vitiligo.26–30 Here, we review all nano-drug delivery systems that can be used for the treatment of vitiligo, hoping to offer useful resources and spark inspiration for future research focused on novel vitiligo treatments (Figure 2).
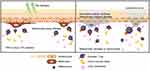 |
Figure 1 Illustration of the pathogenesis of vitiligo. |
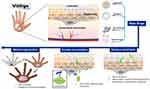 |
Figure 2 A scheme of the nano-drug delivery systems for vitiligo therapy. |
Therapeutic Approaches
Since the mechanics and dynamics of vitiligo are not fully understood, it has been challenging to develop an effective treatment for vitiligo. The ultimate goals for vitiligo treatment are to prevent the expansion of existing white spots on the skin, promote repigmentation and reduce psychological burden. Currently, strategies that are typically used for vitiligo treatment mainly include drug therapy and phototherapy (Table 1). Corticosteroids, such as betamethasone dipropionate, clobetasol dipropionate and mometasone furoate, have been first-line options for vitiligo treatment due to their anti-inflammatory and immunosuppressive effects.31 In recently published guidelines for vitiligo treatment, calcineurin inhibitors such as tacrolimus and pimecrolimus were also recommended as first-line approaches for the management of vitiligo.32–34 Some clinical trials have shown that calcineurin inhibitors have a similar effect as glucocorticoids, especially for facial leukomas.35,36 In addition, the accumulation of oxidative products in the skin is also an important factor contributing to melanocyte dysfunction. Hence, various antioxidants that remove excess ROS and hydrogen peroxide from the epidermis of vitiliginous skin have been used to restore the oxidation-antioxidant system of the skin.37 Oral antioxidants such as polypodium leucotomos, vitamin E, vitamin C and minocycline are used for an antioxidation treatment strategy against vitiligo. Separate from drug therapy, phototherapy including narrow-band ultraviolet (NB-UVA) and psoralen ultraviolet A (PUVA) are relatively safe physical-based approaches.38–40 Since phototherapy alone and in combination with the drugs mentioned are highly successful in inducing repigmentation, these strategies have been extensively adopted in clinical practice.41
The onset, progression and management of vitiligo is usually a long process ranging from months to years. Thus, long-term use of certain drugs or treatment approaches will inevitably result in a variety of side effects. For example, the side effects of the long-term use of glucocorticoids include skin atrophy, acne and folliculitis.42 The large-scale use of corticosteroids is not feasible and may lead to a high number of side effects. Thus, corticosteroids are not considered appropriate for the treatment of generalized vitiligo. Although glucocorticoids and calcineurin are clinically complementary and have similar therapeutic effects, a recent study emphasized that tacrolimus showed no apparent therapeutic effects in mitigating segmental vitiligo.32 The most common side effect of calcineurin inhibitors is a burning sensation during the first two weeks of treatment. In addition, the financial costs of calcineurin inhibitors are much higher than the costs of corticosteroids, which can be a significant financial burden for patients.37,43,44 Comparing these different treatments, antioxidant drugs are milder with no obvious side effects. Nonetheless, accepted consensus guidelines do not recommend the use of topical antioxidants as a single therapy for vitiligo since most studies were restricted by a limited number of patients.45 In terms of a non-drug treatment, phototherapy for vitiligo requires frequent treatment (two or three times weekly), which brings inconvenience to patients. Despite the higher success rates that these strategies have for repigmentation, recurrence rates still remain high. Over 50% of the patients are prone to recurrent white spots on regimented skin within the first year after therapy is discontinued.25,46 In addition, side effects such as headaches, nausea and ocular as well as renal toxicity may occur as a result of phototherapy treatment.47,48 In addition, these therapies are also associated with a risk of skin cancer. Thus, phototherapy is recommended only when major treatments are found to be ineffective by the NHS.49
With the development of nanotechnology, voluminous nano-drug delivery systems have emerged and been applied to enhance drug penetration through the skin. These systems include microemulsions, nanoemulsions, nanoparticles, lipid carriers and many other nanovesicles and exhibit prominent advantages over conventional methods. In the field of nanomedicine, the number of existing literature focused on the treatment of vitiligo is relatively small.
 |
Table 1 Treatment Approaches for Vitiligo |
Nano-Drug Delivery System in the Treatment of Vitiligo
Human skin is the largest of the body's organ and helps to carry out many essential functions including such as acting as a physical barrier, aiding in immune defense, temperature maintenance, UV protection, and moisture retention.50 Three layers comprise the skin: stratum including the corneum, dermis, and hypodermis. The exposed layer and the stratum corneum contain 70–90% protein and 5–15% lipids, and aid in the process of influencing the role of protecting the body from external and environmental stressors and pollutants.51 One of the most significant challenges of transdermal delivery such as to treat afflictions such as vitiligo is that only a few therapeutic compounds possess ideal penetration behaviors and characteristics.52 The best existing technology merely allows drugs with a molecular mass of 100 Da and good lipophilicity to permeate the skin successfully.53–56 In addition, another obstacle in achieving the desired therapeutic effects is the incompetence of the current dosage forms including creams, ointments, lotions, gels, and other vectors.57 Generally, all the problems associated with conventional topical preparations suggest that reform and innovation of transdermal drug delivery for the treatment of vitiligo are urgently needed to improve the understanding of this affliction and to increase the possibility of better patient outcomes.
Recent advancements in cutting-edge nanotechnology provide an unprecedented opportunity to overcome shortcomings related to conventional methodologies (Figure 3). In particular, by taking advantage of some features of the stratum corneum, there is potential for the use of a nano-drug delivery system that can enhance the transdermal penetration of drugs and increase therapeutic effects.58 A large number of novel nano-drug delivery systems carrying therapeutic agents are being developed, such as liposomes, polymeric nanoparticles, microspheres, solid lipid nanoparticles and nanofibrous structures (Table 2)
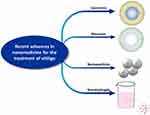 |
Figure 3 Existing nano-drug delivery system for vitiligo therapy. |
 |
Table 2 Nano-Drug Delivery System for Vitiligo Therapy |
Liposomes and Niosomes
Liposomes are particles formed by lipids that consist of a double layer similar to the natural cell membrane enclosing an aqueous core. Containing non-toxic, biodegradable properties, liposomes are regarded as the most promising nanocarrier for topical drug delivery.69 The apparent success of liposomes may be the result of lipid vesicles fusing into the stratum corneum, allowing the therapeutic to permeate into deeper skin layers.70 Liposomes also increase the dissolution of the applied drugs and augment drug solubility on skin surfaces. Furthermore, liposomes are seen as drug repositories that allow for sustained rates of drug release and help reduce the frequency of treatments.71 One group59 prepared an 8-methoxypsoralen-loaded liposomal treatment through a film hydration method. After a series of purges, it was found that the diameter of the final vehicle ranged from 100 to 500 nm and showed excellent penetration behavior when compared to controls treated with hydroalcoholic solution. All nano vehicles displayed high capacities for sustaining the levels of 8-MOP release and its permeation––accumulation into skin layers.
