Back to Journals » Clinical Ophthalmology » Volume 15
Real-World Study on Patient Satisfaction and Tolerability After Switching to Preservative-Free Latanoprost
Authors Erb C, Stalmans I, Iliev M, Muñoz-Negrete FJ
Received 4 December 2020
Accepted for publication 12 February 2021
Published 2 March 2021 Volume 2021:15 Pages 931—938
DOI https://doi.org/10.2147/OPTH.S295821
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Carl Erb,1 Ingeborg Stalmans,2 Milko Iliev,3 Francisco José Muñoz-Negrete4
1Eye Clinic Wittenbergplatz, Berlin, Germany; 2Department of Ophthalmology, University Hospitals Leuven, Leuven, Belgium; 3Ophthalmology Department, University of Bern, Inselspital, Bern, Switzerland; 4Servicio de Oftalmología, Hospital Universitario Ramón y Cajal, Universidad de Alcalá, IRYCIS, Madrid, España
Correspondence: Francisco José Muñoz-Negrete
Servicio de Oftalmología, Hospital Universitario Ramón y Cajal, Universidad de Alcalá, IRYCIS, Madrid, España
Tel +34 913 36 80 00
Email [email protected]
Purpose: Patient satisfaction is important in the treatment of glaucoma. Suboptimal compliance and impaired long-term outcome are a likely result of poor tolerability. The present multicentre, international, transverse, epidemiological survey was conducted to assess the satisfaction of patients who had received preservative-free latanoprost (PFL) for at least 3 months.
Patients and Methods: A total of 1872 patients from 6 European countries, treated with PFL for at least 3 months, were included in this survey. Prior to PFL treatment, patients were to be treatment naïve or currently treated for their glaucoma. During a single routine consultation, patients completed a questionnaire concerning global satisfaction and satisfaction based on tolerability.
Results: In total, 76.2% had been previously treated; 69.4% had received preserved and 6.8% preservative-free (PF) topical treatment. After 3 months of PFL treatment, a large majority of patients (95.3%) were satisfied or very satisfied with their PFL treatment and were, overall, significantly (p< 0.0001) more satisfied with PFL than with their previous treatment; 4.2% were either unsatisfied or very unsatisfied. Overall, 97.3% of originally treatment-naïve patients were satisfied (50.1%) or very satisfied (47.2%) with their PFL. Ocular surface disease was diagnosed in 9.2% of patients (n=173) and was mainly mild (76.9%). Patient satisfaction with PFL was very high.
Conclusion: PFL may be considered a valuable first-choice treatment in glaucoma patients.
Keywords: glaucoma, prostaglandin, preservative-free latanoprost, patient satisfaction, tolerability, tear substitutes, intra-ocular pressure, ocular surface disease, conjunctival hyperaemia
Introduction
Glaucoma is a chronic disorder that requires long-term treatment. In common with other insidious diseases such as hypertension or type-2 diabetes, obtaining patient compliance and long-term adherence with treatment is a key factor in achieving a good clinical outcome.
Whilst efficacy is a rewarding goal for both the patient and physician, many factors can impact the patient’s compliance and treatment regime. Among these factors, which include convenience, comfort and ease of use, the tolerability of the medication is a main issue.1 According to the European Glaucoma Society “A patient who complains about side effects is usually not adherent to therapy”.2 The basic problem with glaucoma patients is that in most cases they do not perceive visual disturbances and, therefore, do not feel impaired in their daily life. However, glaucoma therapy leads to far-reaching changes on the ocular surface, which not only leads to inflammatory changes on the cellular level, but also subjectively cause discomfort for the patients, limiting their quality of life.3–7 Ocular surface toxicity is frequently associated with not only the active ingredient in the eye drops, but also with the preservative contained in the formulation, which prevents bacterial growth.8
Topically applied latanoprost has become the first line treatment for glaucoma and ocular hypertension.9 Not only is it effective and well tolerated, but patients adhere to their treatment significantly more easily with latanoprost than with bimatoprost, travoprost or timolol.10–13 The advent of a patented topical latanoprost formulation without preservative offered a higher tolerability of prostaglandin glaucoma medication, with relatively fewer symptoms of ocular surface disease that could compromise adherence to treatment.14–19
Given the importance of long-term adherence to treatment in glaucoma and the impact of tolerability upon it, surprisingly little attention has been paid to the patient’s experience of topical preservative-free (PF) glaucoma medications. The present study was conceived to determine the degree of satisfaction among treatment-naïve patients or those who had recently switched to PF latanoprost in single-use dose units (PFL, Monoprost®, Laboratoires Théa, France)
Methods
The study was conducted between 2013 and 2015, according to the principles of Good Epidemiological Practices.20 Approvals were obtained from local or National Ethics Committees in Berlin (Charité’s Ethics committee, Berlin, Germany), Bern (Inselspital Bern, Switzerland), Leuven (Université catholique Leuven, Belgium) and from the National Data Protection Committee of Spain (CNPD) Madrid, Spain,) according to the regulations at the investigational sites. According to local regulations, no ethics committee approvals were obtained for the Netherlands and Portugal. All patients provided written informed consent before participating in the study.
