Back to Journals » Clinical Ophthalmology » Volume 14
Real-World Outcomes in Patients with Diabetic Macular Edema Treated Long Term with Ranibizumab (VISION Study)
Authors Van Aken E, Favreau M, Ramboer E , Denhaerynck K , MacDonald K , Abraham I , Brié H
Received 11 September 2020
Accepted for publication 16 November 2020
Published 2 December 2020 Volume 2020:14 Pages 4173—4185
DOI https://doi.org/10.2147/OPTH.S281501
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Elisabeth Van Aken,1,2 Mérédis Favreau,3 Eva Ramboer,3 Kris Denhaerynck,4,5 Karen MacDonald,4 Ivo Abraham,4,6– 8 Heidi Brié3
1Department of Ophthalmology, AZ Sint-Elisabeth, Zottegem, Belgium; 2Department of Head and Skin, Ghent University, Ghent, Belgium; 3Medical Department, Novartis Pharma, Vilvoorde, Belgium; 4Department of Research and Consulting, Matrix45, Tucson, AZ, USA; 5Department of Public Health, University of Basel, Basel, Switzerland; 6Center for Health Outcomes and Pharmacoeconomic Research, University of Arizona, Tucson, AZ, USA; 7Department of Pharmacy Practice and Science, College of Pharmacy, University of Arizona, Tucson, AZ, USA; 8Department of Family and Community Medicine, College of Medicine – Tucson, University of Arizona, Tucson, AZ, USA
Correspondence: Heidi Brié
N.V. Novartis Pharma S.A, Medialaan 40, B-1800 Vilvoorde, Belgium
Tel +32.2.246.1774
Email [email protected]
Aim: Evaluate long-term real-world treatment patterns and associated effectiveness and safety outcomes in patients with diabetic macular edema (DME) treated ≥ 36 months with 0.5mg ranibizumab.
Methods: Open-label observational effectiveness study in 9 Belgian clinics. Included were primary treated eyes of 55 DME patients between August 2014 and March 2015 and followed for 3.5± 1.8 years. Eyes were 21.8% treatment (TX)-naïve, 9.1% non-naïve with exclusive prior anti-VEGF treatment (PRIOR-anti-VEGF), and 63.6% non-naïve with other prior treatments (PRIOR-other). Intravitreal injections with ranibizumab were administered per ophthalmologists’ best clinical judgment. Trend testing of changes in best-corrected visual acuity (BCVA) and central retinal thickness (CRT) over time occurred using mixed regression analysis.
Results: The mean±SD number of treatments in the first year was 5.1± 3.0 (TX-naïve), 4.5± 2.7 (PRIOR-anti-VEGF) and 5.6± 3.1 (PRIOR-other). At 12 months, BCVA increased by 8.9± 16.4 letters from 59.7± 9.3 at baseline in TX-naïve (p< 0.0001), by 11.8± 9.9 from 61.6± 8.5 in PRIOR-anti-VEGF (p=0.03), and by 4.2± 10.6 from 58.2± 14.6 in PRIOR-other groups (p=0.0002). BCVA remained stable for the remainder of follow-up in all groups. CRT decreased over the first 2 months by monthly rates of − 43.8μm in TX-naïve (p=0.04), − 75.7μm in PRIOR-anti-VEGF (p=0.02), and − 65.8μm in PRIOR-other eyes (p=0.0003), showing stability afterwards. No unknown adverse events were recorded; a painful eye following injection was registered with a possible relationship to the treatment.
Conclusion: This real-world study confirms the effectiveness of ranibizumab in preventing a decline in BCVA and demonstrated initial improvement and subsequent retention of BCVA in DME patients ≥ 36 months. Ranibizumab initially reduced and then maintained CRT. However, these data reveal that treatment intensity and BCVA and CRT outcomes are lower than those found in early efficacy trials. Under-treatment likely accounts for this efficacy-effectiveness gap. Yet, intravitreal ranibizumab is an effective and safe long-term treatment for DME under conditions of significant heterogeneity in patients and treatment patterns.
Keywords: diabetic macular edema, ranibizumab, best-corrected visual acuity, central retinal thickness, long-term effectiveness
Introduction
Diabetic macular edema (DME) is a microvascular complication and one of the major vision-threatening causes in the working-age population affecting approximately 7% of patients with diabetes worldwide.1 Although, the pathogenesis of DME has not been fully elucidated yet, vascular endothelial growth factor (VEGF) has been identified as one of the main factors contributing in the breakdown of the blood-retinal barrier.2,3 Ranibizumab (Lucentis®, Novartis, Basel, Switzerland and Genentech Inc., South San Francisco, CA, USA) represents a standard first-line therapy for several retinal diseases, including DME. Its efficacy and safety in patients with DME were evaluated in several trials.4–9 They demonstrated that the efficacy of ranibizumab in the first year is associated with injection frequency, but also noted that visual outcomes were maintained in later years despite less frequent injections.