Another group61 reported the effects of both psoralen and resveratrol that were co-loaded with ultradeformable liposomes (UDL) for the treatment of vitiligo. Psoralen is a natural furanocoumarin derivative used in combination with UV or PUVA for the treatment of various skin diseases.72,73 Due to the presence of a surfactant, the vesicle changes its shape to respond to conditions from the external environment.74 Resveratrol stimulates mitogen-activated protein kinase signaling and has been demonstrated to have antioxidant activity.75 Other groups76 have conducted optimization studies and the most appropriate formula was selected based on particle size, PDI and zeta potential. Co-loaded liposomes were prepared through modified film hydration and liposome particle size was 120 nm with drug loading efficiencies between 2.5% and 5%. In vitro studies and kinetics showed that the carrier can sustain the release of both drugs over prolonged periods of time. In vitro cell studies showed that the carrier significantly stimulated melanin and tyrosinase activity without affecting the antioxidant capacity of psoralen and resveratrol.
One group60 prepared baicalin and berberine samples that were co-loaded with ultradeformable vesicles and demonstrated their potential as adjuvants for the treatment of vitiligo. Baicalin and berberine were selected as curative agents due to their antioxidant, anti-inflammatory and proliferative effects.76–78 In vitro studies showed that preparation enhanced permeation of drugs and antioxidants. Photoprotective effect evaluations showed that co-loaded vesicles increased melanin and tyrosinase activities.
Others63 constructed an elastic cationic noisome, loading the human tyrosinase plasmid pMEL34. The maximum loading amount of pMEL34 in the elastic vehicle was 150 mg/16 mg of niosomal determined by gel electrophoresis and documentation. Due to the enhancement effects of ethanol on vesicle elasticity, cumulative amounts and flux at 6 h post-treatment with pMEL34 were significantly increased in comparison with pMEL34 loaded into nonelastic cationic niosomes. The level of tyrosinase-associated activities were found to be four times higher than the respective levels for free plasmid and plasmid loaded into nonelastic niosomes, indicating that elastic cationic niosomes have potential as efficient gene topical delivery systems for vitiligo treatment. In 2012, a human tyrosinase plasmid pMEL34 by Tat peptide was described and found to enhance melanin production. The vesicular size and zeta potential were proved that the carrier is still in the range of stable dispersion. In vitro studies have indicated that this type of preparation promotes both tyrosinase gene expression and melanin production with little to no cytotoxic side effects.62
Another group66 prepared 8-MOP ethosomes through a central composite design (CCD). Conventional liposomes deliver drugs to superficial layers of the skin, while ethosomes improve the permeation and retention in skin layers of the drugs.79 The in vitro skin permeation study showed that this preparation induced enhanced transdermal efficacy, optimized ethosomal formulation and produced significant accumulation of 8-MOP, likely as a result of ethosome deformation. In vivo studies were consistent with in vitro results. Among all the elaborated topical transdermal delivery approaches, lipid nanocarrier is the most optimal one for its high biocompatibility and drug encapsulation efficiency. An increasing number of researchers in this field are paying attention to various novel vesicle delivery systems that possess percutaneous penetration and better therapeutic effects.
Microemulsions
Microemulsions are thermodynamically stable colloidal systems with a transparent appearance. The conventional formula of microemulsions consists of water and oil stabilized by surfactant and sometimes also contains a cosurfactant.80 Based on the physical and chemical properties of surfactants and ingredients of microemulsions, microstructures of microemulsions vary. The advantages of microemulsions used as transdermally delivered drugs show them as being an attractive technological platform for novel pharmaceutical formulations.81–84 One group85 reported a clobetasol propionate-loaded microemulsion-based gel that successfully overcame the poor solubility of clobetasol propionate. The effectiveness of the preparation indicated that the stratum corneum swelled due to water retention caused by gel formulation thereby assisting clobetasol propionate penetration into the skin. A clinical study indicated that the extent of repigmentation in patients treated with a clobetasol propionate-loaded microemulsion-based gel was faster and more profound when compared to control groups. Numerous studies and literatures have shown that microemulsion can significantly enhance the percutaneous permeability of drugs though the exact mechanisms that have not been fully understood. It is worth noting that apart from the internal microstructure, the composition or internal phase structure largely determines the permeation of microemulsion. Microemulsions have been a promising approach in the transdermal delivery of anti-inflammatory drugs (NSAIDs) with a fairly obvious improvement in the permeation rate.
Nanoparticles
The emerging use of nanoparticles is one of the most impactful components of nanomedicines. Due to large surface-to-volume ratios, nanoparticles not only enhance the contact area between a drug and target tissue but also permits drug release in a controlled manner.86 In recent years, nanoparticles have been incorporated into common and daily goods. Nanoparticles penetrate the skin based on its size, charge and overall structure.87 Metal nanoparticles such as palladium and platinum boost enzyme activity and exhibit anti-inflammatory capacity in UV-treated HaCaT keratinocytes. In addition, the rate of apoptosis in cells pretreated with nano-Pt was significantly reduced compared to controls.88 A mix of Pd and Pt nanoparticles termed PAPLAL has a history as a treatment for chronic diseases such as burns, gastric ulcers and rheumatoid arthritis.89 PAPLAL also exhibited SOD catalase activity and significantly lowered O2- generation levels in mouse skin tissue. Both in vivo and in vitro results showed that PAPLAL effectively suppressed endogenous superoxide levels through SOD and catalase activity via the AHR and NRF2 pathways.67 Further investigation using palladium and platinum nanoparticles in the treatment of vitiligo should be performed with caution since metals may possess allergens.90 By coating or other means of modification, nanoparticles can overcome their inherent shortcomings to achieve efficient transdermal delivery and even release drugs with targeting behavior.
Principles of Nano-Drug Delivery Systems Designed for Vitiligo Therapy
Enhancing the Penetrate Capacity of Therapeutic Agents
Similarities between lipid particles and the stratum corneum make liposomes excellent vectors for delivery of therapeutics.91 Conventional liposomes are thought to be inadequate for transdermal delivery, but more advanced liposomes such as invasomes, transferosomes, ethosomes and niosomes can overcome disadvantages seen with original methods (Table 3).