Eligible adult subjects had to have a documented diagnosis of glaucoma or ocular hypertension and had been treated with PFL for at least 3 months at the time of the study visit or could have been treatment-naive.
The study was a multicentre, transverse, epidemiological survey, conducted during a single consultation with a routine clinical examination. It was performed in 337 private ophthalmological practices. Ophthalmologists were chosen from national databases on the basis of feasibility as well as geographical and national balance.
Ophthalmologists were asked to consecutively recruit 10 patients (5 in Switzerland) who had received PFL for glaucoma or ocular hypertension for at least 3 months at the time of the study visit.
Gender, age, ophthalmological and other medical history, date of glaucoma diagnosis or ocular hypertension, type and stage of glaucoma based on visual field damage were recorded, along with the patient’s intraocular pressure (IOP). The investigator also recorded the history of the patients’ previous glaucoma therapy (if applicable) and the reason for switching to PF latanoprost. Intraocular pressure, presence of ocular surface disease (OSD) and Tear-film Break-Up-Time (TBUT, classified into three groups (>10sec, 5 to 10 sec and <5 sec) were assessed.
For patients who used tear substitutes, the investigator documented whether such use had increased, decreased or remained unchanged after the switch to PFL and whether the patient was using eye drops containing preservatives or not. Moreover, the ophthalmologists assessed ocular signs such as redness, lid swelling, lid scale or crusts, conjunctival hyperaemia, chemosis, positive corneal and conjunctival fluorescein staining on a 4-point ordinal scale from 0 (absent) to 3 (severe). In addition, the investigators were asked to answer the question: “Regarding tolerability, is the patient satisfied with his/her preservative-free latanoprost treatment?” (Very satisfied, satisfied, unsatisfied, very unsatisfied).
Figure 1 shows the questionnaire used to conduct the study. The patient’s subjective experience of tolerability to PFL was defined as the primary variable. Patients reported their subjective experience of the tolerability of their previous glaucoma treatment and of their current PFL on a Visual Analogue Scale (VAS, from 0 mm [very bad tolerance] to 100 mm [very good tolerance]). Moreover, they reported their opinion concerning the tolerability of PFL compared to their previous treatment (much better tolerated, better tolerated, identically tolerated, less well tolerated or much less well tolerated) and their experience of the ease of use of current PF latanoprost compared to their previous treatment (much easier to use/easier to use/same/less easy to use/much less easy to use).
 |
Figure 1 Questionnaire used during the study. |
Continuous variables were described in terms of numbers, mean, standard deviation (SD), median with minimum and maximum, as appropriate. Categorical variables were given as absolute frequency and percentage per category. Confidence intervals at 95% were given where applicable. A logistic regression analysis was used to identify parameters associated with patients’ satisfaction with their current PFL treatment. Odds ratios and p-values were determined, as appropriate. An ad-hoc analysis was performed on treatment-naïve patients who had received no previous treatment prior to their current PFL.
Results
Patient Disposition, Demographics and Disease Characteristics
A total of 1872 patients were recruited (Spain, 1303; Germany, 213; Portugal, 168; Belgium 104; Switzerland 59; Netherlands, 25). Age was 66.8±12.11 years; mainly women participated (60.9%). Patients had mostly early glaucoma (41.5%) or ocular hypertension (29.9%); (Table 1).
