Back to Journals » Clinical Ophthalmology » Volume 13
Real-world retrospective comparison of 0.19 mg fluocinolone acetonide and 0.7 mg dexamethasone intravitreal implants for the treatment of diabetic macular edema in vitrectomized eyes
Authors Coelho J , Malheiro L , Melo Beirão J , Meireles A , Pessoa B
Received 15 January 2019
Accepted for publication 25 June 2019
Published 9 September 2019 Volume 2019:13 Pages 1751—1759
DOI https://doi.org/10.2147/OPTH.S201611
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
João Coelho1, Luísa Malheiro1, João Melo Beirão1,2, Angelina Meireles1,2, Bernardete Pessoa1,2
1Unit of Ophthalmology, Centro Hospitalar Universitário Do Porto, Porto, Portugal; 2Unit of Ophthalmology, Universidade Do Porto-Instituto Ciências Biomédicas Abel Salazar, Porto, Portugal
Correspondence: João Coelho
Unit of Ophthalmology, Centro Hospitalar Universitário do Porto, Largo Prof. Abel Salazar, Porto 4099-001, Portugal
Tel +351 91 765 6826
Email [email protected]
Purpose: The aim of this study was to evaluate the long-term real-world effectiveness of FAc and DEX implants in vitrectomized DME eyes in a real-world setting.
Methods: This was a non-interventional, retrospective, comparative study of 46 vitrectomized eyes in 33 patients with persistent or recurrent DME quantified best-corrected visual acuity (BCVA), central foveal thickness (CFT) and intraocular pressure (IOP) over up to 37 months.
Results: Both FAc and DEX treatment led to statistically and clinically significant improvements in BCVA and CFT. FAc >10-letter improvement on the Early Treatment Diabetic Retinopathy Study [ETDRS] chart over months 3–24 and a sustained ∼200 μm CFT reduction over months 1–24; DEX: >5-letter improvement on the ETDRS chart at months 1 and 3 and >100 μm CFT reduction at month 1. FAc demonstrated sustained, stable and predictable effects on BCVA and CFT over 24 months and also improved BCVA and decreased CFT in a cohort of DME eyes that was refractory to DEX over 6 months.
Conclusion: This real-world study demonstrates long-term effectiveness of FAc in vitrectomized DME eyes and sustained effectiveness in DME eyes that did not respond to DEX therapy.
Keywords: diabetic macular edema, fluocinolone acetonide, dexamethasone implant, intravitreal implants, real-world, vitrectomy
Background
Diabetes is a growing global health challenge. It is estimated that 424.9 million adults were living with diabetes in 2017, and this number is expected to rise to 628.6 million by 2045.1
Approximately one-third of diabetic patients have signs of diabetic retinopathy, out of which one-third develop vision-threatening conditions such as diabetic macular edema (DME).2 DME presents in 14–25% of diabetics within 10 years of the initial diabetes diagnosis.3 Factors that contribute to the increasing prevalence of DME include an aging population, increasing prevalence of diabetes and longer life expectancy of patients with diabetes.4
Although there is currently no cure for DME, treatments that aim to halt or slow down disease progression are available, and include laser therapy, intravitreal anti-vascular endothelial growth factor (anti-VEGF) injections (ranibizumab, aflibercept and off-label bevacizumab) and intravitreal corticosteroid implants (dexamethasone [DEX], fluocinolone acetonide [FAc] and off-label injections of triamcinolone acetonide [TA] such as the I-vation intravitreal triamcinolone acetonide implant.5–8 Switching between different DME therapy types is common, and is often prompted by suboptimal efficacy, adverse events, or patient or physician preferences.9
Intravitreal corticosteroid implants that elute intravitreal DEX (700 µg) or FAc (190 µg) are effective for the treatment of DME, with reduced frequencies of injection and clinical appointments compared with anti-VEGF therapies.6,7,10–12 ILUVIEN® (FAc) implant is a non-biodegradable intravitreal implant that measures approximately 3.5 mm by 0.37 mm in size. It is injected into the vitreous using a 25-gauge injector and designed to release 0.2 µg/day of FAc over a 3-year period. In Europe it is indicated for the treatment of vision impairment associated with chronic DME considered insufficiently responsive to available therapies (that is, DME that persists or recurs despite treatment).13,14 The DEX implant is a biodegradable copolymer of polylactic-co-glycolic acid that measures approximately 6 mm by 0.46 mm. It is injected into the vitreous with a 22-gauge needle and releases DEX for up to 6 months.