Invasomes were first proposed as novel modified liposomes in 2003.92 The presence of soy-phosphatidylcholine (SPC), ethanol and terpenes make significant improvements in percutaneous permeability compared to traditional liposomes.93,94 One group investigated the transdermal ability of invasomes loaded with ferulic acid. Skin permeation experiments revealed that invasomes possess good permeation capacity and are an ideal carrier for the transdermal delivery of ferulic acid.95
Transfersomes were first reported for transdermal delivery in 1992. Surfactants such as sodium cholate, spans and tweens make these carriers more elastic in comparison to traditional liposomes. Another group prepared a transferosome-encapsulating dexamethasone and evaluated its in vivo performance in a carrageenan-induced rat paw edema model. After a series of formula optimization studies, Span-80 was selected as the optimum edge activator that offered maximum deformability. These results demonstrated that drug-loaded transferosomes have better antiedema activity when compared to liposomes, indicating that transferosomes possess better penetration ability.87
 |
Table 3 Brief Introduction of Novel Liposomal Systems |
Microneedles (MN) are micron-sized needle projections with a height of 10–2000 μm and a width of 10–50 μm that painlessly penetrates the skin.98 In recent years, transdermal drug delivery using microneedles has been widely studied.99–101 A combination of microneedles and nanocarriers has also been considered for the treatment of diabetes,102 cancer therapy103 and immunotherapy.104 Various nanoparticles with antioxidant or anti-inflammatory properties can be used as therapeutic agents to remove excess ROS and control vitiligo progression. However, poor water dispersibility and weak percutaneous permeability restrict application.105 The combination of microneedles and nanoparticles brings hope for vitiligo therapy. Kim et al reported gold nanoparticles that were successfully able to be delivered into the hamster cheek at a depth of 100–200μm.106 Nevertheless, the main drawback of treating vitiligo with microneedle is that the frequent use of microneedle will inevitably lead to stubborn skin damage and even inflammation. A comprehensive microneedle drug delivery strategy deserves attention and development.
By applying a mild electrical current, iontophoresis allows ionized substances to pass through the adjacent skin or tissue without invasion.107,108 The combination of nano-drug delivery systems and iontophoresis was first reported in 1996. However, unsatisfactory results were obtained using this method.109 Another group further demonstrated the mechanisms behind the combination strategy. This group observed connexin43 phosphorylation and filamentous actin depolymerization when charged liposomes crossed the intercellular space. The Ca2+ inflow was also stimulated by the electrical stimulus. All evidence suggested that the electric current changed the physiological properties of the skin and enhanced the permeation of liposomes.110
Increasing Therapeutic Agent Accumulation: The Receptor Ligand Binding Mechanism
Melanocytes are dendritic cells that synthesize and secrete melanin and are located in the basal cell layer of the epidermis. There are plenty of ways therapeutic agents can overcome the stratum corneum barrier and penetrate deeper into the skin. However, driving and accumulating the drug to the target site still remains a challenge. The affinity of the receptor and ligand that encompasses active targeting is widely utilized to exert specific retention. Many receptors on the surface of melanocytes enable the construction of such active targeting vectors.
Melanocortin-1 receptor (MC1R) is a G protein-coupled receptor specifically distributed on the surface of melanocytes (Figure 4). Endogenous ligands of this receptor include α-MSH, β-MSH, γ-MSH and adrenocorticotropic hormone (ACTH). Among these ligands, α-MSH shows the strongest affinity with MC1R.111 The amino acid sequence of α-MSH is simple, allowing it to serve as a target molecule enabling nano-drug delivery systems to aggregate in the vicinity of melanocytes.
The endothelin receptor is another receptor located on melanocytes that participates in a variety of physiological activities. During the transformation of melanocytes into melanoma cells, endothelin B receptor expression gradually increases.112 One group reported that Endothelin-1 (ET-1) promotes melanin synthesis and the formation of dendrites in normal human epidermal melanocytes.113 Similarly, a seven-transmembrane receptor on keratinocytes termed protease-activated receptor-2 (PAR-2) plays a central role in the transportation of melanosomes.114 SLIGRL and SLIGKV are two PAR-2-activating peptides that have the same activation capacity as PAR-2 without receptor cleavage.115,116
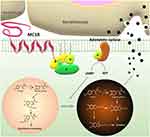 |
Figure 4 Schematic diagram of the role of MC1R in melanin synthesis and secretion. |
Promoting Melanin Regeneration
Therapeutic goals for vitiligo treatment consist of inhibiting the inflammatory response and promoting melanin regeneration. Many therapeutic regimens only focus on inhibiting the inflammatory response rather than promoting repigmentation. Thus, there has been a growing demand for drug delivery systems that inhibit the inflammatory response while promoting melanin regeneration.
Tacrolimus is a calcineurin inhibitor that weakens T cell activity and the secretion of proinflammatory cytokines, thus promoting melanocyte migration and repigmentation.117,118 However, its application is restricted due to the risk of lymphoproliferative disease, narrow therapeutic index and low solubility.119,120 One group prepared a nanolipid carrier containing lipophilic solubilizers to improve drug solubility. This carrier exhibited a high entrapment efficiency of 96.66% and a significantly higher drug release in vitro and in vivo compared to the commercial ointment. Meanwhile, a skin irritation study revealed less irritation using this carrier when compared to the control group.121
As described, many receptors specifically located on the surface of melanocytes and keratinocytes play important roles in controlling melanocyte proliferation, melanin synthesis and melanin migration. On the other hand, their endogenous ligands or the analogues can be used as peptide drugs to activate biochemical reactions associated with melanocytes. α-Melanocyte-stimulating hormone (a-MSH), a tridecanoic peptide derived from pro-opiomelanocortin (POMC), plays a significant role in stimulating melanogenic pigmentation in skin tissues.122 After binding to MC1R, the receptor typically sends signals by increasing cyclic adenosine monophosphate (cAMP) and exhibits powerful anti-inflammatory effects.123 The short fragments of α-MSH or its derivatives Lys-Pro-Val (KPV) and (CKPV)2 are reported to possess similar or even stronger biological activity.124 ET-1 can also regulate the synthesis of melanin, promote melanocyte proliferation and help form dendrites. Accordingly, these endogenous molecules or their analogues can be anchored on transdermal delivery systems to ameliorate transdermal efficiency as well as promote melanin regeneration.125
Conclusion
Vitiligo is a common depigmenting disease affecting 1% of the population worldwide. In some regions, the white spotting caused by vitiligo is confounded with leprosies and has devastating effects on patient psychological health and social activities.126 Treatment and management of vitiligo still shows intractable challenges for both scientists as well as dermatologists. More evidence suggests that the dynamics and pathogenesis of vitiligo are closely related to the immune response of skin and melanocyte response to the immune response. Thus, vitiligo should be treated as a chronic immune disease rather than a problem mainly regarding an approach from a regenerative medicine perspective.127 Nevertheless, the rapid development of nano-drug delivery systems has brought new insights and ideas for the treatment of vitiligo. These novel methods facilitate the ability for developed drugs to possess sustained or controlled release behavior, enhances the therapeutic efficiency and reduces side effects.