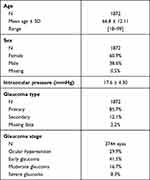 |
Table 1 Demographic and Baseline Disease Characteristics |
In total, 76.2% had been treated for their glaucoma prior to their current PFL use; 69.4% had received a preservative-containing treatment and 6.8% had received PF eye drops.
On average, patients who were previously treated switched treatment 2.4 ± 1.86 times; although some had experienced up to 20 treatment switches. The most common reason for switching to PFL was local intolerance (61.8%), followed by insufficient efficacy (49.1%). Insufficient compliance, systemic intolerance, patient request and other reasons were cited by fewer than 10%.
The mean exposure to PFL was 149.5 ± 83.9 days; mean IOP was 18.4 ± 4.87 mmHg (n=3744 eyes).
Patient Satisfaction
Overall, 95.3% of the patients were satisfied (55.2%) or very satisfied (40.1%) with PFL regarding tolerability. Only 3.5% were unsatisfied and 0.7% very unsatisfied; data were missing for 0.5%. The proportion of patients satisfied with PFL was similar among patients who had previously received preserved medication (95%) and among those who were treatment-naive (97.3%).
Patients were significantly (p<0.0001) more satisfied with the tolerability to PFL (VAS score 83.5 ± 16.5), than with that of their previous treatment (VAS score 57.7 ± 27.2). Among patients who had previously received a preservative-containing topical glaucoma therapy, tolerability scores on the VAS increased from 56.6 ± 27.2 to 82.6 ± 16.8, corresponding to an improvement of 45.9%. For treatment-naive patients, the mean tolerance to PFL, evaluated with VAS, was even higher (86.7 ± 13.9); refer to Figure 2 for details. According to the different types of previous preserved treatments, improvement of tolerability differed.
After having switched to PFL from the most current treatments, the individual improvement of VAS score was 82% from bimatoprost 0.3%, 70% from bimatoprost 0.1%, 63% from travoprost, 42% from preserved latanoprost, 35% from tafluprost and 38% frombeta-blockers.
Tolerability Compared to Previous Treatment
The proportion of patients who provided a positive assessment of tolerance to PFL was 75.2%, with 58.7% of patients in the preserved and preservative-free sub-groups. The proportion of patients who reported tolerance to PFL to be better, much better or the same as their previous treatment was therefore 95.8% for the preserved sub-group and 93.6% for the preservative-free sub-group. A lower proportion of patients in the preserved and preservative-free sub-groups considered tolerance to PFL to be less (2.7% and 4.8%, respectively) and much less (0.5% and 1.6%, respectively) than their previous treatments.
Ease of Use
In total, 48.8% of patients rated PFL to be as easy to use as their previous treatment in drop bottles, 29.4% rated it easier and 11.3% much easier to use. Only 9.3% of patients considered it to be less easy to use; for 1.2%, data were missing.
Ocular Surface Disease
OSD was diagnosed in 9.2% of patients (n=173) and was mainly mild (76.9%). Lid redness was the most common ocular sign among patients with OSD. Lid redness, lid swelling, lid scale or crusts and chemosis showed no statistically significant difference between patients with preserved therapy and patients with PFL (Figure 3).
TBUT, Conjunctival Hyperaemia and Fluorescein Staining
TBUT was performed for 1649 eyes. It was inferior to 5 seconds for 10.8% and inferior to 10 seconds for 52.3% of the eyes.
Even though the fluorescein staining of the cornea and conjunctiva as well as the conjunctival hyperaemia showed no statistical difference between the individual therapy groups, the ocular status was slightly better in treatment naïve patients compared to that of patients who had previously been treated (Figures 4 and 5).
Use of Tear Substitutes
Overall, 45.4% of patients used tear substitutes concomitantly. This use decreased for 24.1% of these patients after they had switched to PFL.
Among patients who had previously received a preserved therapy, 28.1% reported reduced tear substitute use following the switch to PFL. A total of 30% of treatment-naïve patients used tear substitutes concomitantly with PFL.