The critical importance of the frequency of injections in patients with DME was underscored in the Protocol I and Protocol T trials by the Diabetic Retinopathy Clinical Research Network (DRCR.net). The Protocol I study, a randomized controlled trial of ranibizumab plus prompt or deferred laser therapy, showed that first-year treatment with a median of 8 injections in the prompt laser group and 9 in the deferred laser group resulted in a mean overall +9.0 letter advantage in both groups.10 The five-year results from this study revealed that long-term outcomes (mean letter gain of +7.2 in the prompt laser group and +9.8 in the deferred group) were achieved with diminished median number of injections in subsequent years (13 total injections over 5 years in the prompt laser group and 17 in the deferred group).11 The Protocol T study, a comparative effectiveness trial of the three VEGF inhibitors aflibercept, bevacizumab, or ranibizumab likewise showed that the first year mean improvement of +11.2 letters following treatment with a median of 10 injections in the ranibizumab arm12 was maintained at the end of year 2 with a median of 6 additional injections.13
Randomized controlled clinical trials have provided a strong evidence base of ranibizumab regimens in the treatment of DME, with especially the DRCR.net Protocol I and Protocol T studies10–13 demonstrating the association between frequency of injections and short- and long-term outcomes. However, there are only limited data on actual treatment patterns and outcomes in daily clinical practice. Real-world data are essential in understanding how treatments, previously shown to be efficacious in pivotal studies characterized by significant homogeneity in patients, settings, and regimens, perform under conditions of greater heterogeneity. The VISION study therefore aimed to assess the long-term (36 months) treatment patterns and associated effectiveness and safety outcomes of ranibizumab in patients with DME in real-world clinical practice.
Materials and Methods
Design, Sample and Setting
VISION was an open-label, observational, multicenter study conducted in nine Belgian outpatient ophthalmology clinics in patients with visual impairment due to DME treated with intravitreal ranibizumab (0.5 mg) per their ophthalmologist’s best clinical judgment. Being an observational study, there were no fixed time points for follow-up visits or required treatments. Follow-up intervals and treatment decisions, including retreatment, were at the discretion of the ophthalmologist. The study included, retrospectively, DME patients treated with ranibizumab in a compassionate care program prior to reimbursement approval and subsequently followed prospectively, as well as patients in whom treatment was initiated after reimbursement approval. Excluded were patients treated with a VEGF inhibitor other than ranibizumab in the 90 days prior to study enrollment, and those concurrently using other investigational drugs. Eligible patients were included in the study after providing written informed consent, or, if incapable of doing so, after such consent had been provided by a legal representative of the patient.
Data Collection
All data were collected as available from routine clinical practice. There were no mandatory assessments and tests. For retrospective data, patients’ medical records were the source and data were entered into an electronic case report form. Prospective data collection was performed by the treating physician after each visit, and entered into the electronic case report form.
Variables included at baseline were: age, gender, comorbidities, smoking history, ocular history, prior treatments, time since diagnosis, baseline effectiveness parameters of the primary eye (i.e. best-corrected visual acuity [BCVA] via Early Treatment Diabetic Retinopathy Study (ETDRS) letters or equivalent, and central retinal thickness [CRT] by optical coherence tomography), and initial ranibizumab treatment. The primary eye was defined as the first eye treated; or in case of concurrent treatment initiation, the eye with lowest visual acuity (VA); or, if VA was equal, a randomly chosen eye. The primary eye was classified into 1 of 3 groups based on pre-treatment status: 1) Treatment-naïve eyes (TX-naïve), which had not been pre-treated with any intravitreal medication (ranibizumab/other VEGF inhibitor/corticosteroid) or laser; 2) Eyes previously treated with VEGF inhibitors only (PRIOR-anti-VEGF), which had been treated with at least 1 treatment of ranibizumab or other VEGF inhibitor(s); and 3) Eyes previously treated with other treatments (PRIOR-other), which had been pre-treated with at least one ocular treatment other than VEGF inhibitors, regardless whether this treatment also included VEGF inhibition.
Data recorded during follow-up were: administration date of ranibizumab, relevant concomitant ocular therapies, (serious) adverse events ([S]AE), reasons for discontinuation, and effectiveness parameters. We calculated the number of ranibizumab injections per year, omitting the last uncompleted calendar year to prevent underreporting. Safety evaluations comprised the monitoring and assessment of occurrence, relationship, and severity of non-ocular and ocular (S)AEs during the study period.
Note that some patients may have had DME in both eyes. Only data on the primary eye are included in the analyses.