Despite the great potential of the available nano-drug delivery systems, certain limitations exist. Most research regarding novel drug delivery systems for the treatment of vitiligo settle for the minimum control of symptoms rather than optimal outcome to completely cure the disease. Despite these challenges, it is inevitable that scientists will perform and complete the research needed to further optimize nano-drug delivery system efficiency. Various receptors on the surfaces of melanocytes or surrounding keratinocytes including granulocyte colony-stimulating factor (G-CSFR) and melanocortin receptors 1~5 (MC1R~MC5R) increase the feasibility of developing targeted agents. Their endogenous ligands or analogues exhibit great potential for the treatment of vitiligo with minimum side effects. One group proposed ultrashort peptides containing cysteine that spontaneously self-assembled into hydrogels. In vivo studies revealed that one of the formulas exhibits great biocompatibility and limited allergenic potential.128 In the following studies, self-assembly behavior, nanostructure formation, hydrogelation and phase transition of peptide nanostructures of two selected tripeptides were investigated by X-ray crystalline diffraction and scanning electron microscopy.129 A consistent hydrophobic backbone of acetylated Leu-Ile-Val-Ala-Gly plays the role of self-assembly and the C-terminal residue has an obvious impact on the rate and extent in which the peptide fibril interacts with an SMD.130 By elucidating the crosslink and polymerization mechanisms of small molecule peptide drugs, various peptide-based topical drug delivery systems may be an inevitable trend for vitiligo treatment.
Here, we reviewed the pathogenesis, present therapeutic methods and existing nano-drug delivery systems that have been used and that will be used in the future for the treatment of vitiligo. Vitiligo can eventually be better controlled, managed and even cured considering the significant progress that has been made and the ongoing development of strategies to tackle this malady.
Acknowledgment
This study was supported in part by the New Century 151 Talent Project of Zhejiang Province.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ezzedine K, Lim HW, Suzuki T, et al. Revised classification/nomenclature of vitiligo and related issues: the vitiligo global issues consensus conference. Pigm Cell Res. 2012;25(3):E1–E13. doi:10.1111/j.1755-148X.2012.00997.x
2. Sehgal VN, Srivastava G. Vitiligo: compendium of clinico-epidemiological features. Leprology. 2007;73(3):149.
3. Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview: part I. Introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65(3):473–491. doi:10.1016/j.jaad.2010.11.061
4. Asem A, Fain PR, Anthony T, et al. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigm Cell Res. 2010;16(3):208–214.
5. Zhang Z, Xu SX, Zhang FY, et al. The analysis of genetics and associated autoimmune diseases in Chinese vitiligo patients. Arch Dermatol Res. 2009;301(2):167–173. doi:10.1007/s00403-008-0900-z
6. Lerner AB. Part V: clinical applications of psoralens, and related materials: vitiligo. J Invest Dermatol. 1959;32(2):285–310. doi:10.1038/jid.1959.49
7. Mohammed GF. Highlights in pathogenesis of vitiligo. World J Clin Cases. 2015;3(3):221. doi:10.12998/wjcc.v3.i3.221
8. Ying J, Mailloux CM, Katherine G, et al. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. 2007;356(12):1216–1225. doi:10.1056/NEJMoa061592
9. Spritz RA. The Genetics of Vitiligo. J Invest Dermatol. 2011;131(E1):E18–E20. doi:10.1038/skinbio.2011.7
10. Zhu KJ, Lv YM, Yin XY, et al. Psoriasis regression analysis of MHC loci identifies shared genetic variants with vitiligo. PLoS One. 2011;6(11):e23089–e23089. doi:10.1371/journal.pone.0023089
11. Wang X, Erf GF. Melanocyte-specific cell mediated immune response in vitiliginous Smyth line chickens. J Autoimmun. 2003;21(2):149–160. doi:10.1016/S0896-8411(03)00087-8
12. Slominski A, Paus R, Bomirski A. Hypothesis: possible role for the melatonin receptor in vitiligo: discussion paper. J R Soc Med. 1989;82(9):539–541. doi:10.1177/014107688908200911
13. Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386(9988):74–84. doi:10.1016/S0140-6736(14)60763-7
14. Rashighi M, Agarwal P, Richmond JM, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. 2014;6(223):223ra23. doi:10.1126/scitranslmed.3007811
15. Harris JE, Harris TH, Weninger W, et al. A Mouse model of vitiligo with focused epidermal depigmentation requires IFN-γ for autoreactive CD8+ T-cell accumulation in the skin. J Invest Dermatol. 2012;132(7):1869–1876. doi:10.1038/jid.2011.463
16. Gregg RK, Nichols L, Chen Y, et al. Mechanisms of spatial and temporal development of autoimmune vitiligo in tyrosinase-specific TCR transgenic mice. J Immuno. 2010;184(4):1909–1917. doi:10.4049/jimmunol.0902778
17. Bertolotti A, Boniface K, Vergier B, et al. Type I interferon signature in the initiation of the immune response in vitiligo. Pigment Cell Melanoma Res. 2014;27(3):398–407. doi:10.1111/pcmr.12219
18. Wang X, Wang Q, Wu J, et al. Increased expression of CXCR3 and its ligands in vitiligo patients and CXCL10 as a potential clinical marker for vitiligo. Br J Dermatol. 2016;84(1):e101–e101.