The efficacy and safety of the FAc implant in DME was demonstrated in the FAME studies, which were conducted under a single protocol as randomized, double-masked, sham injection-controlled, parallel-group, multicenter studies. The main outcome, evaluated at month 36, was the percentage of patients with an improvement in best-corrected visual acuity (BCVA) of ≥15 letters, and results showed that a significantly higher percentage of patients achieved this after therapy with the FAc implant than with sham control (34% versus 13%; p<0.001).6
The biodegradable intravitreal implant Ozurdex® (DEX) is applied using a pre-loaded 22-gauge intravitreal injector system. It contains 0.7 mg preservative-free DEX and remains effective for up to 6 months and a recent systematic review of real-world DEX studies indicates the mean retreatment average time is shorter than this and occurs around 5 months.15 The implant is indicated for the treatment of adult patients with visual impairment due to DME who are pseudophakic or who are considered insufficiently responsive to, or unsuitable for, non-corticosteroid therapy. The effectiveness and safety of DEX in DME was demonstrated in the pivotal MEAD study.7
Intravitreal administration of corticosteroids reduces the risk of potential systemic side effects. The longer treatment intervals that are associated with DEX and FAc implants, compared with anti-VEGF therapies, also reduce treatment costs, increase patient compliance and lower the risk of endophthalmitis and traumatic cataract.16 These benefits have to be balanced against a higher risk of ocular hypertension and cataract, which are well-known and manageable undesired effects of both DEX and FAc implants.14
To the best of our knowledge, no study has so far directly compared the effectiveness and safety of the FAc and DEX implants for the treatment of DME. In the study presented here, we compared the real-world effectiveness and safety of FAc and DEX intravitreal implants in patients with DME who have previously undergone vitrectomy.
Methods
Aim
The primary aim of this real-world study was to investigate the changes over time in BCVA, central foveal thickness (CFT) and intraocular pressure (IOP) in DME patients treated with either the FAc or DEX intravitreal implant.
Study design
This was a non-interventional, retrospective, comparative analysis of 46 vitrectomized eyes in 33 patients with persistent or recurrent DME. The study was conducted at the Centro Hospitalar Universitario do Porto, a tertiary referral center in Oporto, Portugal. The clinical records of patients who had received prior treatment of DME with the FAc or DEX implant were retrieved.
Two patient groups were identified: group 1 included 29 eyes (26 patients) that had received an intravitreal injection of the 0.2 µg/day FAc implant, while group 2 included 17 eyes (14 patients) that had received an intravitreal injection of the 0.7 mg DEX implant. All patients provided informed consent for treatment, and the study protocol complies with the requirements of the institute’s committee on human research.
Study endpoints
Patient demographic data were recorded, as well as the following parameters: visual acuity which was recorded and converted to ETDRS letters; CFT; IOP; duration of DME; number of intravitreal injections received; and number of topical IOP-lowering medications received.
Inclusion and exclusion criteria
The inclusion criteria were based on prior FAc and/or DEX treatment in line with the European indications for each drug.
Statistical analysis
The statistical analysis involved measures of descriptive statistics (absolute and relative frequencies, averages and respective standard deviations [SD]) and inferential statistics. The level of significance to accept or reject the null hypothesis was set at (α) ≤0.05. Student’s t-test was used for independent samples, and Student’s t-test for paired samples was used when comparing quantitative variables between the baseline and each of the several moments of observation. The assumption of normal distribution was analysed with Shapiro–Wilk tests. When the assumptions were not satisfied, the Mann–Whitney test was used as an alternative to the Student t-test for independent samples and the Wilcoxon test as an alternative to the Student’s t-test for paired samples. The Chi-square test and the Fischer’s test were used to test the difference between the two proportions. Statistical analysis was performed with SPSS (Statistical Package for the Social Sciences) version 24.0 for Windows. Data are reported as mean ± SD unless stated otherwise.
Results
Patient demographics
The baseline demographics of the FAc (29 eyes in 26 patients) and DEX (17 eyes in 14 patients) implant groups are summarized in Table 1. The FAc and DEX implant groups were well matched, with no significant differences in participant age, duration of DME, mean number of previous anti-VEGF injections, BCVA, CFT or IOP (Table 1). The FAc and DEX implant groups differed significantly in mean follow-up time (16.9 vs 5.5 months), and both the percentage of patients receiving prior steroid injections and the mean number of prior steroid injections per se were higher in the FAc than the DEX implant group (Table 1). The refractory nature of the DME study eyes included in this study is reflected by the high treatment burden (prior anti-VEGF and steroid treatments) at baseline for both study groups (Table 1).