Effectiveness Measures
The primary effectiveness parameter used for long-term evaluation was change in BCVA since baseline, expressed in ETDRS letters gained. If the VA was recorded using Snellen fractions, conversion to an approximate letters score was accomplished using the formula [85 + (50 * log10 Snellen fraction)] rounded to the nearest integer.14 The secondary effectiveness endpoint was change in CRT since baseline, expressed in µm.
As available and per clinical schedule, effectiveness parameters were recorded monthly for the first three months within a time window of ±0.5 month and subsequently semi-annually within a time window of ±2.5 months. These time windows allowed capturing the visit closest to the selected time point provided it fell within 0.5 or 2.5 months at either sides of that point. The data recorded closest to the start of treatment were considered the baseline data. BCVA change from baseline to the last measurement was expressed nominally as letters recorded, but also classified into the following categories: gains of 15 or more letters, 10–14 letters, 5–9 letters, or 1–4 letters; or losses of 0–9 letters, 10–14 letters, or 15 or more letters.
Statistical Analysis
Patient demographics, medical history, ocular disease history, prior treatments, and other baseline characteristics were analyzed using standard descriptive statistics under consideration of applicable levels of measurement. Testing for significance of change over time in BCVA and CRT was done using linear mixed regression analysis, employing random intercepts to separate within-center from between-center and within-patient from between-patient variability. Evaluation of change over time between the TX-naïve, PRIOR-anti-VEGF, and PRIOR-other treatment subgroups was achieved by entering an interaction of subgroup and time variables and specifying contrasts for specific hypotheses to be tested, e.g. omnibus or pairwise comparisons. The level of statistical significance was set at 0.05. All statistical analyses were performed using the SAS® v.9.4 statistical software package (SAS Institute Inc., Cary, NC, USA).
Compliance
VISION was designed, implemented, and reported in accordance with the Guidelines for Good Pharmacoepidemiology Practices of the International Society for Pharmacoepidemiology, the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guideline, and with the ethical principles laid down in the Declaration of Helsinki. The protocol and proposed informed consent form were reviewed and approved by a properly constituted Independent Ethics Committee of the University Hospital KU Leuven, Belgium (BE322201421737, July 16, 2014) before study initiation. All study subjects or their legally authorized proxy provided written informed consent.
Results
Sample
The VISION evaluable sample consisted of primary treated eyes of 55 patients with DME. Almost half of the patients also developed DME in a secondary eye (49.1%; n=27). The safety sample included both the primary and secondary eyes (n=82).
The median duration of follow-up of primary eyes was 42 months (interquartile range [IQR] 21–60) with a mean±SD of 41.6±21.3 months (Table 1). Study discontinuation was reported in a total of 26 (47.3%) DME patients. Switch to other anti-angiogenic agents (n=8, 14.6%) or another treatment (n=4, 7.3%), death (n=6, 10.9%) and patient not seen in over one year (n=4, 7.3%) were the primary reasons for premature study stop. Follow-up was discontinued at median of 25.5 months (IQR 12–39) for 26 (47.3%) eyes (mean 27.4±17.6 months). At inclusion, patients were on average 67.3±8.6 years old and two-thirds were male (65.5%; n=36), and 47 (85.5%) had type 2 diabetes. The majority of patients also had hypertension (70.9%; n=39) and/or hypercholesterolemia (56.4%; n=31). Over half had pseudophakic implants (58.2%, n=32), 16.4% had undergone vitrectomy (n=9) and 16.4% had glaucoma (n=9). In terms of prior treatment experience, 21.8% were treatment-naïve (n=12), 9.1% had been previously treated with anti-VEGF (n=5) and 63.6% were previously treated with other treatments (n=35). At baseline, mean±SD BCVA was 59.3±12.8 letters and CRT was 453.1±119.9 µm.
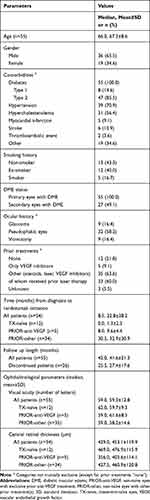 |
Table 1 Patient Demographic and Clinical Characteristics at Start of Ranibizumab Treatment (n=55) |
Treatment Patterns
The average number of visits in the first year was 9.9±3.5 (Table 2). Ranibizumab was administered on average in 5.2±3.0 of these visits, without significant differences between prior treatment subgroups (p=0.40), with administrations occurring on average 5.1±3.0 in the TX-naïve, 4.5±2.7 in the PRIOR-anti-VEGF, and 5.6±3.1 in the PRIOR-other subgroups. Treatment frequency decreased over time in the TX-naïve (p=0.01) and PRIOR-other (p<0.0001) but not in the PRIOR-anti-VEGF (p=0.51) subgroups of eyes. Intervals between treatments in the first year were on average 7.1±5.6 weeks apart for TX-naïve, 9.9±8.9 weeks for PRIOR-anti-VEGF, and 7.3±6.0 weeks for the PRIOR-other eyes. Intervals generally lengthened over time in accordance with the decrease in treatment frequencies over time (Figure 1).