19. Bassiouny DA, Shaker O. Role of interleukin‐17 in the pathogenesis of vitiligo. Clin Exp Dermatol. 2011;36(3):292–297. doi:10.1111/j.1365-2230.2010.03972.x
20. Wang CQF, Cruzinigo AE, Fuentesduculan J, et al. Th17 cells and activated dendritic cells are increased in vitiligo lesions. PLoS One. 2011;6(4):e18907–e18907. doi:10.1371/journal.pone.0018907
21. Elela MA, Hegazy RA, Fawzy MM, et al. Interleukin 17, interleukin 22 and FoxP3 expression in tissue and serum of non-segmental vitiligo: a case- controlled study on eighty-four patients. Eur J Dermatol. 2013;23(3):350–355. doi:10.1684/ejd.2013.2023
22. Méry‐Bossard L, Bagny K, Chaby G, et al. New‐onset vitiligo and progression of pre‐existing vitiligo during treatment with biological agents in chronic inflammatory diseases. J Eur Acad Dermatol Venereol. 2016;31(1):181–186. doi:10.1111/jdv.13759
23. Toosi S, Orlow SJ, Manga P. Vitiligo-inducing phenols activate the unfolded protein response in melanocytes resulting in upregulation of IL6 and IL8. J Invest Dermatol. 2012;132(11):2601–2609. doi:10.1038/jid.2012.181
24. Schallreuter KU, Moore J, Wood JM, et al. In vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB-activated pseudocatalase. J Invest Dermatol. 1999;4(1):91. doi:10.1038/sj.jidsp.5640189
25. Speeckaert R, Geel N. Vitiligo: an update on pathophysiology and treatment options. Am J Clin Dermatol. 2017;18(6):1–12.
26. Laddha NC, Mitesh D, Mohmmad Shoab M, et al. Role of oxidative stress and autoimmunity in onset and progression of vitiligo. Exp Dermatol. 2014;23(5):352–353. doi:10.1111/exd.12372
27. Mosenson JA, Zloza A, Nieland JD, et al. Mutant HSP70 reverses autoimmune depigmentation in vitiligo. Sci Transl Med. 2013;5(174):ra28. doi:10.1126/scitranslmed.3005127
28. Kroll TM, Bommiasamy H, Boissy RE, et al. 4-tertiary butyl phenol exposure sensitizes human melanocytes to dendritic cell-mediated killing: relevance to vitiligo. J Invest Dermatol. 2005;124(4):798. doi:10.1111/j.0022-202X.2005.23653.x
29. Denman CJ, Mccracken J, Hariharan V, et al. HSP70i accelerates depigmentation in a mouse model of autoimmune vitiligo. J Invest Dermatol. 2008;128(8):2041–2048. doi:10.1038/jid.2008.45
30. Harris JE. Cellular stress and innate inflammation in organ-specific autoimmunity: lessons learned from vitiligo. Immunol Rev. 2016;269(1):11–25. doi:10.1111/imr.12369
31. Van GN, Desmedt V, De SS, et al. Cessation of spread as a treatment objective in vitiligo: perception from the patients‘ point of view. Br J Dermatol. 2016;174(4):922. doi:10.1111/bjd.14283
32. Daniel BS, Wittal R. Vitiligo treatment update. Br J Dermatol. 2015;56(2):85–92.
33. Westerhof W, Nieuweboer-Krobotova L, Mulder PG, et al. Left-right comparison study of the combination of fluticasone propionate and UV-A vs. either fluticasone propionate or UV-A alone for the long-term treatment of vitiligo. Arch Dermatol. 1999;135(9):1061. doi:10.1001/archderm.135.9.1061
34. Boone B, Ongenae K, Van GN, et al. Topical pimecrolimus in the treatment of vitiligo. Eur J Dermatol. 2007;17(1):55. doi:10.1684/ejd.2007.0093
35. Taieb A, Alomar A, BHm M, et al. Guidelines for the management of vitiligo: the European dermatology forum consensus. Br J of Dermatol. 2013;168(1):5–19. doi:10.1111/j.1365-2133.2012.11197.x
36. Veronica L, Benjamin M, Juan Pablo CC, et al. A double-blind randomized trial of 0.1% tacrolimus vs 0.05% clobetasol for the treatment of childhood vitiligo. Arch Dermatol. 2003;139(5):581. doi:10.1001/archderm.139.3.369
37. Iraj E, Ali E, Saeedeh F, et al. The efficacy of pimecrolimus 1% cream plus narrow-band ultraviolet B in the treatment of vitiligo: a double-blind, placebo-controlled clinical trial. J Dermatolog Treat. 2009;20(1):14–18. doi:10.1080/09546630802155057
38. Khan R, Satyam A, Gupta S, et al. Circulatory levels of antioxidants and lipid peroxidation in Indian patients with generalized and localized vitiligo. Arch Dermatol Res. 2009;301(10):731–737. doi:10.1007/s00403-009-0964-4
39. Colucci R, Dragoni F, Conti R, et al. Evaluation of an oral supplement containing Phyllanthus emblica fruit extracts, vitamin E, and carotenoids in vitiligo treatment. Dermatol Ther. 2015;28(1):17–21. doi:10.1111/dth.12172
40. Middelkamp‐Hup MA, Bos JD, Rius‐Diaz F, et al. Treatment of vitiligo vulgaris with narrow‐band UVB and oral Polypodium leucotomos extract: a randomized double‐blind placebo‐controlled study. J Eur Acad Dermatol Venereol. 2007;21(7). doi:10.1111/j.1468-3083.2006.02132.x.
41. Parsad D, Kanwar A. Oral minocycline in the treatment of vitiligo – A preliminary study. Dermatol Ther. 2010;23(3):305–307. doi:10.1111/j.1529-8019.2010.01328.x
42. Sunil D, Bhushan K. Repigmentation in vitiligo universalis: role of melanocyte density, disease duration, and melanocytic reservoir. Dermatol Online. 2005;11(3):30.
43. Kathuria S, Khaitan BK, Ramam M, Sharma V. Segmental vitiligo: a randomized controlled trial to evaluate efficacy and safety of 0.1% tacrolimus ointment vs 0.05% fluticasone propionate cream. Indian J Dermatol Venereol Leprol. 2011;78(1):68.