 |
Table 1 Baseline characteristics of the study participants in the FAc and DEX implant treatment groups |
Patient disposition
This real-world study was made up of five different patient cohorts (Figure 1) The “DEX cohort” consisted of 17 eyes (in 14 patients) that were initially treated with DEX. Fourteen of these eyes (in 13 patients) completed 6 months of follow-up (the “DEX 6-month cohort”). The “FAc cohort” included all 29 eyes (from 26 patients) that received at least one FAc implant with a minimum follow-up of 6 months. The “FAc 24 months FU cohort” was defined as FAc cohort eyes that completed 24 months of follow-up post FAc implantation (8 eyes in 8 patients). Subgroup analyses were performed to compare outcomes in the same patients. The “One DEX-to-FAc cohort” consisted of 5 eyes in 5 patients with at least 12 months of post-FAc implant follow-up that had been treated with a single DEX implant and then switched to an FAc implant.
DEX changes in BCVA and CFT over time
In the DEX cohort, 14 eyes completed six months of follow-up. (Figure 2A and B) Relative to the 40.6 ETDRS letter baseline, 6.5 letters were gained at month 1, 9.0 letters at month 3 and 2.8 letters at month 6. Relative to the 470.5 µm baseline, CFT decreased by 133.1 µm at month 1, 48 µm at month 3 and 14.7 µm at month 6.
Fac implant changes in BCVA and CFT over time
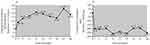
Eight eyes from 8 patients completed 24 months of follow-up (Figure 3A and B). Relative to the 31.5 ETDRS letter baseline, 8.1 letters were gained at month 1, 12.9 letters at month 3, 16.3 letters at month 6, 18.8 letters at month 12 and 18.0 letters at month 24. Relative to the 594.8 µm baseline, CFT decreased by 201.5 µm at month 1, 206.3 µm at month 3, 195.7 µm at month 6, 240.5 µm at month 12 and 257.0 µm at month 24.
In some cases, FAc and DEX implants were administered to the same patients. In this subgroup, patients were selected if they had received a single prior DEX implant and subsequently had 12 months of follow-up after being treated with a FAc implant. Five eyes in five patients were initially treated with a single DEX implant before switching to the FAc implant. Relative to the 38.0 ETDRS letter baseline, 12.5 letters were gained at month 1, and 5.0 and 1.3 letters were lost at months 3 and 6, respectively (Figure 4A). Relative to the 499.4 µm baseline, CFT decreased by 70.0 µm at month 1, 6.7 µm at month 3 and increased by 59.3 µm at month 6 (Figure 4B). After a single DEX treatment, the 5 DEX patients were switched to an FAc implant: Following the switch to the FAc implant, 12.6 letters were gained at month 1, 14.6 letters at month 3, 13.3 letters at month 6, 24.0 letters at month 9 and 13.0 letters at month 12 (Figure 4C). Similarly, CFT decreased by 173.0 µm at month 1, 138.4 µm at month 3, 23.3 µm at month 6, 107.7 µm at month 9 and 114.0 µm at month 12 following the switch from DEX to a FAc implant (Figure 4D).
Safety and supplemental therapies
In the “FAc 24 months FU cohort” (n=8) where IOP was 15.0±2.4 mmHg at baseline and a peak occurred (17.0±2.8 mmHg) between weeks 2 and 4 (Table 2). This was similar to the “DEX 6-month cohort” (n=13 of 14 with an IOP measurement at baseline) where IOP was 16.0±4.1 mmHg at baseline and reached a maximum of 19.2±5.4 mmHg between weeks 2 and 4 (Table 2).
 |
Table 2 Mean IOP pressures and treatments in the participants in the DEX (A) and FAc implant (B) treatment groups |
At their final study visit, one eye in the FAc implant treated group and one eye in the DEX implant treated group had IOP ≥21 mmHg, and five eyes in the FAc implant treated group and eight eyes in the DEX implant treated group were receiving IOP-lowering medication (Table 2). It is notable that a similar proportion of eyes in both groups were receiving IOP-lowering drops at baseline (Table 2).
In the FAc implant treated patients, none of the eyes required surgery to control IOP pressure and two eyes in the DEX implant group had a significant increase in IOP that required IOP-lowering treatment and subsequent surgical intervention.
Post-DEX implant administration, eight eyes from the “DEX 6-month cohort” were subsequently treated with a FAc implant after month 6 and none received a second DEX implant. In the FAc implant treated group, two eyes received intravitreal injections of anti-VEGF (bevacizumab and aflibercept in the first case and ranibizumab in the second case) which started at months 9 and 21, respectively. One patient was treated with panretinal photocoagulation at month 15.