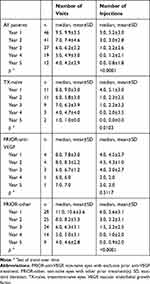 |
Table 2 Ranibizumab Treatment Patterns (n=55) |
 |
Figure 1 Time intervals between injections. |
Visual Acuity
Figure 2 and Table 3 present the mean monthly changes in ETDRS letters read from baseline by prior treatment status. The overall trend line in Figure 2 shows an initial increase in visual acuity during the first six months. At one year, on average primary eyes of TX-naïve patients gained +8.9±16.4 letters (median=15), those of PRIOR-anti-VEGF patients gained +11.8±9.9 letters (median=11.3), and those of PRIOR-other patients gained +4.2±10.6 letters (median=3) (Table 3). These first-year improvements in visual were statistically significant at an annual rate of β=13.01 (95% CI: 6.71, 19.32; p<0.0001) for TX-naïve, β=8.46 (95% CI: 0.64, 16.28; p=0.03) for PRIOR-anti-VEGF, and β=6.19 (95% CI: 2.93, 9.45; p=0.0002) for PRIOR-other primary eyes. Visual acuity remained stable beyond the first year, as indicated by the subsequent statistically non-significant annual rates of β=−0.47 (95% CI: −1.89, 0.95; p=0.52) for TX-naïve, β=0.24 (95% CI: −1.43, 1.92; p=0.76) for PRIOR-anti-VEGF, and β=−0.23 (95% CI: −0.82, 0.36; p=0.45) for PRIOR-other eyes. This is also illustrated by the fitted trend line in Figure 2 that shows overall gains maintained through study end. At the end of follow-up, 11/12 (92%) of TX-naïve eyes showed gains of at least +5 letters and 1/12 (8%) maintained VA with 0 letters gained/lost. Similarly, in PRIOR-anti-VEGF eyes, 4/5 (80%) showed gains of at least 1 letter and 1/5 (20%) maintained VA with 0 letters gained/lost. By contrast, in PRIOR-other eyes, only 16/35 (46%) showed gains of at least +1 letter, 7/35 (20%) maintained VA with 0 letters gained/lost, while 12/35 (34%) had a loss of 1 or more letters (Table 4).
 |
Table 3 Functional and Anatomical Outcomes Across Selected Time Points (n=55) |
 |
Table 4 Categorized VA Change at End of Follow Up Relative to Baseline |
Central Retinal Thickness
Table 3 and Figure 3 show the mean monthly changes in CRT from baseline by prior treatment status. The overall trend line in Figure 3 shows an initial decrease in CRT during the first 2 months. CRT changed at statistically significant monthly rates of −43.8 µm in TX-naïve eyes (95% CI: −86.1, −1.4; p=0.04), −75.7 µm in PRIOR-anti-VEGF eyes (95% CI: −183.0, −13.5; p=0.02), and −65.8 µm in PRIOR-other eyes (95% CI: −96.8, −34.8; p=0.0003) over these 2 months. Afterwards, CRT remained stable long term for TX-naïve (β= –0.2, 95% CI: –0.9, 0.6; p=0.62) and PRIOR-anti-VEGF eyes (β= –0.4, 95% CI: –1.4, 0.6; p=0.61), and slightly decreased further for PRIOR-other eyes (β= –1.15, 95% CI: –1.50, –0.79, p=<.0001). This is also illustrated by the fitted trend line in Figure 3 that shows overall improvements in CRT maintained through study end.
Safety
In the safety sample of 82 primary and secondary eyes, 52 ocular and 55 non-ocular (107 total) AEs were registered (Table 5) in 35 patients. Only one (non-serious) AE was suspected to be treatment-related (painful eye some days after the injection). Of the 107 AEs, 3 resulted in treatment discontinuation (Table 6). Thirty-five were considered serious AEs (Table 7), included 7 fatal or life-threatening, 25 requiring hospitalization or prolonging existing hospitalization, and 3 classified as otherwise medically significant events. Six patients died, however, none of these deaths were suspected to be related to ranibizumab treatment (Table 8).
 |
Table 5 Adverse Events: MedDRA Coded Organ Classes |
 |
Table 6 Events Leading to Discontinuation |
 |
Table 7 Classes of Serious Adverse Events per Prior Treatment Status a |
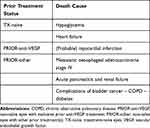 |
Table 8 Causes of Death |
Discussion
Observational studies of real-world effectiveness and safety of treatments previously deemed efficacious and (in as far as possible considering the typical sample sizes in randomized controlled trials) safe have an important role in understanding who is being treated in routine clinical practice and how then patients respond to treatment. This is certainly the case with biologicals such as anti-VEGF agents as we, among others, have previously shown for ranibizumab in the neovascular age-related macular degeneration indication.15 The VISION study reported here is among the first studies aiming to evaluate the long-term real-world effectiveness of ranibizumab in DME.