44. Grimes PE, Morris R, Avaniss-Aghajani E, et al. Topical tacrolimus therapy for vitiligo: therapeutic responses and skin messenger RNA expression of proinflammatory cytokines. J Am Acad Dermatol. 2004;51(1):52–61. doi:10.1016/j.jaad.2003.12.031
45. Lo YH, Cheng GS, Huang CC, et al. Efficacy and safety of topical tacrolimus for the treatment of face and neck vitiligo. J Dermatol. 2010;37(2):125–129. doi:10.1111/j.1346-8138.2009.00774.x
46. Nicolaidou E, Antoniou C, Stratigos AJ, et al. Efficacy, predictors of response, and long-term follow-up in patients with vitiligo treated with narrowband UVB phototherapy. J Am Acad Dermatol. 2007;56(2):0–278. doi:10.1016/j.jaad.2006.09.004
47. Sitek JC, Loeb M, Ronnevig JR. Narrowband UVB therapy for vitiligo: does the repigmentation last? J Eur Acad Dermatol Venereol. 2007;21(7):891–896. doi:10.1111/j.1468-3083.2007.01980.x
48. Roelandts. Photo(chemo) therapy for vitiligo. Photomedicine. 2010;19(1):1–4.
49. Ghafourian A, Ghafourian S, Sadeghifard N, et al. Vitiligo: symptoms, pathogenesis and treatment. Int J Immunopathol Pharmacol. 2014;27(4):485–489. doi:10.1177/039463201402700403
50. Palmer BC, Delouise L. Nanoparticle enabled transdermal drug delivery systems for enhanced dose control and tissue targeting. Molecules. 2016;21(12):1719. doi:10.3390/molecules21121719
51. Marwah H, Garg T, Goyal AK. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 2014;23(2):564. doi:10.3109/10717544.2014.935532
52. Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26(11):1261–1268. doi:10.1038/nbt.1504
53. Stinchcomb AL. Editors. Transdermal drug delivery 2nd Edition, revised and expanded (2003) Marcel Dekker,New York 383 pp. J Controlled Release. 2005;104(1):234–235. doi:10.1016/j.jconrel.2004.09.032
54. Watkinson AC. Transdermal and Topical Drug Delivery Today. Wiley; 2012.
55. Prausnitz MR, Samir M, Robert L. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004;3(2):115. doi:10.1038/nrd1304
56. Wester RC, Maibach H. In vivo methods for percutaneous absorption measurements. J Toxicol Cutaneous Ocul Toxicol. 2001;20(4):411–422.
57. Garg B, Saraswat A, Bhatia A, Katare O. Leprology. Topical treatment in vitiligo and the potential uses of new drug delivery systems. Indian J Dermatol Venereol Leprol. 2010;76(3):231. doi:10.4103/0378-6323.62961
58. Franzé S, Donadoni G, Podestà A, et al. Tuning the extent and depth of penetration of flexible liposomes in human skin. Mol Pharm. 2017;14(6):1998. doi:10.1021/acs.molpharmaceut.7b00099
59. Sinico C, Valenti D, Manconi M, et al. Cutaneous delivery of 8-methoxypsoralen from liposomal and niosomal carriers. J Drug Deliv Sci Technol. 2006;16(2):115–120. doi:10.1016/S1773-2247(06)50017-6
60. Mir-Palomo S, Nácher A, Busó OV, et al. Baicalin and berberine ultradeformable vesicles as potential adjuvant in vitiligo therapy. Colloids Surf B Biointerfaces. 2019;175:654–662.
61. Doppalapudi S, Mahira S, Khan W. Development and in vitro assessment of psoralen and resveratrol co-loaded ultradeformable liposomes for the treatment of vitiligo. J Photochem Photobiol B. 2017;174:44–57. doi:10.1016/j.jphotobiol.2017.07.007
62. Jiradej M, Narinthorn K, Friedrich GT, et al. Potent melanin production enhancement of human tyrosinase gene by Tat and an entrapment in elastic cationic niosomes: potential application in vitiligo gene therapy. Chem Biol Drug Des. 2015;80(6):953–960.
63. Jiradej M, Narinthorn K, Worapaka M, et al. Enhancement of transdermal absorption, gene expression and stability of tyrosinase plasmid (pMEL34)-loaded elastic cationic niosomes: potential application in vitiligo treatment. J Pharm Sci. 2010;99(8):3533–3541. doi:10.1002/jps.22104
64. Patel HK, Barot BS, Parejiya PB, et al. Topical delivery of clobetasol propionate loaded microemulsion based gel for effective treatment of vitiligo – part II: rheological characterization and in vivo assessment through dermatopharmacokinetic and pilot clinical studies. Colloids Surf B Biointerfaces. 2014;119(1):145–153.
65. Patel HK, Barot BS, Parejiya P. Topical delivery of clobetasol propionate loaded microemulsion based gel for effective treatment of vitiligo: ex vivo permeation and skin irritation studies. Colloids Surf B Biointerfaces. 2013;102(102):86–94. doi:10.1016/j.colsurfb.2012.08.011
66. Garg BJ, Garg NK, Beg S, Singh B, Katare OP. Nanosized ethosomes-based hydrogel formulations of methoxsalen for enhanced topical delivery against vitiligo: formulation optimization, in vitro evaluation and preclinical assessment. J Drug Target. 2016;24(3):233–246. doi:10.3109/1061186X.2015.1070855
67. Tsuji G, Hashimoto-Hachiya A, Takemura M, et al. Palladium and platinum nanoparticles activate AHR and NRF2 in human keratinocytes-implications in vitiligo therapy. J Invest Dermatol. 2017;137(5):S0022202X17312198. doi:10.1016/j.jid.2017.02.981
68. Huang Y, Li Y, Hu Z, et al. Mimicking melanosomes: polydopamine nanoparticles as artificial microparasols. ACS Cent Sci. 2017;3(6):564–569. doi:10.1021/acscentsci.6b00230
69. Wang W, Lu KJ, Yu CH, et al. Nano-drug delivery systems in wound treatment and skin regeneration. J Nanobiotechnology. 2019;17(1):82. doi:10.1186/s12951-019-0514-y
70. Bouwstra JA, Hofland HEJ, Spies F, et al. Changes in the structure of the human stratum corneum induced by liposomes. In Braun-Falco O, Korting HC, Maibach HI, editors. Liposome Dermatics. Griesbach Conference. Berlin, Heidelberg: Springer; 1992.