Discussion
The aim of the study was to investigate the effects of FAc and DEX intravitreal implantation in in difficult-to-treat vitrectomized eyes with DME. Twenty-nine eyes were treated with a FAc implant and followed for up to 37 (range: 6–37) months; 17 eyes were treated with a DEX implant and followed for up to 6 (range: 3–6) months.
The baseline characteristics of the FAc and DEX implant treatment groups were similar, with the exception of mean follow-up time, mean number of prior steroid injections, the time since vitrectomy, and number of DEX intravitreal injections, which were all greater in the FAc implant treatment group.
Following a single FAc implantation, treatment resulted in a sustained increase in BCVA and sustained reduction in CFT over up to 24 months, with only transient increases in IOP. An adequate response from DEX implantation was initially observed (BCVA gains at months 1 and 3, and a CFT decrease at month 1), but this response was not sustained through to month 6, when both BCVA and CFT returned to close to baseline values. Furthermore, a subset of five DEX-treated eyes that responded inadequately to DEX implantation when followed over 6 months responded well to FAc implantation, with sustained improvements in BCVA and CFT over 12 months post-FAc implantation. It is important to note that the small number of study eyes at certain time points in this real-world study of difficult-to-treat DME eyes may add noise to the interpretation of effect of DEX and FAc implant treatments on BCVA and CFT. Larger study cohorts of difficult-to-treat DME eyes are needed, however, to validate the findings reported in this study.
In the FAc implant treated group, CFT decreased by approximately 200 µm in the first 12 months, which is in line with the CFT reduction reported by a UK-based real-world study by Alfaqawi et al,17 but almost double the CFT reduction reported by 2 other UK based real-world studies.18,19 Baseline CFT was slightly higher in this study (FAc cohort: 513.7 µm; DEX cohort: 462 µm) (Table 1), compared with other real-world FAc implant studies (451–494 µm).17–20 which may reflect a higher DME disease activity in our study population.
In the DEX-treated eyes that completed 6 months’ follow-up, CFT decreased by approximately 133.1 µm at month 1, which is more than what was reported in similar real-world studies such as REINFORCE, a large (n=180 eyes) US-based multicenter Phase 4 observational study, and CHROME, a Canadian multicenter, retrospective cohort study of 120 DME eyes.21,22 The timing of the CFT nadir in the DEX-treated group in the present study was at 1–3 months, which is similar to the two-month CFT nadir reported in REINFORCE.22 In contrast, in the FAc implant treated eyes, the increase in BCVA and decrease in CFT was sustained, stable and predictable for up to 24 months, which aligns well with previously reported long-term effects of the FAc implant on CFT.6 After 24 months, the number of patients treated with a FAc implant started to decline, which prevented meaningful effectiveness analyses post-24 months. Further real-world studies with larger patient cohorts are needed to assess outcomes after 3 years of follow-up, which is the duration of action for which the FAc implant has been designed.6,14
It is difficult to evaluate the effect of FAc and DEX implants on IOP in this real-world study, as study participants in both the FAc and DEX implant cohorts were being treated with IOP-lowering medication at baseline.
No head-to-head studies comparing the safety and efficacy of FAc and DEX implants have been published to date. This may, at least partly, be because FAc and DEX implants have different indications for the treatment of DME, which precludes direct comparisons of their effectiveness and safety.8,14
In this real-world study, treatment with FAc and DEX intravitreal implants led to statistically and clinically significant improvements in both BCVA and CFT in vitrectomized DME eyes. The FAc implant demonstrated sustained, stable and predictable effects on BCVA and CFT over 24 months. Importantly, the FAc implant also improved BCVA and decreased CFT in a cohort of DME eyes that displayed inadequate response to the DEX implant over 6 months.
In conclusion, our study demonstrates long-term effectiveness of the 0.2 µg/day FAc implant in vitrectomized DME eyes in a real-world setting, as well as demonstrating sustained effectiveness in previously treatment-refractory vitrectomized DME eyes.
Ethics statement
Subjects (or their parents or guardians) have given their written informed consent and the study protocol has been approved by the research ethics committee of Centro Hospitalar Universitário do Porto.
Acknowledgment
Writing support was provided by Hayward Medical Communications and funded by Alimera Sciences Ltd. This paper was funded by Alimera Sciences Ltd.