Anti-VEGF considerably changed the treatment, management and prognosis of DME, with ranibizumab being the first anti-VEGF agent approved for this indication. Randomized controlled clinical trials have provided a strong evidence base of ranibizumab regimens in the treatment of DME; however, there are only limited data on actual treatment patterns and outcomes in daily clinical practice. Therefore, the VISION study provides valuable data about the real-world long-term safety and efficacy (median follow-up of 42 months, i.e., 3.5 years) in a heterogeneous sample of Belgian DME patients treated with ranibizumab.
Overall, in VISION, injections decreased from an average of 5.2 in year 1 to 0.8 in year 5. BCVA gains over baseline included a mean of +5.3 letters at year 1, +8.9 at year 2 and +5.8 at year 3. The results for the three subgroups of eyes (TX-naïve; PRIOR-anti-VEGF; PRIOR-other) suggest variation by treatment status, though caution is advised due to the limited sample sizes in, especially, the TX-naïve and PRIOR-anti-VEGF groups. Eleven of the 12 TX-naïve eyes administered ranibizumab showed a gain in letters peaking at a median +15 letters over baseline at 3, 6, and 9 months; with decreases in CRT reaching a median of −144.50 µm over baseline at 24 months. This suggests that eyes not previously treated may be more likely to show fast or strong response to ranibizumab. Four of the 5 PRIOR-anti-VEGF treated eyes evidenced a gain in letters as high as a median +15 letters at 48 months, and a peak decline in CRT by a median of −99 µm at 6 months – though with marked variation over time in both BCVA and CRT. Representing only 5 eyes or 9% of the study sample and lacking detailed information as to type and intensity of prior anti-VEGF treatment permits at most a hypothesis of cumulative and/or residual anti-VEGF effect in these few patients. In the PRIOR-other subgroup, which included 35/55 (63.6%) eyes, only 16/35 showed a gain in BCVA and 7 maintained BCVA while 12 evidenced a loss. BCVA peaked at a median of +7 letters gained at 3 months, while CRT declined by as much as a median of −125 µm at 24 months. In year 1 an average gain of +4.2 letters was achieved with a mean number of 5.6 treatments.
These real-world effectiveness results diverge from the efficacy findings of the initial randomized controlled trials, which had higher treatment intensity, while aligning with those with less frequent treatment. The initial Phase II and III clinical studies indicated that a higher injection frequency in the first year of treatment is associated with better VA outcomes. This can be attributed to the intensity of the initiation treatment, a strict re-treatment algorithm, and close monitoring during the active treatment phase. For instance, in RESOLVE an average of 10.2 injections yielded a +10.3 letter benefit during the first year of treatment;4 in RISE and RIDE monthly injections for 24 months resulted in at least a +10.9 letter gain;7 a median of 8 (in the prompt laser group) or 9 injections (deferred laser group) in the first year produced a mean overall +9.0 letter advantage in both groups in the Protocol I study,10 and a median of 10 injections in the first year of the Protocol T study induced an overall mean improvement of +11.2 letters.12 In contrast, in RESTORE patients receiving 7 injections of ranibizumab in year 1 had a mean gain of +6.1 letters.5 Likewise, the lower visual acuity outcomes in RELIGHT (+4.8 letters),16 REVEAL (+5.9 letters),17 READ-2 (+6.6 letters),18 and RETAIN (+7.4 letters)19 followed fewer first year injections than in the initial trials.