Association of Study Parameters with Patient Satisfaction
Patient satisfaction with PFL was significantly associated with improved tolerance after use (p<0.0001), tolerability regarding the current medication (p<0.0001), ease-of-use (p<0.0001), reduction of tear substitute use after having switched to PFL (p<0.0001), absence of ocular symptoms (p<0.0004), absence of OSD (p<0.0001), use of prior treatment (p<0.05) and use of tear substitutes (p<0.02).
Discussion
Glaucoma treatment is life-long and, as with all long-term prophylactic treatments, compliance is a major issue.3,8 Preservatives in topical glaucoma medication have well-described toxic effects which trigger OSD and impact treatment compliance.5,8,21–23 A study showed that OSD are common among glaucoma patients receiving topical therapy and that it is both more common and more severe in older patients and in those receiving multiple treatments. Moreover, the severity of OSD has been associated with low effective control of IOP.24
The majority of patients who entered the present study had switched several times from preserved monotherapy. However, the relatively high number of included treatment-naive patients allowed for an ad-hoc subgroup analysis.
Surprisingly, 6.8% of patients who switched to PFL, previously used preservative-free drops. This change may be due to tolerability issues as reported by Duru et al for preservative-free brimonidine tartrate.25 However, study results are based on a very small sample size and a comparative study may help to shade some light on the observed phenomenon.
Regarding tolerability, patients were more satisfied with PFL than with their previous preserved treatment (45.9% improvement on average on the VAS); 75.2% of patients considered the tolerability of PFL as better or much better than their previous preserved treatment, although it was changed from drop bottles to PFL.
Overall, 42% assessed the PFL as easier or much easier to use than their previous treatment. Furthermore, the use of PFL led to a lower percentage of patients using tear substitutes in the subgroup of treatment-naïve patients, and a decreased use in a quarter of patients after switching from a previous treatment. Artificial tears are currently used to treat dry eye, known to be caused by preservatives such as benzalkonium chloride.26 Eliminating preservatives in glaucoma treatments may, therefore, reduce the use of artificial tears.
Not surprisingly, the most common reason for switching to PFL was local intolerance, a well-known issue in the context of the local toxicity of preserved treatments previously used by a large majority of the included patients. The second most common reason was insufficient efficacy, which could be potentially explained by poor treatment adherence, due to local tolerability issues.
More than 97% of treatment-naïve patients were satisfied with PFL. OSD was reported in less than 7% of patients and, when it occurred, severity was generally mild. Conjunctival hyperaemia is a recognized adverse event of topical prostaglandin analogues, although it appears to be a less frequent problem with latanoprost than with other prostaglandin analogues, and its incidence remained relatively high after the switch (58.7%).27,28 The incidence of conjunctival hyperaemia was relatively low among patients in this study and was rarely severe.
Despite these encouraging results, the study has several limitations and bias:1. This was not a comparative, randomised study making a direct comparison between preserved drops and PFL impossible.2. The low number of patients in the subgroups previously treated by PF glaucoma treatment (126 patients), representing only 6.8% of the overall study population compared to other sub-groups requires further investigations in order to support the benefits of PFL in glaucoma for all different patient profiles. Additional randomised studies comparing the prevalence in the preserved and PF groups or switching treatments are needed, in order to provide unequivocal evidence of an improvement of signs/symptoms and the long-term benefit of PF treatments.3. Investigators asked their patients to estimate their treatment satisfaction without asking for the reason for dissatisfaction. This may cause a bias, which may potentially lead to an overestimation of patient satisfaction, as patients might not have wanted to contradict their ophthalmologist and, thereby, tended to minimize potential symptoms, which limits the understanding of the reasons for dissatisfaction.4. A logistic regression was used to determine whether patient satisfaction was related to other variables. Each variable was analysed in a separate logistic regression model as a predictor for being satisfied with PFL. With satisfaction potentially being multifactorial, the analysis was not performed with multiple comparison methods or used for adjusting the overall α-level. The poor tolerability of glaucoma medication may lead to poor treatment compliance and, therefore, to treatment failure. Patients’ evaluation of their tolerance to glaucoma treatment is key to assessing treatment efficacy. Moreover, the usability of the dropper device is worthy of consideration; a study suggests that the force required to expel a drop from some devices is beyond the physical capability of many patients.28
The study shows that preservative-free latanoprost may be a valuable choice of therapy when switching patients from preserved treatment due to tolerability issues, and may be considered as first choice in newly diagnosed glaucoma patients.29
In conclusion, after at least 3 months of treatment with PF latanoprost, patient satisfaction was very high and potentially led to a reduced use of tear substitutes.