Author contributions
All authors contributed towards data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. International Diabetes Federation. IDF diabetes atlas - 8th Edition. Available from: http://diabetesatlas.org/resources/2017-atlas.html
2. Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond). 2015;2:17. doi:10.1186/s40662-015-0026-2
3. Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XV. The long-term incidence of macular edema. Ophthalmology. 1995;102(1):7–16. doi:10.1016/s0161-6420(95)31052-4
4. El-Ghrably I, Steel DHW, Habib M, Vaideanu-Collins D, Manvikar S, Hillier RJ. Diabetic macular edema outcomes in eyes treated with fluocinolone acetonide 0.2 microg/d intravitreal implant: real-world UK experience. Eur J Ophthalmol. 2017;27(3):357–362. doi:10.5301/ejo.5000929
5. Wells JA, Glassman AR, Ayala AR, et al.; Diabetic Retinopathy Clinical Research N. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193–1203. doi:10.1056/NEJMoa1414264
6. Campochiaro PA, Brown DM, Pearson A, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119(10):2125–2132. doi:10.1016/j.ophtha.2012.04.030
7. Boyer DS, Yoon YH, Belfort R
8. Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, et al. Guidelines for the management of diabetic macular Edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica. 2017;237(4):185–222. doi:10.1159/000458539
9. Hussain RM, Ciulla TA. Treatment strategies for refractory diabetic macular edema: switching anti-VEGF treatments, adopting corticosteroid-based treatments, and combination therapy. Expert Opin Biol Ther. 2016;16(3):365–374. doi:10.1517/14712598.2016.1131265
10. Campochiaro PA, Brown DM, Pearson A, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118(4):626–35 e2. doi:10.1016/j.ophtha.2010.12.028
11. Danis RP, Sadda S, Li XY, Cui H, Hashad Y, Whitcup SM. Anatomical effects of dexamethasone intravitreal implant in diabetic macular oedema: a pooled analysis of 3-year phase III trials. Br J Ophthalmol. 2016;100(6):796–801. doi:10.1136/bjophthalmol-2015-306823
12. Quhill F. BA. Cost advantage of fluocinolone Acetonide Implant (ILUVIEN® versus Ranibizumab in the treatment of chronic disbetic macular oedema. Glob Reg Health Technol Assess. 2017;4(1):e155–64.
13. Campochiaro PA, Nguyen QD, Hafiz G, et al. Aqueous levels of fluocinolone acetonide after administration of fluocinolone acetonide inserts or fluocinolone acetonide implants. Ophthalmology. 2013;120(3):583–587. doi:10.1016/j.ophtha.2012.09.014
14. Alimera Sciences Limited. Iluvien summary of product characteristics. Available from: https://www.medicines.org.uk/emc/product/3061/smpc
15. Bucolo C, Gozzo L, Longo L, Mansueto S, Vitale DC, Drago F. Long-term efficacy and safety profile of multiple injections of intravitreal dexamethasone implant to manage diabetic macular edema: a systematic review of real-world studies. J Pharmacol Sci. 2018;138(4):219–232. doi:10.1016/j.jphs.2018.11.001
16. Nurozler Tabakci B, Unlu N. Corticosteroid treatment in diabetic macular edema. Turk J Ophthalmol. 2017;47(3):156–160. doi:10.4274/tjo.56338
17. Alfaqawi F, Lip PL, Elsherbiny S, Chavan R, Mitra A, Mushtaq B. Report of 12-months efficacy and safety of intravitreal fluocinolone acetonide implant for the treatment of chronic diabetic macular oedema: a real-world result in the United Kingdom. Eye (Lond). 2017;31(4):650–656. doi:10.1038/eye.2016.301
18. Bailey C, Chakravarthy U, Lotery A, Menon G, Talks J, Medisoft Audit G. Real-world experience with 0.2 mug/day fluocinolone acetonide intravitreal implant (ILUVIEN) in the United Kingdom. Eye (Lond). 2017;31(12):1707–1715. doi:10.1038/eye.2017.125
19. Fusi-Rubiano W, Mukherjee C, Lane M, et al. Treating Diabetic Macular Oedema (DMO): real world UK clinical outcomes for the 0.19mg Fluocinolone Acetonide intravitreal implant (Iluvien) at 2 years. BMC Ophthalmol. 2018;18(1):62. doi:10.1186/s12886-018-0726-1
20. Holden SE, Currie CJ, Owens DR. Evaluation of the clinical effectiveness in routine practice of fluocinolone acetonide 190 microg intravitreal implant in people with diabetic macular edema. Curr Med Res Opin. 2017;33(sup2):5–17. doi:10.1080/03007995.2017.1366645
21. Lam WC, Albiani DA, Yoganathan P, et al. Real-world assessment of intravitreal dexamethasone implant (0.7 mg) in patients with macular edema: the CHROME study. Clin Ophthalmol. 2015;9:1255–1268. doi:10.2147/OPTH.S80500
22. Singer MA, Dugel PU, Fine HF, Capone A
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.