Importantly, several of the published studies with higher intensity treatment in the first year and better corresponding outcomes also demonstrated sustained outcomes in subsequent years but with less frequent treatment in those following years.6,9,11,13 This suggests that number of injections alone does not determine treatment outcome but that treatment intensity during the initial treatment phase may be a critical factor. Timing of treatment initiation may also play an important role as Bressler and colleagues20 observed that eyes receiving deferred ranibizumab did not achieve the same 5-year improvement as did eyes treated first-line. In addition to delaying ranibizumab therapy, such factors as chronic DME recurrences and laser-related structural damage may cause irreversible vision loss due to neural cell loss, retinal atrophy, or other permanently induced changes.20
In VISION, where treatment regimens were set per the prescribing ophthalmologist’s best clinical judgment, the number of treatments in year 1 and subsequent years was lower than in the several randomized trials4,7,10,12 and more aligned with the randomized studies using a milder initiation treatment and more moderate retreatment criteria.5,16–19 This raises the question as to whether the milder treatment patterns observed in VISION (as well as those in the later randomized controlled trials) may have been insufficient to maximize initial letter gains in eyes in general and especially in eyes that were treatment-naïve. Additionally, at 59 letters the median baseline BCVA in VISION was lower than what was observed in the large prospective randomized DME trials,4,5,10,16,19,21 suggesting delayed treatment initiation and, more generally, a pattern of under-treatment. Conceivably, better outcomes could be achieved with greater treatment intensity and greater sustained treatment in the longer term.
Anatomic improvements, evidenced by changes in CRT, were not as pronounced as those seen in the earlier efficacy trials. In the sample at large, decreases in CRT were −62 µm at 12 months compared to changes ranging from −118.7 to −194.2 µm in the randomized clinical trials.4,5,8,10,12 In VISION, declines in CRT peaked at −111 µm at 24 months. As with the BCVA outcomes seen in this VISION real-world study that fall short of those of earlier efficacy trials, the smaller reductions in CRT may be due to less frequent injection schedule.
Several real-world studies on ranibizumab in DME have evaluated treatment patterns and outcomes over periods of 9 to 24 months.22–35 Mean number of ranibizumab injections ranged from 3.1 to 7.2. VA gains varied between +0.0 and +8.4 letters with CRT reductions ranging from −83.9 µm to −164.6 µm. Recently, Epstein and Amrén reported on a real-world study in Sweden with 4 years of follow-up. Quite similarly to our findings, an average of 4.7 injections resulted in a 12-month +6.4 letter advantage, which was maintained over the 4 years of follow-up together with a decline in the number of injections.36 Also in the LUMINOUSTM study, which represents the largest real-world study in medical retina across all approved ranibizumab indications, preliminary results trend to demonstrate similar effectiveness and treatment patterns in the DME cohort.35 Overall, a gain of 3.5 letters obtained with an average of 4.5 injections was observed in the first year. Despite VA acuity being maintained at year 2, injection numbers varied widely across regions and remained generally low.35 It is evident that, as in our study, real-world clinical practice of ranibizumab in DME is characterized by a degree of under-treatment, including lower intensity (i.e., lower injection rates and longer durations between treatments), relative to the treatment regimens evaluated in major clinical trials.
Several factors may contribute to the lower injection rates and lower treatment intensity and the associated outcomes noticed in the everyday clinical practice. Comorbidities and concomitantly or previously received treatments, in general excluded in clinical trials, may limit the real-world effectiveness outcomes obtained with ranibizumab. Patients in real-world effectiveness studies are likely to be more diverse compared to the defined patient populations enrolled in efficacy trials in terms of stage of the disease, baseline characteristics, adherence to appointments and treatments; comorbidities with specific additional disease burdens; while clinical practice may vary in terms of frequency of injections, frequency of monitoring, and re-treatment criteria. Specific to Belgium, the VISION study was initiated when the reactive (in response to outcomes) as-needed dosing regimen prevailed and the pro-active personalized treat-and-extend-treatment approach was still novel.
Our study has limitations. As a real-world study, VISION utilized a convenience sample which may introduce selection bias. Likewise, attrition bias cannot be excluded due to the high drop-out rate. Furthermore, as this was an observational study on ranibizumab practice patterns and treatments, all patients were treated with ranibizumab and there was no randomization or comparator group of untreated patients or patients treated exclusively with other therapies. Lastly, the limited number of TX-naïve and PRIOR-anti-VEGF eyes may limit the reliability of the results particularly beyond 24 months. Taken together, caution should be taken when interpreting the results beyond the study sample. Future research could address some of these issues by incorporating a larger sample, comparative groups (e.g., with other pharmacotherapies and/or laser treatment), and a longer follow-up duration. Also, missed follow-up visits could be assessed to see if patient adherence to the scheduled follow-up plan affects long-term outcomes.
Conclusion
VISION is among the first studies reporting long-term real-world clinical data in DME patients treated with ranibizumab, albeit in a small sample from a single country. These data demonstrated significant improvements in visual acuity and CRT in the majority of the DME patients in the first few months after initiating ranibizumab treatment, followed by a stabilization of these achieved improvements and a reduction of injections in the subsequent years. The incidence of ocular and non-ocular AEs was low and no unknown safety signals were identified. Findings support the well-characterized benefit profile of ranibizumab treatment in DME within a real-world clinical setting, though with a pattern of relative under-treatment and lower treatment intensity relative to previous randomized controlled clinical trials.