71. Zhong-Kai C, Anne B, Nicolas C, et al. Formation of pH-sensitive cationic liposomes from a binary mixture of monoalkylated primary amine and cholesterol. Langmuir. 2012;28(38):13668–13674. doi:10.1021/la302278q
72. Pathak MA, Fitzpatrick T. The evolution of photochemotherapy with psoralens and UVA (PUVA): 2000 BC to 1992 AD. J Photochem Photobiol B. 1992;14(1–2):3–22. doi:10.1016/1011-1344(92)85080-E
73. Wu X, Hu X, Hamblin M. Ultraviolet blood irradiation: is it time to remember “the cure that time forgot”? J Photochem Photobiol B. 2016;157:89–96. doi:10.1016/j.jphotobiol.2016.02.007
74. El Maghraby GM, Williams AC, Barry BW. Oestradiol skin delivery from ultradeformable liposomes: refinement of surfactant concentration. Int J Pharm. 2000;196(1):63–74. doi:10.1016/S0378-5173(99)00441-X
75. Vidolin AP, Aurizi C, Leone G. Phototherapy for vitiligo, what’s new? G Ital Dermatol Venereol. 2017;152(5):474–488. doi:10.23736/S0392-0488.17.05721-2
76. Mir-Palomo S, Nácher A, Díez-Sales O, et al. Inhibition of skin inflammation by baicalin ultradeformable vesicles. Int J Pharm. 2016;511(1):23–29. doi:10.1016/j.ijpharm.2016.06.136
77. Manconi M, Manca ML, Caddeo C, et al. Nanodesign of new self-assembling core-shell gellan-transfersomes loading baicalin and in vivo evaluation of repair response in skin. Nanomedicine. 2017;14(2):569. doi:10.1016/j.nano.2017.12.001
78. Manconi M, Manca ML, Caddeo C, et al. Preparation of gellan-cholesterol nanohydrogels embedding baicalin and evaluation of their wound healing activity. Eur J Pharmaceutics Biopharmaceutics. 2018;127:S0939641117315382. doi:10.1016/j.ejpb.2018.02.015
79. Elsayed MM, Abdallah OY, Naggar VF, Khalafallah NM. Deformable liposomes and ethosomes as carriers for skin delivery of ketotifen. Pharmazie die. 2007;62(2):133–137.
80. Santos P, Watkinson AC, Hadgraft J, Lane M. Application of microemulsions in dermal and transdermal drug delivery. Skin Pharmacol Physiol. 2008;21(5):246. doi:10.1159/000140228
81. Barot BS, Patel HK, Gohel MC, et al. Microemulsion-based gel of terbinafine for the treatment of onychomycosis: optimization of formulation using d-optimal design. AAPS PharmSciTech. 2012;13(1):184–192. doi:10.1208/s12249-011-9742-7
82. Huabing C, Xueling C, Danrong D, Jin L, Huibi X, Xiangliang Y. Microemulsion-based hydrogel formulation of ibuprofen for topical delivery. Int J Pharm. 2009;315(1):52–58.
83. Zhao X, Liu JP, Zhang X, et al. Enhancement of transdermal delivery of theophylline using microemulsion vehicle. Int J Pharmaceutics. 2006;327(1):58–64. doi:10.1016/j.ijpharm.2006.07.027
84. Hathout RM, Woodman TJ, Mansour S, et al. Microemulsion formulations for the transdermal delivery of testosterone. Eur J Pharm Sci. 2010;40(3):188–196. doi:10.1016/j.ejps.2010.03.008
85. Patel HK, Barot BS, Parejiya PB, et al. Topical delivery of clobetasol propionate loaded microemulsion based gel for effective treatment of vitiligo–part II: rheological characterization and in vivo assessment through dermatopharmacokinetic and pilot clinical studies. Colloids Surf B Biointerfaces. 2014;119(1):145–153. doi:10.1016/j.colsurfb.2014.02.005
86. Valenzuela P, Simon JAJN. Nanoparticle delivery for transdermal HRT. Nanomedicine. 2012;8(1):S83–S89. doi:10.1016/j.nano.2012.05.008
87. Jain S, Jain P, Umamaheshwari RB, Jain NK. Transfersomes–a novel vesicular carrier for enhanced transdermal delivery: development, characterization, and performance evaluation. Sci Pharm. 2003;29(9):1013–1026.
88. Yoko Y, Ayumi H, Qing-Li Z, et al. Protective effects of platinum nanoparticles against UV-light-induced epidermal inflammation. Small. 2010;19(11):1000–1006.
89. Shibuya S, Ozawa Y, Yokote K, et al. 209–palladium and platinum nanoparticles attenuate aging-like skin atrophy via antioxidant activity. PLoS One. 2014;76(10):S90–S90.
90. Kobayashi H, Kumagai K, Eguchi T, et al. Characterization of T cell receptors of th1 cells infiltrating inflamed skin of a novel murine model of palladium-induced metal allergy. PLoS One. 2013;8(10):e76385. doi:10.1371/journal.pone.0076385
91. Sun L, Liu Z, Cun D, HY Tong, Zheng Y. Application of nano- and micro-particles on the topical therapy of skin-related immune disorders. Curr Pharm Des. 2015;21(19):2643–2667.
92. Ashtikar M, Nagarsekar K, Fahr A. Transdermal delivery from liposomal formulations - Evolution of the technology over the last three decades. J Controlled Release. 2016;242:126. doi:10.1016/j.jconrel.2016.09.008
93. Dragicevic-Curic N, Scheglmann D, Albrecht V, Fahr A. Temoporfin-loaded invasomes: development, characterization and in vitro skin penetration studies. J Controlled Release. 2008;127(1):59–69. doi:10.1016/j.jconrel.2007.12.013
94. Dwivedi M, Sharma V, Pathak K. Pilosebaceous targeting by isotretenoin loaded invasomal gel for the treatment of eosinophilic pustular folliculitis: optimization, efficacy and cellular analysis. Drug Dev Ind Pharm. 2016;43(2):293–304. doi:10.1080/03639045.2016.1239628
95. Chen M, Liu X, Fahr A. Skin delivery of ferulic acid from different vesicular systems. J Biomed Nanotechnol. 2010;6(5):577–585. doi:10.1166/jbn.2010.1154
96. Touitou E, Dayan N, Bergelson L, et al. Ethosomes — novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Controlled Release. 2000;65(3):403–418. doi:10.1016/S0168-3659(99)00222-9