Acknowledgments
The authors acknowledge the participation of the PASSY study team and of their patients and the writing assistance of Karl Patrick Göritz, SMWS, France.
Funding
The study was funded by Laboratoires Théa, Clermont-Ferrand, France.
Disclosure
Prof. Carl Erb received consultancies from Aerie, Alcon, Allergan, Bayer, Bausch&Lomb, Thea, Santen, OmniVision, Ophtaprotect, Oculus, Glaukos and Zeiss. Prof. Ingeborg Stalmans received consultancies and research grants from Aerie, Alcon, Allergan, EyeD Pharma, EyeTechCare, Horus Pharma, Oculis, Santen, Laboratoires Théa, and Zeiss. Prof. Milko Iliev received consultancies and research grants from Thea, Santen, and Allergan. Prof. Francisco José Muñoz-Negrete received consultancies and research grants from Alcon, Avizor, Bayer, Esteve, Laboratoires Théa, and Santen. The authors report no other conflicts of interest in this work.
References
1. Vélez-Gómez MC, Vásquez-Trespalacios EM. Adherence to topical treatment of glaucoma, risk and protective factors: a review. Arch Soc Esp Oftalmol. 2018;93(2):87–92. doi:10.1016/j.oftal.2017.07.012
2. Molina-Castañer S. Terminology and guidelines for glaucoma. Arch Soc Esp Oftalmol. 2009;84(10):543–544.
3. Lemij HG, Hoevenaars JG, van der Windt C, Baudouin C. Patient satisfaction with glaucoma therapy: reality or myth? Clin Ophthalmol. 2015;9:785–793. doi:10.2147/OPTH.S78918
4. Yeaw J, Benner JS, Walt JG, Sian S, Smith DB. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm. 2009;15(9):728–740. doi:10.18553/jmcp.2009.15.9.728
5. Baudouin C, Liang H, Hamard P, et al. The ocular surface of glaucoma patients treated over the long term expresses inflammatory markers related to both T-helper 1 and T-helper 2 pathways. Ophthalmology. 2008;115(1):109–115. doi:10.1016/j.ophtha.2007.01.036
6. Kestelyn PA, Kestelyn PG, De Bacquer D, Stevens AM. Switch from BAK-preserved to preservative-free latanoprost decreases anterior chamber flare in POAG patients. Int Ophthalmol. 2019;39(1):105–109. doi:10.1007/s10792-017-0792-z
7. Inoue D, Mohamed YH, Uematsu M, Kitaoka T. Corneal damage and its recovery after instillation of preservative-free versus preserved latanoprost eye drops. Cutan Ocul Toxicol. 2020;39(2):158–164. doi:10.1080/15569527.2020.1752228
8. Baudouin C, Labbe A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29(4):312–334. doi:10.1016/j.preteyeres.2010.03.001
9. European glaucoma society terminology and guidelines for glaucoma, 4th edition - chapter 3: treatment principles and options supported by the EGS foundation: part 1: foreword; introduction; glossary; chapter 3 treatment principles and options. Br J Ophthalmol. 2017;101(6):130–195.