Abbreviations
BCVA, best-corrected visual acuity; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CRT, central retinal thickness; DME, diabetic macular edema; ETDRS, Early Treatment Diabetic Retinopathy Study; IQR, interquartile range; PRIOR-anti-VEGF, eyes previously treated with VEGF inhibitors only; PRIOR-other, eyes previously treated with other treatments; [S]AE, (serious) adverse events; SD, standard deviation; TX-naïve, treatment-naïve eyes; VA, visual acuity; VEGF, vascular endothelial growth factor.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study and manuscript were funded by Novartis Pharma. The funding organization participated in the design and implementation of the study and the preparation, review and approval of the manuscript.
Disclosure
Van Aken E has served on advisory boards for Novartis, the study sponsor. Favreau M, Ramboer E, and Brié H are employees of Novartis. Denhaerynck K is affiliated with Matrix45 which was hired by Novartis Pharma to develop the study protocol, conduct the statistical analysis and develop the study report and manuscript. Abraham I and MacDonald K are affiliated with and have equity in Matrix45 which was hired by Novartis Pharma to develop the study protocol, conduct the statistical analysis and develop the study report and manuscript. The authors report no other conflicts of interest in this work.
References
1. Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi:10.2337/dc11-1909
2. Aiello LP, Bursell S-E, Clermont A, et al. Vascular Endothelial Growth Factor-Induced Retinal Permeability Is Mediated by Protein Kinase C In Vivo and Suppressed by an Orally Effective -Isoform-Selective Inhibitor. Diabetes. 1997;46(9):1473–1480. doi:10.2337/diab.46.9.1473
3. Vinores SA, Youssri AI, Luna JD, et al. Upregulation of vascular endothelial growth factor in ischemic and non-ischemic human and experimental retinal disease. Histol Histopathol. 1997;12(1):99–109.
4. Massin P, Bandello F, Garweg JG, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33(11):2399–2405. doi:10.2337/dc10-0493
5. Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE Study. Ophthalmology. 2011;118(4):615–625. doi:10.1016/j.ophtha.2011.01.031
6. Schmidt-Erfurth U, Lang GE, Holz FG, et al. Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE Extension Study. Ophthalmology. 2014;121(5):1045–1053. doi:10.1016/j.ophtha.2013.11.041
7. Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 Phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. doi:10.1016/j.ophtha.2011.12.039
8. Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120(10):2013–2022. doi:10.1016/j.ophtha.2013.02.034
9. Boyer DS, Nguyen QD, Brown DM, Basu K, Ehrlich JS. Ride and Rise Research Group. Outcomes with as-needed ranibizumab after initial monthly therapy: long-term outcomes of the phase III RIDE and RISE trials. Ophthalmology. 2015;122(12):2504–2513. doi:10.1016/j.ophtha.2015.08.006
10. Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064–1077.e35. doi:10.1016/j.ophtha.2010.02.031
11. Elman MJ, Ayala A, Bressler NM, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology. 2015;122(2):375–381. doi:10.1016/j.ophtha.2014.08.047
12. Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193–1203.
13. Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123(6):1351–1359. doi:10.1016/j.ophtha.2016.02.022
14. Gregori NZ, Feuer W, Rosenfeld PJ. Novel method for analyzing snellen visual acuity measurements. Retina. 2010;30(7):1046–1050. doi:10.1097/IAE.0b013e3181d87e04
15. Jacob J, Brié H, Leys A, et al. Six-year outcomes in neovascular age-related macular degeneration with ranibizumab. Int J Ophthalmol. 2017;10(1):81.