97. Cevc G. Preparation for drug application in minute droplet form. 1996.
98. Ma G, Wu C. Microneedle, bio-microneedle and bio-inspired microneedle: a review. J Controlled Release. 2017;251(Complete):11–23. doi:10.1016/j.jconrel.2017.02.011
99. Cai X, Yang X, Wang F, et al. Multifunctional pH-responsive folate receptor mediated polymer nanoparticles for drug delivery. J Biomed Nanotechnol. 2016;12(7):1453. doi:10.1166/jbn.2016.2287
100. Lim HJ, Kim JK, Park J, et al. Complexation of apoptotic genes with polyethyleneimine (PEI)-Coated Poly-(DL)-Lactic-Co-Glycolic acid nanoparticles for cancer cell apoptosis. J Biomed Nanotechnol. 2015;11(2):211. doi:10.1166/jbn.2015.1880
101. Ribeiro N, Costa-Pinheiro P, Henrique R, et al. Comprehensive analysis of secreted protein, acidic and rich in cysteine in prostate carcinogenesis: development of a 3D nanostructured bone-like model. J Biomed Nanotechnol. 2016;12(8):1667. doi:10.1166/jbn.2016.2276
102. Liu X, Li X, Zhang N, et al. Bioengineering strategies for the treatment of type i diabetes. J Biomed Nanotechnol. 2016;12(4):581. doi:10.1166/jbn.2016.2176
103. Yu H, Tang Z, Li M, et al. Cisplatin Loaded Poly (L-glutamic acid)-g-Methoxy Poly(ethylene glycol) complex nanoparticles for potential cancer therapy: preparation, in vitro and in vivo evaluation. J Biomed Nanotechnol. 2016;12(1):69. doi:10.1166/jbn.2016.2152
104. Votavova P, Tomala J, Subr V, et al. Novel IL-2-Poly (HPMA)nanoconjugate based immunotherapy. J Biomed Nanotechnol. 2015;11(9):1662. doi:10.1166/jbn.2015.2114
105. Hu B, Liu X, Zhang C, et al. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. J Food Drug Anal. 2017;25(1):3. doi:10.1016/j.jfda.2016.11.004
106. Kim CS, Ahn YC, Wilder P, Oh S, Chen Z, Kwon YJ. Efficient and facile delivery of gold nanoparticles in vivo using dissolvable microneedles for contrast-enhanced optical coherence tomography. Biomed Opt Express. 2010;1(1):106–113. doi:10.1364/BOE.1.000106
107. Gratieri T, Kalia Y. Mathematical models to describe iontophoretic transport in vitro and in vivo and the effect of current application on the skin barrier. Adv Drug Deliv Rev. 2013;65(2):315–329. doi:10.1016/j.addr.2012.04.012
108. Byrne JD, Yeh JJ, JM D. Use of iontophoresis for the treatment of cancer. J Controlled Release. 2018;284:144–151. doi:10.1016/j.jconrel.2018.06.020
109. Vutla NB, Betageri GV, Banga AK. Transdermal iontophoretic delivery of enkephalin formulated in liposomes. J Pharm Sci. 1996;85(1):5–8. doi:10.1021/js950349y
110. Hama S, Kimura Y, Mikami A, et al. Electric stimulus opens intercellular spaces in skin. J Biol Chem. 2014;289(4):2450–2456. doi:10.1074/jbc.M113.514414
111. Wolf Horrell Erin M, Boulanger Mary C, D’Orazio John A. Melanocortin 1 receptor: structure, function, and regulation. Front Genet. 2016;7:95.
112. Lahav R. Endothelin receptor B is required for the expansion of melanocyte precursors and malignant melanoma. Int J Dev Biol. 2005;49(2–3):173–180. doi:10.1016/j.colsurfb.2018.12.055
113. Hirobe T, Shinpo T, Higuchi K, et al. Life cycle of human melanocytes is regulated by endothelin-1 and stem cell factor in synergy with cyclic AMP and basic fibroblast growth factor. J Dermatol Sci. 2010;57(2):123–131. doi:10.1016/j.jdermsci.2009.11.006
114. Boissy RE. Melanosome transfer to and translocation in the keratinocyte. Exp Dermatol. 2003;12(s2):5–12. doi:10.1034/j.1600-0625.12.s2.1.x
115. Nystedt S, Emilsson K, Larsson AK, et al. Molecular cloning and functional expression of the gene encoding the human proteinase‐activated receptor 2. Eur J Biochem. 1995;232(1). doi:10.1111/j.1432-1033.1995.tb20784.x.
116. Bohm SK, Kong W, Bromme D, et al. Molecular cloning, expression and potential functions of the human proteinase-activated receptor-2. Biochem J. 1996;314(3):1009–1016. doi:10.1042/bj3141009
117. Jung H, Oh ES. FK506 positively regulates the migratory potential of melanocyte-derived cells by enhancing syndecan-2 expression. Pigment Cell Melanoma Res. 2016;29(4):434–443. doi:10.1111/pcmr.12480
118. Jung H, Chung H, Chang SE, et al. FK506 regulates pigmentation by maturing the melanosome and facilitating their transfer to keratinocytes. Pigment Cell Melanoma Res. 2016;29(2):199–209. doi:10.1111/pcmr.12443
119. Wijnen RMH, Ericzon BG, Tiebosch ATMG, et al. Toxicology of FK506 in the cynomolgus monkey: a clinical, biochemical, and histopathological study. Transpl Int. 1992;5(Suppl 1):S454–S458. doi:10.1111/tri.1992.5.s1.454
120. Tzu JH, Utine CA, Stern ME, et al. Topical calcineurin inhibitors in the treatment of steroid-dependent atopic keratoconjunctivitis. Cornea. 2012;31(6):649–654. doi:10.1097/ICO.0b013e31822481c2
121. Pople PV, Singh KK. Development and evaluation of colloidal modified nanolipid carrier: application to topical delivery of tacrolimus. Eur J Pharm Biopharm. 2013;79(1):0–94.
122. Eberle AN. Proopiomelanocortin and the Melanocortin Peptides. Humana Press; 2000.
123. Semenza G. The melanotropins: chemistry, physiology and mechanisms of action. FEBS Lett. 1989;242:449–450. doi:10.1016/0014-5793(89)80523-X
124. Lipton J. Anti-inflammatory actions of the neuroimmunomodulator alpha-MSH. Immunol Today. 1997;18:140–145. doi:10.1016/S0167-5699(97)01009-8
125. Yamaguchi Y, Hearing VJ. Physiological factors that regulate skin pigmentation. Biofactors. 2009;35(2):193–199. doi:10.1002/biof.29
126. Parsad D, Dogra S, Kanwar A. Quality of life in patients with vitiligo. Health Qual Life Outcomes. 2003;1(1):58. doi:10.1186/1477-7525-1-58
127. Taïeb A. Vitiligo as an inflammatory skin disorder: a therapeutic perspective. Pigment Cell Melanoma Res. 2011;25(1):9–13. doi:10.1111/j.1755-148X.2011.00939.x
128. Seow WY, Salgado G, Lane EB, Hauser CA. Transparent crosslinked ultrashort peptide hydrogel dressing with high shape-fidelity accelerates healing of full-thickness excision wounds. Sci Rep. 2016;6:32670. doi:10.1038/srep32670
129. Chan KH, Xue B, Robinson RC, et al. Systematic moiety variations of ultrashort peptides produce profound effects on self-assembly, nanostructure formation, hydrogelation, and phase transition. Sci Rep. 2017;7(1):12897. doi:10.1038/s41598-017-12694-9
130. Kiat HC, Wei HL, Ming N, et al. C-terminal residue of ultrashort peptides impacts on molecular self-assembly, hydrogelation, and interaction with small-molecule drugs. Sci Rep. 2018;8:1–14.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