10. Alm A. Latanoprost in the treatment of glaucoma. Clin Ophthalmol. 2014;8:1967–1985. doi:10.2147/OPTH.S59162
11. Arias A, Schargel K, Ussa F, Canut MI, Robles AY, Sanchez BM. Patient persistence with first-line antiglaucomatous monotherapy. Clin Ophthalmol. 2010;4:261–267. doi:10.2147/opth.s7971
12. Rouland JF, Traverso CE, Stalmans I, et al. Efficacy and safety of preservative-free latanoprost eyedrops, compared with BAK-preserved latanoprost in patients with ocular hypertension or glaucoma. Br J Ophthalmol. 2013;97(2):196–200. doi:10.1136/bjophthalmol-2012-302121
13. Heo JH, Rascati KL, Wilson JP, Lawson KA, Richards KM, Nair R. Comparison of prostaglandin analog treatment patterns in glaucoma and ocular hypertension. J Manag Care Spec Pharm. 2019;25(9):1001–1010. doi:10.18553/jmcp.2019.25.9.1001
14. Cucherat M, Stalmans I, Rouland JF. Relative efficacy and safety of preservative-free latanoprost (T2345) for the treatment of open-angle glaucoma and ocular hypertension: an adjusted indirect comparison meta-analysis of randomized clinical trials. J Glaucoma. 2014;23(1):e69–e75. doi:10.1097/IJG.0b013e3182a075e6
15. Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002;86(4):418–423. doi:10.1136/bjo.86.4.418
16. Jaenen N, Baudouin C, Pouliquen P, Manni G, Figueiredo A, Zeyen T. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol. 2007;17(3):341–349. doi:10.1177/112067210701700311
17. Thygesen J. Glaucoma therapy: preservative-free for all? Clin Ophthalmol. 2018;12:707–717. doi:10.2147/OPTH.S150816
18. Misiuk-Hojlo M, Pomorska M, Mulak M, et al. The RELIEF study: tolerability and efficacy of preservative-free latanoprost in the treatment of glaucoma or ocular hypertension. Eur J Ophthalmol. 2019;29(2):210–215. doi:10.1177/1120672118785280
19. Munoz Negrete FJ, Lemij HG, Erb C. Switching to preservative-free latanoprost: impact on tolerability and patient satisfaction. Clin Ophthalmol. 2017;11:557–566. doi:10.2147/OPTH.S126042
20. IEA. International Epidemiological Association: Good Epidemiological Practice (GEP), IEA guidelines for proper conduct in epidemiological research. November, 2007 Available from: http://ieaweb.org/good-epidemiological-practice-gep/.
21. Hakkarainen JJ, Reinisalo M, Ragauskas S, Seppänen A, Kaja S, Kalesnykas G. Acute cytotoxic effects of marketed ophthalmic formulations on human corneal epithelial cells. Int J Pharm. 2016;511(1):73–78. doi:10.1016/j.ijpharm.2016.06.135
22. Pérez-Bartolomé F, Martínez-de-la-casa JM, Arriola-Villalobos P, Fernández-Pérez C, Polo V, García-Feijoó J. Ocular surface disease in patients under topical treatment for glaucoma. Eur J Ophthalmol. 2017;27(6):694–704. doi:10.5301/ejo.5000977
23. Martinez-de-la-casa JM, Perez-Bartolome F, Urcelay E, et al. Tear cytokine profile of glaucoma patients treated with preservative-free or preserved latanoprost. Ocul Surf. 2017;15(4):723–729. doi:10.1016/j.jtos.2017.03.004
24. Baudouin C, Renard JP, Nordmann JP, et al. Prevalence and risk factors for ocular surface disease among patients treated over the long term for glaucoma or ocular hypertension. Eur J Ophthalmol. 2013;23(1):47–54. doi:10.5301/ejo.5000181
25. Duru Z, Ozsaygili C. Preservative-free versus preserved brimonidine %0.15 preparations in the treatment of glaucoma and ocular hypertension: short term evaluation of efficacy, safety, and potential advantages. Cutan Ocul Toxicol. 2020;39(1):21–24. doi:10.1080/15569527.2019.1680685
26. Walsh K, Jones L. The use of preservatives in dry eye drops. Clin Ophthalmol. 2019;13:1409–1425. doi:10.2147/OPTH.S211611
27. Feldman RM. Conjunctival hyperemia and the use of topical prostaglandins in glaucoma and ocular hypertension. J Ocul Pharmacol Ther. 2003;19(1):23–35. doi:10.1089/108076803762718088
28. Honrubia F, Garcia-Sanchez J, Polo V, de la Casa JM, Soto J. Conjunctival hyperaemia with the use of latanoprost versus other prostaglandin analogues in patients with ocular hypertension or glaucoma: a meta-analysis of randomised clinical trials. Br J Ophthalmol. 2009;93(3):316–321. doi:10.1136/bjo.2007.135111
29. Economou MA, Laukeland HK, Grabska-Liberek I, Rouland JF. Better tolerance of preservative-free latanoprost compared to preserved glaucoma eye drops: the 12-month real-life FREE study. Clin Ophthalmol. 2018;12:2399–2407. doi:10.2147/OPTH.S176605
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