16. Pearce I, Banerjee S, Burton BJL, et al. Ranibizumab 0.5 mg for diabetic macular edema with bimonthly monitoring after a phase of initial treatment: 18-month, multicenter, phase IIIB RELIGHT Study. Ophthalmology. 2015;122(9):1811–1819. doi:10.1016/j.ophtha.2015.05.038
17. Ishibashi T, Li X, Koh A, et al. The REVEAL Study: ranibizumab monotherapy or combined with laser versus laser monotherapy in Asian patients with diabetic macular edema. Ophthalmology. 2015;122(7):1402–1415. doi:10.1016/j.ophtha.2015.02.006
18. Nguyen QD, Shah SM, Khwaja AA, et al. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2010;117(11):2146–2151. doi:10.1016/j.ophtha.2010.08.016
19. Prünte C, Fajnkuchen F, Mahmood S, et al. Ranibizumab 0.5 mg treat-and-extend regimen for diabetic macular oedema: the RETAIN study. Br J Ophthalmol. 2016;100(6):787–795. doi:10.1136/bjophthalmol-2015-307249
20. Bressler SB, Glassman AR, Almukhtar T, et al. Five-year outcomes of ranibizumab with prompt or deferred laser versus laser or triamcinolone plus deferred ranibizumab for diabetic macular edema. Am J Ophthalmol. 2016;164:57–68. doi:10.1016/j.ajo.2015.12.025
21. Berger A, Sheidow T, Cruess AF, Arbour JD, Courseau A-S, de Takacsy F. Efficacy/safety of ranibizumab monotherapy or with laser versus laser monotherapy in DME. Can J Ophthalmol. 2015;50(3):209–216. doi:10.1016/j.jcjo.2014.12.014
22. Brynskov T, Laugesen CS, Sorensen TL. Intravitreal ranibizumab for diabetic macular oedema: 1-year experiences in a clinical setting. Acta Ophthalmol. 2013;91(3):e243–244. doi:10.1111/aos.12014
23. Calugaru D, Calugaru M. Real-world outcomes of ranibizumab treatment for diabetic macular edema in a United Kingdom National Health Service setting. Am J Ophthalmol. 2017;174:175–176. doi:10.1016/j.ajo.2016.10.013
24. Egan C, Zhu H, Lee A, et al. The United Kingdom Diabetic Retinopathy Electronic Medical Record Users Group, Report 1: baseline characteristics and visual acuity outcomes in eyes treated with intravitreal injections of ranibizumab for diabetic macular oedema. Br J Ophthalmol. 2017;101(1):75–80. doi:10.1136/bjophthalmol-2016-309313
25. Farinha C, Martins A, Neves A, et al. Ranibizumab for the treatment of diabetic macular oedema in the real-world clinical setting in Portugal: A Multicentre Study. Ophthalmologica. 2018;1–8.
26. Granström T, Forsman H, Olinder AL, et al. Patient-reported outcomes and visual acuity after 12 months of anti-VEGF-treatment for sight-threatening diabetic macular edema in a real world setting. Diabetes Res Clin Pract. 2016;121:157–165. doi:10.1016/j.diabres.2016.09.015
27. Hodzic-Hadzibegovic D, Sander BA, Monberg TJ, Larsen M, Lund-Andersen H. Diabetic macular oedema treated with intravitreal anti-vascular endothelial growth factor - 2-4 years follow-up of visual acuity and retinal thickness in 566 patients following Danish national guidelines. Acta Ophthalmol. 2018;96(3):267–278. doi:10.1111/aos.13638
28. Holekamp NM, Campbell J, Almony A, et al. Vision Outcomes Following Anti–Vascular Endothelial Growth Factor Treatment of Diabetic Macular Edema in Clinical Practice. Am J Ophthalmol. 2018;191:83–91. doi:10.1016/j.ajo.2018.04.010
29. Koc İ, Kadayifcilar S, Eldem B. Real-world results of intravitreal ranibizumab, bevacizumab, or triamcinolone for diabetic macular edema. Ophthalmologica. 2018;239(2–3):85–93. doi:10.1159/000481180
30. Patrao NV, Antao S, Egan C, et al. Real-world outcomes of ranibizumab treatment for diabetic macular edema in a United Kingdom National Health Service setting. Am J Ophthalmol. 2016;172:51–57. doi:10.1016/j.ajo.2016.09.002
31. Stefanickova J, Cunha-Vaz J, Ulbig M, et al. A noninterventional study to monitor patients with diabetic macular oedema starting treatment with ranibizumab (POLARIS). Acta Ophthalmol. 2018;96(8):e942–949.
32. Vorum H, Olesen TK, Zinck J, Storling Hedegaard M. Real world evidence of use of anti-VEGF therapy in Denmark. Curr Med Res Opin. 2016;32(12):1943–1950. doi:10.1080/03007995.2016.1221803
33. Wecker T, Ehlken C, Buhler A, et al. Five-year visual acuity outcomes and injection patterns in patients with pro-re-nata treatments for AMD, DME, RVO and myopic CNV. Br J Ophthalmol. 2017;101(3):353–359.
34. Callanan DG, Loewenstein A, Patel SS, et al. A multicenter, 12-month randomized study comparing dexamethasone intravitreal implant with ranibizumab in patients with diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2017;255(3):463–473. doi:10.1007/s00417-016-3472-1
35. Mitchell P, Parikh S, Macfadden W. Effectiveness of ranibizumab for the treatment of patients with diabetic macular edema in a real-world setting: 1- and 2-year results from the LUMINOUS study. Invest Ophthalmol Vis Sci. 2018;59(9):3617.
36. Epstein D, Amrén U. Long-time outcome in patients treated with ranibizumab for diabetic macular edema. A 4-year study. Retina. 2018;38(1):183–186. doi:10.1097/IAE.0000000000001501
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


